Abstract
UiO-66-NH2, a zirconium-based functional metal–organic framework (MOF), was postsynthetically modified via Schiff base reaction between aldehyde groups in glutaraldehyde and amino groups in UiO-66-NH2 and CO2-preabsorbed polyethyleneimine (PEI). The resulting PEI-modified MOFs, abbreviated as PEIC@UiO, were characterized with 1H NMR, Fourier transform infrared, powder X-ray diffraction, Brunauer–Emmett–Teller, scanning electron microscopy, and thermogravimetric analysis and evaluated as CO2 adsorbents. In comparison with pristine UiO-66-NH2, the PEIC@UiO adsorbents have reduced specific surface area (7–150 m2/g) but maintained the same crystal structure. Particularly, the PEIC96@UiO adsorbent exhibited significantly improved CO2/N2 adsorption selectivity (48 vs 25) and higher CO2 adsorption capacity (3.2 vs 2.7 mmol/g). The adsorbent also displayed moderate desorption energy (68 kJ/mol CO2), superior moisture endurance, and recyclability, which are very desirable for practical applications.
1. Introduction
To control the concentration of carbon dioxide (CO2) in the air, carbon capture, utilization, and storage have received extensive attention in recent years.1−3 Currently, the chemical absorption using aqueous organic amines like monoethanolamine as absorbent is the most important method for CO2 capture from industrial waste gases.4−6 However, some inherent drawbacks such as degradation or volatilization of organic amines, equipment corrosion, and high energy consumption limit its practical application. Therefore, various kinds of solid porous adsorbents have been proposed for CO2 capture.
Metal–organic frameworks (MOFs) are three-dimensional porous crystalline materials made up of metal ions or clusters coordinated to organic linkers.7−10 As a new type of promising adsorbent materials,11,12 MOFs have shown enormous potential for CO2 capture due to large specific surface area, high crystallinity, tunable chemical structure, and pore properties.8 However, it remains a challenge to design MOFs with quite high selectivity as well as CO2 adsorption capacity because most MOFs do not possess high CO2 adsorption selectivity. Therefore, much research has been done to improve the CO2 adsorption selectivity of MOFs, including incorporation of open metal sites,13 construction of size/shape with specific pores,14 ligand functionalization to introduce strongly polar functional groups,15 and amine grafting.16
As a zirconium(IV)-based MOF, UiO-66 has attracted great interest because of its superior thermal and chemical stability.17 By functionalizing the ligand of UiO-66 with polar groups such as −NH2, −Br, −NO2, −(CF3)2, −SO3H, and −CO2H,18−20 the CO2 adsorption selectivity was improved while retaining the stability, especially when amino group was used as the functional group.20 Cmarik et al. reported that UiO-66-NH2 showed enhanced CO2 adsorption capacity (2.98 vs 1.75 mmol/g) and adsorption selectivity over N2 (13.2 vs 12.5) at 298 K and 1 bar20 when compared with UiO-66. The selectivity obtained by applying ideal adsorption solution theory (IAST) for a 15:85 CO2/N2 gas mixture was 52.20 Higher CO2 adsorption capacity (3,21 3.522 mmol/g) and CO2/N2 selectivity (3121) of UiO-66-NH2 were reported by Molavi et al.21 and Huang et al.22 Improvement of CO2 capture performance of UiO-66 by polyethyleneimine (PEI) impregnation23 or ethanolamine grafting24 was also reported.
For UiO-66-NH2, postsynthetic modification can further improve the CO2 capture performance.21,25,26 Molavi et al. modified UiO-66-NH2 with glycidyl methacrylate (GMA) by epoxy-amino reaction, resulting in improvement in CO2 adsorption capacity from 3.0 to 4.3 mmol/g and CO2/N2 selectivity from 31 to 46.21 The resulting GMA-UiO-66 was further functionalized with ethylenediamine through aza-Michael addition reaction to improve CO2 adsorption capacity up to 5.0 mmol/g.25 Piperazine-grafted UiO-66-NH2 was reported to have IAST selectivity up to 19 for a 15:85 CO2/CH4 gas mixture at 298 K at the expense of lower CO2 adsorption capacity.26 Interaction between amines with the open metal sites of MOFs has also been reported to improve CO2 adsorption capacity of MOFs at low pressure and room temperature, such as N,N′-dimethylethylenediamine (mmen)/[Mg2(dobpdc)],27 ethylenediamine/ZIF-8,28 tetraethylenepentamine (TEPA)/MIL-101-NH2,29 polyethyleneimine (PEI)/MIL-101,30,31 PEI/MIL-101-NH2,3232 and PEI/HKUST33 composites. In all these amine-modified MOFs, the MOFs act as supports with large specific surface area and the amine groups adsorb CO2. The synergism between them results in enhancement of the CO2 adsorption capacity and selectivity. PEI is a suitable polyamine with highly dense amine groups, especially accessible primary amine sites at chain ends, which can react with CO2 by forming carbamates.34 But as a viscous liquid, PEI itself is not a suitable candidate for CO2 adsorption. Therefore, PEI is usually supported onto porous materials such as zeolites,35 microporous/mesoporous silica,36 and porous polymers37 as well as MOFs23,30−33 to improve their CO2 sorption. Because of the higher porosity and specific surface area, MOFs seems to be more suitable for PEI incorporation to reach high CO2 adsorption capacity.
In this work, we report PEI-modified UiO-66-NH2 in an attempt to prepare CO2 adsorbent with high adsorption capacity, kinetics, and selectivity. UiO-66-NH2 was postsynthetically modified with PEI in the presence of glutaraldehyde (GD) via Schiff base reaction. The adsorbents were characterized with 1H NMR, Fourier transform infrared (FTIR), powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), and thermogravimetric analysis (TGA). CO2 adsorption was tested under different conditions to evaluate the adsorbents for potential application in CO2 capture from flue gas.
2. Results and Discussion
2.1. Synthesis and Structural Characterization
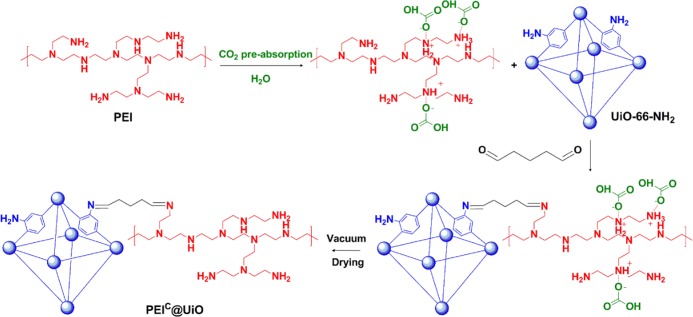
Powdery UiO-66-NH2 was prepared according to a previously reported procedure and then postsynthetically modified with PEI in the presence of GD, which linked UiO-66-NH2 and PEI together through Schiff base reaction, as illustrated in Scheme 1. He et al.42 reported that a CO2-imprinted solid amine adsorbent prepared by cross-linking CO2-preabsorbed PEI with glutaraldehyde showed a 25% higher CO2 adsorption capacity. In this study, CO2-preabsorbed PEI was also used in the postsynthetic modification to prepare three adsorbents named as PEIC@UiOx, in which x (48, 72, and 96%) means mass percentage of PEI based on UiO-66-NH2. For comparison, PEI72@UiO was prepared using PEI without CO2 preabsorption and PEI96+UiO was prepared by impregnation of PEI in the absence of glutaraldehyde. Detailed preparation conditions and structural characteristics of the adsorbents are summarized in Table 1. The PEI content in the adsorbent (PEIexp) was calculated by the following formula
where W is the weight gain based on the unit mass of UiO-66-NH2, N is the nitrogen content of the adsorbent measured by elemental analysis, and N(PEI) is the theoretical nitrogen content (30.74 wt %) of PEI. For the three PEIC@UiO samples, PEI content (10.6–22.4 wt %) increased with increasing PEI feeding (48–96 wt %) while the color changes from yellow to grayish brown. The weight gain is less than the PEI feeding for all the samples. It seems to be independent of CO2 preabsorbing PEI (PEIC72@UiO vs PEI72@UiO) or the presence of glutaraldehyde (PEIC96@UiO vs PEI96+UiO). But, the nitrogen content of PEI96+UiO is clearly higher than that of PEIC96@UiO because both PEI and nitrogen-free glutaraldehyde are gained in PEIC96@UiO but only PEI is gained in PEI96+UiO. From these results, it is concluded that only partial PEI has been incorporated onto the surface of the MOF, especially in the chemically modified PEI@UiO and PEIC@UiO series. The incomplete chemical grafting of PEI may be attributed to the steric hindrance imposed by partial pendant −NH2 groups, which point to the MOF cavities other than those outside.38 In other words, these −NH2 groups in the MOF did not contribute to graft PEI. Another possibility is that partial glutaraldehyde reacted with the MOF or PEI itself, resulting in intra-cross-linking of MOF or PEI instead of PEI grafting onto the MOF.
Scheme 1. Schematic Diagram for the Synthesis and Hypothesized Structure of the PEI-Modified UiO-66-NH2 Adsorbents (PEIC@UiO).
Table 1. Preparation Conditions and Results of Pristine and PEI-Modified UiO-66-NH2.
| sample | PEItheoa (wt %) | PEItheob (wt %) | Wc (wt %) | Nd (wt %) | PEIexpe (wt %) | Apf (m2/g) | Vpf (cm3/g) | Dpf (nm) |
|---|---|---|---|---|---|---|---|---|
| UiO-66-NH2 | 0 | 0 | 0 | 5.0 | 0 | 987 | 0.51 | 2.05 |
| PEIC48@UiO | 48 | 26 | 41 | 6.8 | 10.6 | 151 | 0.14 | 3.58 |
| PEIC72@UiO | 72 | 34 | 64 | 9.0 | 19.4 | 12.0 | 0.076 | 25.4 |
| PEIC96@UiO | 96 | 41 | 90 | 9.5 | 22.4 | 7.64 | 0.11 | 55.5 |
| PEI72@UiO | 72 | 34 | 62 | 8.4 | 17.3 | 14.4 | 0.096 | 26.5 |
| PEI96+UiO | 96 | 49 | 89 | 16.0 | 43.4 | 1.40 | 0.0034 | 9.60 |
The mass percentage of PEI used in adsorbent synthesis, based on the mass of UiO-66-NH2.
The mass percentage of PEI used in adsorbent synthesis, based on the sum of UiO-66-NH2, PEI, and GD fed.
Weight gain based on the mass of UiO-66-NH2, calculated from the weight change before and after the modification reaction.
Nitrogen content measured by elemental analysis.
PEI content in adsorbent, calculated by nitrogen content.
Ap: specific surface area; Vp: total pore volume; Dp: average pore size.
Figure 1 shows the 1H NMR spectra of PEI-modified and PEI-impregnated UiO-66-NH2 adsorbents after digestion in NaOH/D2O solution as well as PEI itself. In UiO-66-NH2, as expected, the three proton signals of 7.02, 7.09, and 7.53 ppm are attributed to the benzene ring structure (3 × 1H) of organic ligand in MOFs. The peak at 2.07 ppm is attributed to Zr–OH43 formed during digestion. The reason of the peak at 8.27 ppm is not clear yet. It also exists in the spectrum of digested UiO-66-NH2 reported previously.44 After PEI modification, the multiple broad proton signals of −CH2 in PEI and glutaraldehyde appeared at 2.3–2.6 ppm. The peak intensity shows an increasing trend while the PEI loading increased. However, −N=CH bond formatted by Schiff base reaction of glutaraldehyde was not observed in PEI@UiO & PEIC@UiO. It was possible that −N=CH bonds were unstable under alkaline conditions and ruptured.
Figure 1.
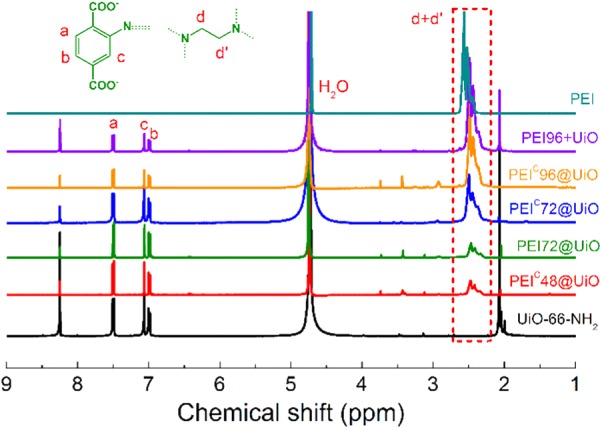
1H NMR spectra of PEI, UiO-66-NH2, and PEI-modified MOFs. The samples except PEI were digested for 24 h in a 1 M solution of NaOH/D2O before NMR measurement.
The FTIR spectra shown in Figure 2 provided more information for the products. For UiO-66-NH2, a strong peak at 1658 cm–1, assigned to the v(C=O) stretching of N,N-dimethylformamide (DMF) can be seen. The absence of this band in PEIC@UiO-66 indicates the complete exchange of DMF with water.45 The 3000–2800 and 3600–3100 cm–1 signals are assigned to the stretching vibration of CH2 and unreacted amino groups, respectively. They became stronger when more PEI was incorporated. The characteristic stretching vibration peaks of NH2 on the MOF appeared at 1436 and 1258 cm–1. The two peaks were weakened because of its Schiff base reaction with aldehyde group in glutaraldehyde to form N=C bond at 1618 cm–1 (stretching vibration), resulting PEI grafting shown in Scheme 1. In addition to the grafting reaction, the intra-cross-linking reactions of PEI and UiO-66-NH2 with glutaraldehyde also occurred unavoidably at the same time. In fact, all these Schiff base reactions contributed to the signal at 1618 cm–1, but could not be identified from each other in the FTIR results. For simplicity and clarity, Scheme 1 only shows the expected grafting structure of the products.
Figure 2.
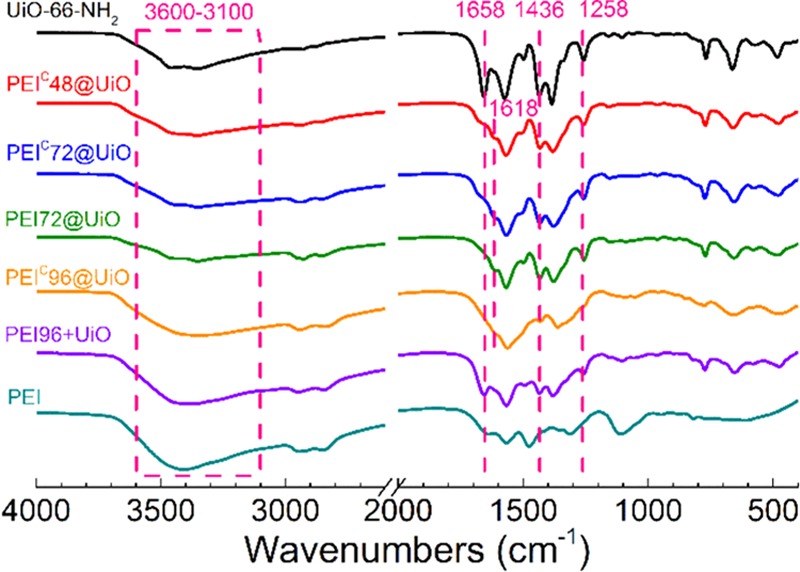
FTIR spectra of PEI, UiO-66-NH2, and PEI-modified MOFs.
The PXRD patterns of UiO-66-NH2 and the five PEI-modified adsorbents are shown in Figure 3. The as-synthesized UiO-66-NH2 showed narrow diffraction peaks in accordance with previously published data,17,38 confirming successful synthesis and high crystallinity of UiO-66-NH2. The PXRD patterns of PEI-modified MOFs were essentially identical to that for the parent UiO-66-NH2, indicating that the crystal structure of UiO-66-NH2 was well maintained after the modification. The peak intensity of UiO-66-NH2 decreased after PEI modification because of the decrease of MOF content and the enhancement of the interaction between the PEI amino groups and the UiO-66-NH2 Zr sites.23 Another reason that cannot be completely excluded is that the MOF framework structure could be destroyed to some extent in alkali amine solution though UiO-66-NH2 is reported to show excellent alkali resistance.38 Similar structure destruction were reported in some amine-impregnated MOFs, such as MIL-101,31 HKUST-1,33 and MOF-74.46
Figure 3.
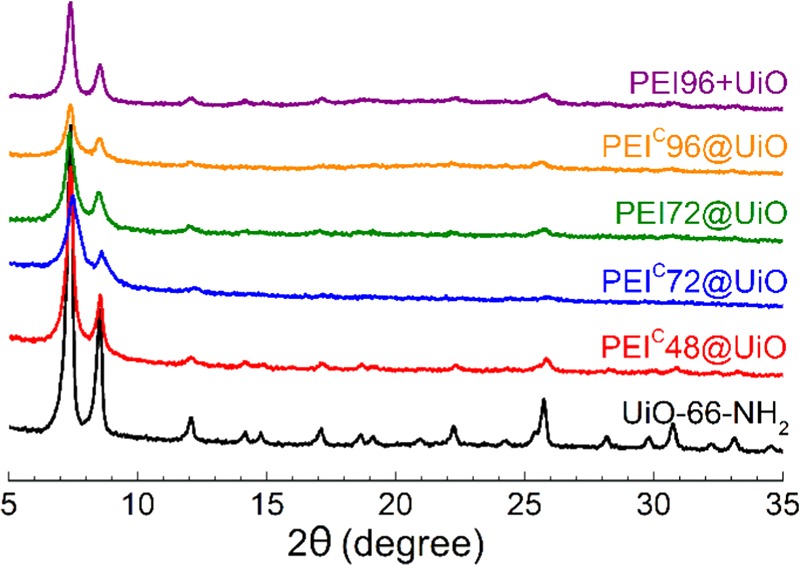
Powder X-ray diffraction (PXRD) patterns of pristine UiO-66-NH2 and PEI-modified adsorbents.
The nitrogen adsorption isotherms of the five PEI-modified adsorbents and UiO-66-NH2 are shown in Figure S1. The specific surface areas calculated from N2 adsorption isotherms are listed in Table 1. It reaches 987 m2/g for UiO-66-NH2 but decreases to 151, 12.0, and 7.6 m2/g for PEIC48@UiO, PEIC72@UiO, and PEIC96@UiO, respectively. At the same time, the average pore size gradually increases and the pore volume decreases with increasing PEI loading. The results suggested that PEI modification remarkably decreased the specific surface area of UiO-66-NH2 due to the pore-filling effect of PEI on the macropores between MOF particles. Whether or not pretreating PEI with CO2 preabsorption did not show clear influence on the pore characteristics (PEIC72@UiO vs PEI72@UiO), but impregnation of UiO-66-NH2 with large amount of PEI (PEI96+UiO) led to serious pore blockage (supported by SEM observation, see next paragraph), making the micropores in MOF crystals no longer accessible for N2 adsorption. Therefore, PEI96+UiO only maintained a very small surface area of 1.40 m2/g.
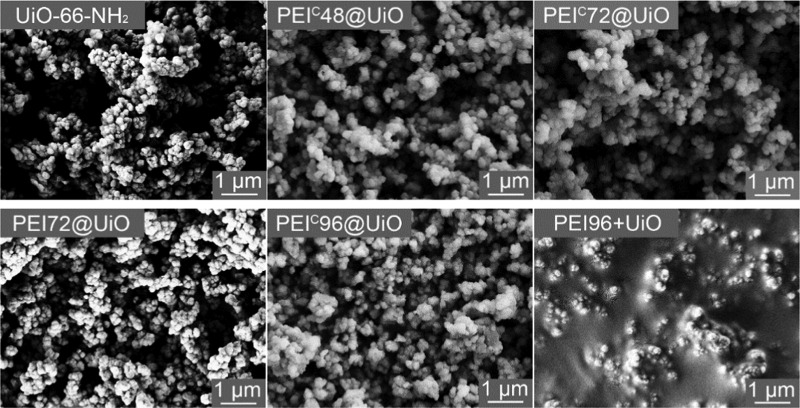
To further understand the effect of PEI modification on the pore characteristic, the morphological structure of the samples was further observed by SEM. As shown in Figure 4, the UiO-66-NH2 appears to be near-spherical crystals. For PEI@UiO or PEIC@UiO series, the morphology of MOF particles did not show an observable change though there might exist PEI in the intercrystal void of UiO-66-NH2. But for PEI96+UiO, the surface and intercrystal void of UiO-66-NH2 crystals are almost fully occupied by PEI. In other words, the UiO-66-NH2 crystals are embedded in the PEI layers. This phenomenon explains why the specific surface area of PEI96+UiO was very low.
Figure 4.
SEM images of pristine UiO-66-NH2 and PEI-modified adsorbents.
CO2 adsorbents must have enough thermal stability to endure high temperature of at least 100–150 °C of flue gas.47 The TGA curves of UiO-66-NH2 and the five PEI-modified adsorbents are shown in Figure 5. The TGA curves of UiO-66-NH2 showed a two-step weight loss. The initial weight loss at 100–280 °C was ascribed to the removal of adsorbed gas and dehydroxylating of the zirconium oxoclusters.38 The second-step weight loss (after 280 °C) was due to the framework decomposition.38 The PEI-modified adsorbents were all stable up to 200 °C, if we ignore the initial weight loss over 100 °C, which possibly results from absorbed water and gases. At temperature higher than 200 °C, the weight loss was caused mainly by PEI decomposition. With the increase of PEI loading, the weight loss curve moves toward low temperature side. The differences in preparation methods do not show a clear influence on thermal stability. In conclusion, the PEI-modified adsorbents have good thermal stability to endure normal flue gas temperature for CO2 separation.
Figure 5.
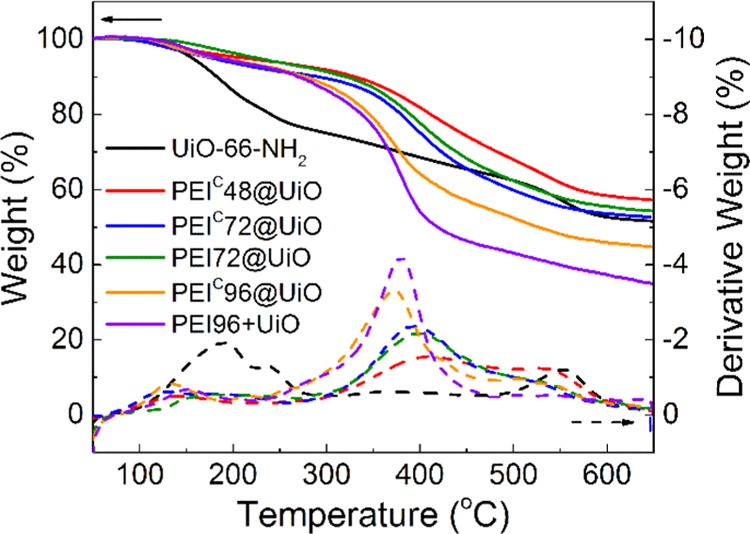
TGA curves of pristine UiO-66-NH2 and PEI-modified adsorbents (N2 atmosphere, heating rate 10 °C/min).
2.2. CO2 Adsorption Capacity, Rate, and Selectivity over N2
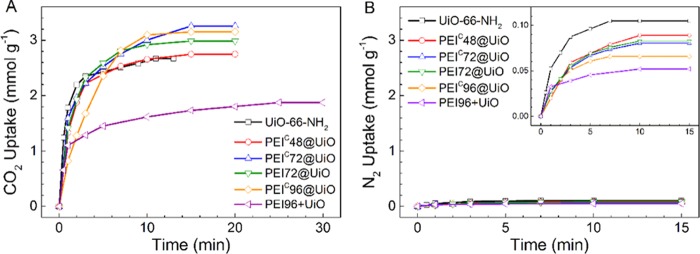
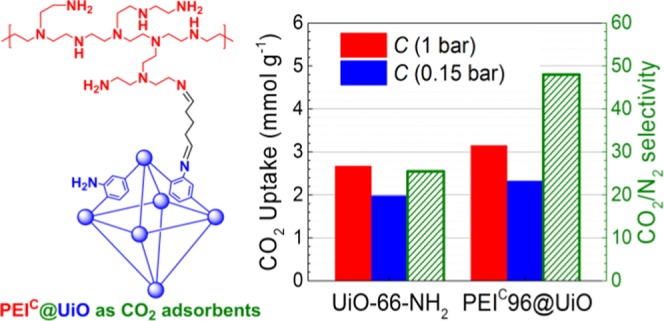
CO2 adsorption performance of UiO-66-NH2 and the five PEI-modified adsorbents at 25 °C and 1 atm is shown in Figure 6A. The saturated adsorption capacity of UiO-66-NH2 reaches up to 2.67 mmol CO2/g. It is roughly in accordance with some of the reported values (2.86–3.15 mmol CO2/g).21 In comparison, the PEIC@UiO adsorbents exhibit higher adsorption capacity ranging from 2.75 to 3.26 mmol CO2/g (Table 2). The NH2 groups in PEI and UiO-66-NH2 contributed to chemical CO2 adsorption, and the pores in UiO-66-NH2 contributed to physical CO2 adsorption. However, partial NH2 groups in UiO-66-NH2 that had converted to N=C bonds no longer contributed to CO2 adsorption and offset part of the contribution of PEI to CO2 adsorption. The amount of incorporated PEI is not positively correlated with the CO2 adsorption capacity. Among them, PEIC72@UiO shows the highest adsorption capacity, 3.26 mmol CO2/g. In comparison with PEI72@UiO, PEIC72@UiO shows 9% higher CO2 adsorption capacity (3.26 vs 2.99 mmol CO2/g). The increment is higher than the experimental error (<3%) but not as high as the value (25%) reported previously.42 The saturated adsorption capacity of PEI96+UiO is much lower than that of PEIC96@UiO (1.87 vs 3.15 mmol CO2/g) because of its much lower specific surface area.
Figure 6.
CO2 (A) and N2 (B) adsorption behaviors of pristine UiO-66-NH2 and PEI-modified adsorbents.
Table 2. CO2 Adsorption/Desorption Properties of Pristine UiO-66-NH2 and PEI-Modified Adsorbents.
|
Cs,1bara (mmol CO2/g) |
||||||
|---|---|---|---|---|---|---|
| adsorbent | dry | moistb | α | Cs,expc(mmol CO2 + N2/g) | Cs,0.15bard(mmol CO2/g) | Qdese(kJ/mol CO2) |
| UiO-66-NH2 | 2.67 | 2.13 | 25.5 | 2.06 | 1.98 | |
| PEIC48@UiO | 2.75 | 2.69 | 30.9 | 2.06 | 2.00 | 63.3 |
| PEIC72@UiO | 3.26 | 3.43 | 40.6 | 2.17 | 2.12 | 67.3 |
| PEI72@UiO | 2.99 | 3.02 | 36.1 | 2.10 | 2.04 | |
| PEIC96@UiO | 3.15 | 3.33 | 48.0 | 2.38 | 2.33 | 68.0 |
| PEI96+UiO | 1.87 | 2.69 | 36.0 | 1.59 | 1.55 | |
Experimental CO2 adsorption capacity.
The CO2 adsorption capacity measured with moist CO2.
Experimental gas adsorption capacity measured in simulated flue gas (14.3 vol % CO2).
CO2 adsorption capacity calibrated by the separation factor α in simulated flue gas.
The CO2 desorption heat.
Furthermore, it can be seen that all the PEI@UiO adsorbents exhibit high adsorption rate, reaching saturated adsorption capacity in 15 min. However, PEI96+UiO need much longer time to reach CO2 saturation due to low specific surface area and consequent slower adsorption rate.
CO2/N2 adsorption selectivity is another important indicator to evaluate a solid sorbent for practical use. For this purpose, the adsorption of pure N2 (60 mL/min) was conducted under the same condition. The results are shown in Figure 6B and listed in Table 2. UiO-66-NH2 adsorbed N2 significantly (0.11 mmol/g). But, the PEIC@UiO adsorbents had a relatively small amount of N2 adsorption, decreasing to 0.06–0.09 mmol/g. The separation factor α (adsorption selectivity of CO2 over N2) was calculated by the formula
where Qi and Pi (Pi = 1 bar here) are the adsorption capacity and the partial pressure of component i, respectively. The separation factor of UiO-66-NH2 is 25.5, which is between the reported values in literature (1320–3221). After PEI modification, the value of PEIC@UiO increased to 30.9–48.0. As expected, it was obviously improved with increasing PEI loading.
To simulate flue gas that usually contains about 15 vol % CO2, a CO2/N2 mixture gas (10 + 60 mL/min, CO2 14.3 vol %) was also used for CO2 adsorption. The result is shown in Figure 7. The saturated gas adsorption capacity of PEIC@UiO was 2.06–2.38 mmol CO2 + N2/g. The true CO2 sorption capacity from the mixed gas (Cs,0.15bar) can be calibrated by the following formula. The adsorption rate and capacity for simulated flue gas both decreased to some extent in comparison with those for pure CO248 due to lower CO2 partial pressure. Among them, PEIC96@UiO reaches the highest adsorption capacity (2.33 mmol CO2/g).
The PEIC96@UiO is compared with some other CO2 adsorbents reported in literature. As shown in Table 3, it can be seen that this functional MOF adsorbent shows a reasonably good CO2 adsorption performance, especially in CO2 selectivity.
Figure 7.
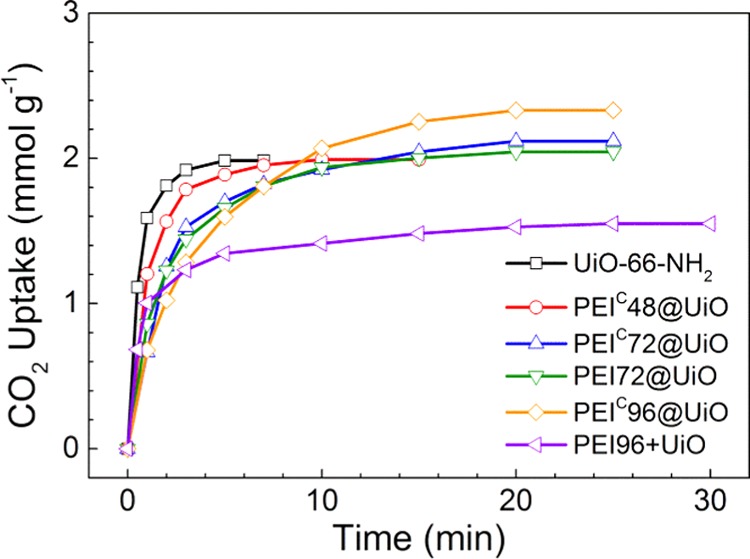
CO2 uptake of pristine UiO-66-NH2 and PEI-modified adsorbents from CO2 + N2 mixture containing CO2 15% at 25 °C.
Table 3. CO2 Adsorption Performance of Different Adsorbents.
|
Csc (mmol/g) |
||||||
|---|---|---|---|---|---|---|
| adsorbentsa | Tb (K) | 1 bar | 0.15 bar | αd | αe | refs |
| PEIC96@UiO | 298 | 3.1 | 2.3 | 48 | this work | |
| PEI (50%) + silica | 348 | 3.1 | 3.0 | (36) | ||
| 0.70PEI@PDVB | 298 | 3.2 | 2.4 | (37) | ||
| PEI@PS polyHIPE | 313 | 3.5 | 26 | (49) | ||
| PEI@poly(GMA)HIPE | 313 | 4.0 | 3.1 | 27 | (50) | |
| PEI + UiO-66 | 298 | 3.3 | 1.6 | (23) | ||
| GMA-UiO-66 | 298 | 4.3 | 1.8 | 46 | (21) | |
| TEPA (50%) + NH2-MIL-101 | 298 | 3.1 | (29) | |||
| PEI (2.5%) + HKUST | 298 | 4.1 | 0.8 | 2 | (32) | |
| en-[Mg2(dobpdc)] | 298 | 4.5 | 3.5 | 230 | (16) | |
| pyrrolic N-enriched carbon | 298 | 3.6 | 1.5 | 115 | (51) | |
PAF: porous aromatic framework; PDVB: nanoporous poly(divinylbenzene). PS: poly(styrene divinylbenzene), modified with polyacrylic acid.
Temperature.
CO2 adsorption capacity at the indicated CO2 partial pressure.
CO2/N2 selectivity: α = nCO2 (1 bar)/nN2 (1 bar).
Calculated by ideal adsorption solution theory (IAST) model, α = [nCO2 (0.15 bar)/nN2 (0.85 bar)] × (0.85/0.15).
2.3. CO2 Desorption Enthalpy
Differential scanning calorimetry (DSC) was used to determine the CO2 desorption heat (Qdes). As shown in Figure 8, a strong endotherm (155–191 J/g CO2-saturated adsorbent) was observed for all the three CO2-saturated PEIC@UiO adsorbents due to CO2 desorption but none for the neat PEIC96@UiO. The CO2 desorption occurred in a wide temperature range from 70 to 230 °C, and the endotherm peak temperature appears at 133–144 °C. From the desorption enthalpy value, the desorption heat Qdes (kJ/mol CO2) of CO2-saturated PEIC@UiO can be calculated by the equation Qdes = E/(Cs/(CsMCO2/1000 + 1)), where E, Cs, MCO2 are the desorption enthalpy (J/g), the CO2 adsorption capacity (mmol/g), and the molar mass of CO2 (44 g/mol), respectively. The value ranges from 63.3 to 68.0 kJ/mol CO2, higher than that of pristine UiO-66-NH2 (ca. 27 kJ/mol).20 The more PEI is incorporated, the higher the CO2 desorption heat is. The values are located in the range of 60–90 kJ/mol of typical chemsorption47 but lower than the typical value, ca. 80 kJ/mol, of alkanolamine aqueous solution.52,53 Therefore, this CO2 adsorbent seems competitive in reducing the regeneration energy consumption.
Figure 8.
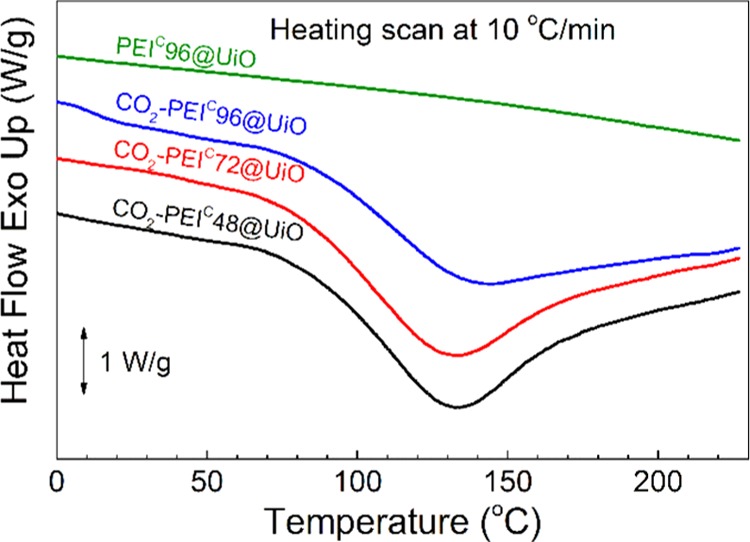
DSC curves of neat PEIC96@UiO and three CO2-saturated PEIC@UiO adsorbents.
2.4. Effect of Moisture on CO2 Adsorption
Moisture is a non-negligible factor that affects practical CO2 capture from flue gas, which usually contains 8–17% water vapor.54 Due to the hydrophilicity, the adsorbents unavoidably adsorb water vapor from the flue gas. To determine the water content in adsorbents during CO2 capture, the water adsorption of PEIC96@UiO at 25 °C was measured. This hygroscopic adsorbent adsorbed 1.7 wt % water in 15 min. To study the influence of moisture on CO2 adsorption, dry CO2 (d-CO2) and moist CO2 (m-CO2) were used to pass through dry adsorbent (d-Sorb) separately. The moist CO2 gas was obtained by feeding dry CO2 gas through water. The results are shown in Figure 9.
Figure 9.
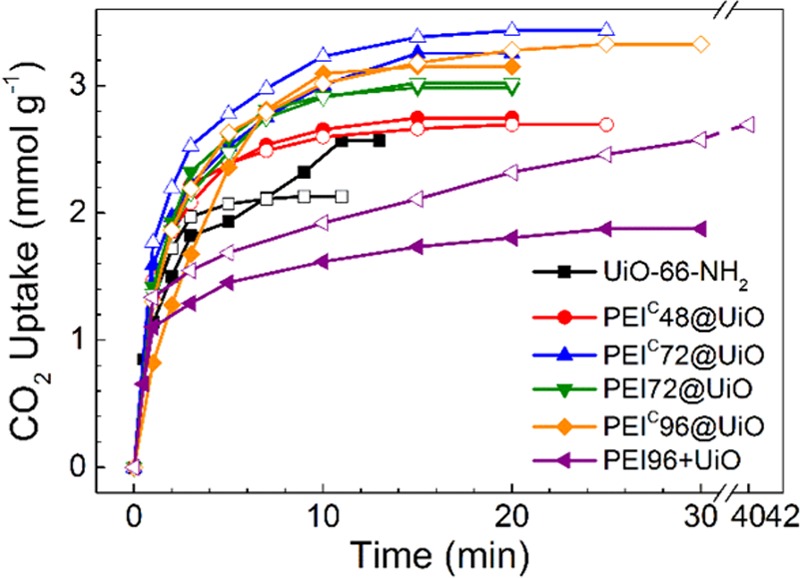
CO2 adsorption of pristine UiO-66-NH2 and PEI-modified adsorbents using dry or moist CO2 gas (solid: d-CO2; hollow: m-CO2).
As hygroscopic adsorbents, UiO-66-NH2 showed a 20.2% decrease in CO2 adsorption capacity in the “m-CO2 + d-Sorb” case because water vapor adsorption competed with CO2 adsorption. The existence of water could also affect the CO2 binding to the open metal sites in MOF.55 The adsorption capacity of PEIC48@UiO slightly decreased by 2.2%, which was within the allowable error range of the adsorption experiment. However, CO2 adsorption capacity of PEIC72@UiO, PEIC96@UiO, and PEI96+UiO increased in the m-CO2 + d-Sorb case by 5.2, 5.7, and 43.8%, respectively. The rise instead of descent in CO2 adsorption is attributed to the tertiary amino groups in PEI (about 12 wt %), which might contribute to CO2 adsorption in the presence of moisture.56 Therefore, the PEIC@UiO adsorbents show excellent moisture endurance in CO2 adsorption.
2.5. Adsorption/Desorption Cycles
Finally, CO2 adsorption/desorption cycles of PEIC96@UiO were conducted to assess the recyclability and durability of the adsorbent. The CO2 desorption was carried out at 120 °C. The same temperature was also used for the CO2 desorption of aqueous monoethanolamine absorbent.52 Pure N2 purging was used instead of vacuum to facilitate the desorption experiment, though the latter seemed more suitable for practical CO2 capture. Figure 10 shows three cycles of CO2 adsorption/desorption of PEIC96@UiO. Because of weak chemical bonding between PEIC96@UiO and CO2 at high temperature, the adsorbed CO2 was desorbed rapidly and nearly completely. In each cycle, the adsorbent effectively captured 3.0 mmol CO2/g. A high adsorption capacity, 97.4%, was retained after three cycles. The preadsorded moisture and N2 might account for the slight loss in adsorption capacity. In conclusion, PEIC96@UiO, showed quite steady CO2 adsorption and superior durability.
Figure 10.
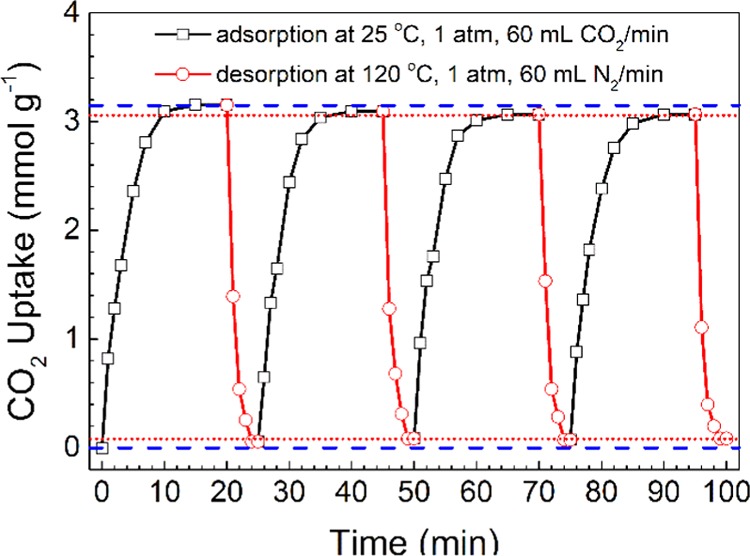
CO2 adsorption/desorption cycles of PEIC96@UiO.
3. Conclusions
UiO-66-NH2, a zirconium-based functional metal–organic framework (MOF), was postsynthetically modified with CO2-preabsorbed polyethyleneimine (PEI) via Schiff base reaction. The resulting PEIC@UiO adsorbents contain PEI up to 22.1 wt % or nitrogen up to 9.5 wt %. They show specific surface area of 7–150 m2/g and maintain unchanged crystal structure of the MOF. When compared with pristine UiO-66-NH2, PEIC96@UiO shows significantly improved CO2/N2 adsorption selectivity (48 vs 25) and higher CO2 adsorption capacity (3.2 vs 2.7 mmol/g), but retains fast CO2 adsorption/desorption in spite of its reduced specific surface area. It also displays moderate desorption heat (68 kJ/mol CO2), superior moisture endurance, and recyclability, which are very desirable for practical applications. Further studies on raising PEI content as well as maintaining high specific surface will be reported elsewhere.
4. Experimental Section
4.1. Materials
Zirconium tetrachloride (ZrCl4, 98%), branched polyethyleneimine (PEI, 99%, Mw = 600, viscous liquid, theoretical amino group content 32.9 wt %, primary amino group content 12.8 wt %), and glutaraldehyde (GD, 50%) were purchased from Aladdin Chemical Co. Ltd. China. 2-Aminobenzene-1,4-dicarboxylic acid (ABDC or H2BDC-NH2, 98%) was supplied by J&K Chemicals. N,N-dimethylformamide (DMF), anhydrous ethanol, anhydrous methanol, sodium hydroxide (NaOH), and hydrochloric acid (HCl, 36.5%) were all analytical grade reagents purchased from Sinopharm Chem. Reagent Co. Ltd. China. All chemicals were used as received. Nitrogen (N2, ≥99.5%) and carbon dioxide (CO2, ≥99.995%) were obtained from Jingong Specialty Gases Co., Ltd. China.
4.2. Synthesis of UiO-66-NH2
[Zr6O4(OH)4(BDC-NH2)6] (UiO-66-NH2) was prepared according to a procedure previously reported,20,38 with some modifications. In brief, ZrCl4 (5 mmol, 1.166 g), DMF (15 mL), and HCl (5 mL) were mixed and sonicated for 20 min until all materials were dissolved fully at room temperature. ABDC (5 mmol, 0.9057 g) and the residual DMF (35 mL) were then added and the mixture was sonicated for another 20 min. The homogeneous mixture was heated at 80 °C for 12 h and then at 100 °C for 24 h. The resulting product precipitated as microcrystalline powder. The mixture was cooled down to room temperature and centrifuged at 8000 rpm for 10 min to separate the solid from the solution. After washing three times with DMF (30 mL) and ethanol (30 mL), respectively, and drying under vacuum at 100 °C for 24 h, yellow powder was finally obtained at a yield of 92%.
4.3. Synthesis of PEI-Modified UiO-66-NH2 Adsorbent (PEIC@UiO and PEI@UiO)
Scheme 1 depicts the preparation process. PEI (0.375, 0.563, or 0.751 g) was dissolved in water (10.0 mL). CO2 was fed into the resulting solution at room temperature to prepare CO2-preabsorbed PEI. After 2 h, UiO-66-NH2 (0.5 mmol, 0.78 g) was added. After stirring at 400 rpm for 30 min, an aqueous solution of GD (0.6007 g) was added dropwise under stirring at room temperature for another 12 h. The resulting solid was isolated via centrifugation and washed with deionized water three times. After vacuum drying at 100 °C for 24 h to remove absorbed CO2 and solvent, a blocky solid product was obtained. It was symbolized as “PEICx@UiO”, where UiO and superscript C represented UiO-66-NH2 and preabsorption of CO2, x was the mass percentage of PEI fed based on UiO-66-NH2. An adsorbent was also prepared without CO2 preabsorption. In this case, the product was named as “PEIx@UiO”. The details of preparation conditions and structural characteristics of the adsorbents are listed in Table 1.
4.4. Preparation of PEI-Impregnated UiO-66-NH2 Adsorbent (PEI + UiO)
A PEI-impregnated UiO-66-NH2 adsorbent was also prepared using an impregnation method according to the procedure previously reported for PEI-impregnated UiO-66 adsorbent.23 PEI (1.5 mmol, 0.716 g) was dissolved in 5 mL anhydrous methanol. UiO-66-NH2 (0.5 mmol, 0.78 g) powder was added into the solution and the mixture was treated under ultrasonication for 1 h. Then, it was dried under nitrogen atmosphere at 40 °C for 6 h and further dried under vacuum at 100 °C for 12 h. Finally, the obtained sample was symbolized as PEI96+UiO, where UiO represented UiO-66-NH2 and 96 was the mass percentage of PEI fed based on UiO-66-NH2.
4.5. Characterization
The samples were characterized by elemental analysis (Flash EA1112, ThermoFinnigan Co.), 1H NMR (Bruker AC-80 spectroscopy, 400 MHz, NaOH/D2O solution), and FTIR (Nicolet 5700). Before 1H NMR measurement, the PEI-modified samples (20 mg) were digested with 0.6 mL of a NaOH/D2O solution (1 M) and allowed to stand for 24 h. After the digestion process, the organic portions of the samples (linkers in the MOF and grafted amines) were dissolved, and the inorganic component of the MOF was converted to zirconium hydroxide, which was separated from the solution by filtration.38 PEI was directly measured using NaOH/D2O solution (1 M) as the solvent. The FTIR spectra of PEI-modified samples were recorded using KBr disk samples.
Powder X-ray diffraction (PXRD) pattern was measured using a PANalytical X’Pert X-ray diffraction system (PANalytical Company) using Cu Kα radiation (1.54 Å), working at 40 kV and 40 mA.
N2 adsorption–desorption isotherms were recorded with a Quantachrome Autosorb-1-C instrument at 77 K. The specific surface area was estimated with the Brunauer–Emmett–Teller (BET) method and the pore volume was calculated with the desorption branch of the isotherms. The samples were degassed at 100 °C for 24 h before BET analysis.
The morphology was recorded with a scanning electron microscope (SEM, Utral 55, Carl zeiss, Germany) at an acceleration voltage of 1.5 kV, using the samples coated with a thin gold layer.
Thermogravimetric analysis (TGA) was recorded with a Perkin-Elmer instrument Pyris 1 TGA analyser under N2 flow (20 mL/min) from 50 to 650 °C at a heating rate of 10 °C min–1.
4.6. CO2 Capture
Before CO2 adsorption, about 1.0 g of adsorbent was outgassed under vacuum at 100 °C. CO2 adsorption/desorption was performed under atmospheric pressure, as previously reported.39,40 Desorption of the adsorbent was conducted under pure N2 (60 mL/min) at a temperature of 120 °C. Some repeated experiments of CO2 adsorption and desorption were done. CO2 desorption heat (Qdes) was recorded with differential scanning calorimetry (DSC, Q200, TA Instrument).41 The samples were scanned at a heating rate of 10 °C/min under nitrogen flow.
Acknowledgments
This work was supported by the National High Technology Research and Development Program (2015BAC04B0103) and 151 Talents Project of Zhejiang Province. The authors thank Mrs. Li Xu, Mrs. Qun Pu and Mrs. Na Zheng for their assistance on in performing BET, DSC, TGA, and SEM analyses at State Key Laboratory of Chemical Engineering (Zhejiang University).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b02319.
N2 adsorption isotherms of pristine UiO-66-NH2 and PEI-modified adsorbents (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Keith D. W. Why capture CO2 from the atmosphere?. Science 2009, 325, 1654–1655. 10.1126/science.1175680. [DOI] [PubMed] [Google Scholar]
- Stauffer P. H.; Keating G. N.; Middleton R. S.; Viswanathan H. S.; Berchtold K. A.; Singh R. P.; Pawar R. J.; Mancino A. Greening coal: breakthroughs and challenges in carbon capture and storage. Environ. Sci. Technol. 2011, 45, 8597–8604. 10.1021/es200510f. [DOI] [PubMed] [Google Scholar]
- Jung H.; Jeon S.; Jo D. H.; Huh J.; Kim S. H. Effect of crosslinking on the CO2 adsorption of polyethyleneimine-impregnated sorbents. Chem. Eng. J. 2017, 307, 836–844. 10.1016/j.cej.2016.09.005. [DOI] [Google Scholar]
- Rochelle G. T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. 10.1126/science.1176731. [DOI] [PubMed] [Google Scholar]
- Han C.; Graves K.; Neathery J.; Liu K. Simulation of the energy consumption of CO2 capture by aqueous monoethanolamine in pilot plant. Energy Environ. Res. 2011, 1, 67–80. 10.5539/eer.v1n1p67. [DOI] [Google Scholar]
- Nittaya T.; Douglas P. L.; Croiset E.; Ricardez-Sandoval L. A. Dynamic modeling and evaluation of an industrial-scale CO2 capture plant using monoethanolamine absorption processes. Ind. Eng. Chem. Res. 2014, 53, 11411–11426. 10.1021/ie500190p. [DOI] [Google Scholar]
- Sumida K.; Rogow D. L.; Mason J. A.; McDonald T. M.; Bloch E. D.; Herm Z. R.; Bae T. H.; Long J. R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–781. 10.1021/cr2003272. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Zhao Y.; Gong Q.; Li Z.; Li J. MOFs for CO2 capture and separation from flue gas mixtures: the effect of multifunctional sites on their adsorption capacity and selectivity. Chem. Commun. 2013, 49, 653–661. 10.1039/C2CC35561B. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Bai J.; Lu Z.; Pan Y.; You X. Finely tuning MOFs towards high-performance post-combustion CO2 capture materials. Chem. Commun. 2016, 52, 443–452. 10.1039/C5CC07751F. [DOI] [PubMed] [Google Scholar]
- Li J. R.; Sculley J.; Zhou H. C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. 10.1021/cr200190s. [DOI] [PubMed] [Google Scholar]
- Belmabkhout Y.; Guillerm V.; Eddaoudi M. Low concentration CO2 capture using physical adsorbents: are metal-organic frameworks becoming the new benchmark materials?. Chem. Eng. J. 2016, 296, 386–397. 10.1016/j.cej.2016.03.124. [DOI] [Google Scholar]
- Madden D. G.; Scott H. S.; Kumar A.; Chen K. J.; Sanii R.; Bajpai A.; Lusi M.; Curtin T.; Perry J. J.; Zaworotko M. J. Flue-gas and direct-air capture of CO2 by porous metal-organic materials. Philos. Trans. R. Soc., A 2017, 375, 20160025 10.1098/rsta.2016.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt D.; Furukawa H.; Wang B.; Glover T. G.; Yaghi O. M. Highly efficient separation of carbon dioxide by a metal-organic framework replete with open metal sites. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 20637–20640. 10.1073/pnas.0909718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H.; Grunder S.; Cordova K. E.; Valente C.; Furukawa H.; Hmadeh M.; Gándara F.; Whalley A. C.; Liu Z.; Asahina S.; et al. Large-pore apertures in a series of metal-organic frameworks. Science 2012, 336, 1018–1023. 10.1126/science.1220131. [DOI] [PubMed] [Google Scholar]
- Deng H.; Doonan C. J.; Furukawa H.; Ferreira R. B.; Towne J.; Knobler C. B.; Wang B.; Yaghi O. M. Multiple functional groups of varying ratios in metal-organic frameworks. Science 2010, 327, 846–850. 10.1126/science.1181761. [DOI] [PubMed] [Google Scholar]
- Lee W. R.; Sang Y. H.; Ryu D. W.; Lim K. S.; Sang S. H.; Moon D.; Choi J.; Chang S. H. Diamine-functionalized metal-organic framework: exceptionally high CO2 capacities from ambient air and flue gas, ultrafast CO2 uptake rate, and adsorption mechanism. Energy Environ. Sci. 2014, 7, 744–751. 10.1039/C3EE42328J. [DOI] [Google Scholar]
- Cavka J. H.; Jakobsen S.; Olsbye U.; Guillou N.; Lamberti C.; Bordiga S.; Lillerud K. P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. 10.1021/ja8057953. [DOI] [PubMed] [Google Scholar]
- Yang Q.; Wiersum A. D.; Llewellyn P. L.; Guillerm V.; Serre C.; Maurin G. Functionalizing porous zirconium terephthalate UiO-66(Zr) for natural gas upgrading: a computational exploration. Chem. Commun. 2011, 47, 9603–9605. 10.1039/c1cc13543k. [DOI] [PubMed] [Google Scholar]
- Kronast A.; Eckstein S.; Altenbuchner P. T.; Hindelang K.; Vagin S. I.; Rieger B. Gated channels and selectivity tuning of CO2 over N2 sorption by post-synthetic modification of a UiO-66-type metal-organic framework. Chem. - Eur. J. 2016, 22, 12800–12807. 10.1002/chem.201602318. [DOI] [PubMed] [Google Scholar]
- Cmarik G. E.; Kim M.; Cohen S. M.; Walton K. S. Tuning the adsorption properties of UiO-66 via ligand functionalization. Langmuir 2012, 28, 15606–15613. 10.1021/la3035352. [DOI] [PubMed] [Google Scholar]
- Molavi H.; Eskandari A.; Shojaei A.; Mousavi S. A. Enhancing CO2/N2 adsorption selectivity via post-synthetic modification of NH2-UiO-66(Zr). Microporous Mesoporous Mater. 2018, 257, 193–201. 10.1016/j.micromeso.2017.08.043. [DOI] [Google Scholar]
- Huang A.; Wan L.; Caro J. Microwave-assisted synthesis of well-shaped UiO-66-NH2 with high CO2 adsorption capacity. Mater. Res. Bull. 2018, 98, 308–313. 10.1016/j.materresbull.2017.10.038. [DOI] [Google Scholar]
- Xian S.; Wu Y.; Wu J.; Wang X.; Xiao J. Enhanced dynamic CO2 adsorption capacity and CO2/CH4 selectivity on polyethylenimine-impregnated UiO-66. Ind. Eng. Chem. Res. 2015, 54, 11151–11158. 10.1021/acs.iecr.5b03517. [DOI] [Google Scholar]
- Li L. J.; Liao P. Q.; He C. T.; Wei Y. S.; Zhou H. L.; Lin J. M.; Li X. Y.; Zhang J. P. Grafting alkylamine in UiO-66 by charge-assisted coordination bonds for carbon dioxide capture from high-humidity flue gas. J. Mater. Chem. A 2015, 3, 21849–21855. 10.1039/C5TA05997F. [DOI] [Google Scholar]
- Molavi H.; Joukani F. A.; Shojaei A. Ethylenediamine grafting to functionalized NH2-UiO-66 using green aza-Michael addition reaction to improve CO2/CH4 adsorption selectivity. Ind. Eng. Chem. Res. 2018, 57, 7030–7039. 10.1021/acs.iecr.8b00372. [DOI] [Google Scholar]
- Vahidi M.; Rashidi A. M.; Tavasoli A. Preparation of piperazine-grafted amine-functionalized UiO-66 metal organic framework and its application for CO2 over CH4 separation. J. Iran. Chem. Soc. 2017, 14, 2247–2253. 10.1007/s13738-017-1161-6. [DOI] [Google Scholar]
- Mcdonald T. M.; Lee W. R.; Mason J. A.; Wiers B. M.; Chang S. H.; Long J. R. Capture of carbon dioxide from air and flue gas in the alkylamine-appended metal-organic framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 2012, 134, 7056–7065. 10.1021/ja300034j. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Xian S.; Xia Q.; Wang H.; Li Z.; Li J. Enhancement of CO2 adsorption and CO2/N2 selectivity on ZIF-8 via postsynthetic modification. AIChE J. 2013, 59, 2195–2206. 10.1002/aic.13970. [DOI] [Google Scholar]
- Huang X.; Lu J.; Wang W.; Wei X.; Ding J. Experimental and computational investigation of CO2 capture on amine grafted metal-organic framework NH2-MIL-101. Appl. Surf. Sci. 2016, 371, 307–313. 10.1016/j.apsusc.2016.02.154. [DOI] [Google Scholar]
- Lin Y.; Yan Q.; Kong C.; Chen L. Polyethyleneimine incorporated metal-organic frameworks adsorbent for highly selective CO2 capture. Sci. Rep. 2013, 3, 1859 10.1038/srep01859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.; Lin H.; Wang H.; Suo Y.; Li B.; Kong C.; Chen L. Enhanced selective CO2 adsorption on polyamine/MIL-101(Cr) composites. J. Mater. Chem. A 2014, 2, 14658–14665. 10.1039/C4TA01174K. [DOI] [Google Scholar]
- Yan Q.; Lin Y.; Kong C.; Chen L. Remarkable CO2/CH4 selectivity and CO2 adsorption capacity exhibited by polyamine-decorated metal-organic framework adsorbents. Chem. Commun. 2013, 49, 6873–6875. 10.1039/c3cc43352h. [DOI] [PubMed] [Google Scholar]
- Aarti A.; Bhadauria S.; Nanoti A.; Dasgupta S.; Divekar S.; Gupta P.; Chauhan R. [Cu3(BTC)2]-polyethyleneimine: an efficient MOF composite for effective CO2 separation. RSC Adv. 2016, 6, 93003–93009. 10.1039/C6RA10465G. [DOI] [Google Scholar]
- Zhang H.; Goeppert A.; Olah G. A.; Prakash G. K. S. Remarkable effect of moisture on the CO2 adsorption of nano-silica supported linear and branched polyethylenimine. J. CO2 Util. 2017, 19, 91–99. 10.1016/j.jcou.2017.03.008. [DOI] [Google Scholar]
- Lee C. H.; Hyeon D. H.; Jung H.; Chung W.; Jo D. H.; Shin D. K.; Kim S. H. Effects of pore structure and PEI impregnation on carbon dioxide adsorption by ZSM-5 zeolites. J. Ind. Eng. Chem. 2015, 23, 251–256. 10.1016/j.jiec.2014.08.025. [DOI] [Google Scholar]
- Li K.; Jiang J.; Tian S.; Yan F.; Chen X. Polyethyleneimine–nano silica composites: a low-cost and promising adsorbent for CO2 capture. J. Mater. Chem. A 2015, 3, 2166–2175. 10.1039/C4TA04275A. [DOI] [Google Scholar]
- Liu F.; Huang K.; Yoo C. J.; Okonkwo C.; Tao D. J.; Jones C. W.; Dai S. Facilely synthesized meso-macroporous polymer as support of poly(ethyleneimine) for highly efficient and selective capture of CO2. Chem. Eng. J. 2017, 314, 466–476. 10.1016/j.cej.2016.12.004. [DOI] [Google Scholar]
- Chavan S. M.; Shearer G. C.; Svelle S.; Olsbye U.; Bonino F.; Ethiraj J.; Lillerud K. P.; Bordiga S. Synthesis and characterization of amine-functionalized mixed-ligand metal-organic frameworks of UiO-66 topology. Inorg. Chem. 2014, 53, 9509–9515. 10.1021/ic500607a. [DOI] [PubMed] [Google Scholar]
- Ren J.; Wu L. B.; Li B. G. Potential for using simple 1,2,4-triazole salt solutions as highly efficient CO2 absorbents with low reaction enthalpies. Ind. Eng. Chem. Res. 2013, 52, 8565–8570. 10.1021/ie4006386. [DOI] [Google Scholar]
- Zhu J.; Wu L. B.; Bu Z.; Jie S.; Li B. G. Synthesis and CO2 capture behavior of porous cross-linked polymers containing pendant triazole groups. Ind. Eng. Chem. Res. 2017, 56, 10155–10163. 10.1021/acs.iecr.7b01961. [DOI] [Google Scholar]
- Gray M. L.; Hoffman J. S.; Hreha D. C.; Fauth D. J.; Hedges S. W.; Champagne K. J.; Pennline H. W. Parametric study of solid amine sorbents for the capture of carbon dioxide. Energy Fuels 2009, 23, 4840–4844. 10.1021/ef9001204. [DOI] [Google Scholar]
- He H.; Zhuang L.; Chen S.; Liu H. Solid amine adsorbent prepared by molecular imprinting and its carbon dioxide adsorption properties. Chem. - Asian J. 2016, 11, 3055–3061. 10.1002/asia.201601031. [DOI] [PubMed] [Google Scholar]
- Devautour-Vinot S.; Maurin G.; Serre C.; Horcajada P.; Cunha D. P. D.; Guillerm V.; Costa E. D. S.; Taulelle F.; Martineau C. Structure and dynamics of the functionalized MOF type UiO-66(Zr): NMR and dielectric relaxation spectroscopies coupled with DFT calculations. Chem. Mater. 2012, 24, 2168–2177. 10.1021/cm300863c. [DOI] [Google Scholar]
- Saleem H.; Rafique U.; Davies R. P. Investigations on post-synthetically modified UiO-66-NH2 for the adsorptive removal of heavy metal ions from aqueous solution. Microporous Mesoporous Mater. 2016, 221, 238–244. 10.1016/j.micromeso.2015.09.043. [DOI] [Google Scholar]
- Valenzano L.; Civalleri B.; Chavan S.; Bordiga S.; Nilsen M. H.; Jakobsen S.; Lillerud K. P.; Lamberti C. Disclosing the complex structure of UiO-66 metal organic framework: a synergic combination of experiment and theory. Chem. Mater. 2011, 23, 1700–1718. 10.1021/cm1022882. [DOI] [Google Scholar]
- Su X.; Bromberg L.; Martis V.; Simeon F.; Huq A.; Hatton T. A. Postsynthetic functionalization of Mg-MOF-74 with tetraethylenepentamine: structural characterization and enhanced CO2 adsorption. ACS Appl. Mater. Interfaces 2017, 9, 11299–11306. 10.1021/acsami.7b02471. [DOI] [PubMed] [Google Scholar]
- Samanta A.; Zhao A.; Shimizu G. K. H.; Sarkar P.; Gupta R. Post-combustion CO2 capture using solid sorbents: a review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. 10.1021/ie200686q. [DOI] [Google Scholar]
- Ren J.; Wu L. B.; Li B. G. Preparation and CO2 sorption/desorption of N-(3-aminopropyl)aminoethyl tributylphosphonium amino acid salt ionic liquids supported into porous silica particles. Ind. Eng. Chem. Res. 2012, 51, 7901–7909. 10.1021/ie2028415. [DOI] [Google Scholar]
- Liu Z.; Du Z.; Zou W.; Mi J.; Li H.; Wang Y.; Zhang C. Moisture-resistant porous polymer from concentrated emulsion as low-cost and high-capacity sorbent for CO2 capture. RSC Adv. 2013, 3, 18849–18856. 10.1039/c3ra43597k. [DOI] [Google Scholar]
- Han J.; Du Z.; Zou W.; Li H.; Zhang C. Moisture-responsive hydrogel impregnated in porous polymer foam as CO2 adsorbent in high-humidity flue gas. Ind. Eng. Chem. Res. 2015, 54, 7623–7631. 10.1021/acs.iecr.5b01305. [DOI] [Google Scholar]
- Wu Y.; Wang J.; Muhammad Y.; Subhan S.; Zhang Y.; Ling Y.; Li J.; Zhao Z.; Zhao Z. Pyrrolic N-enriched carbon fabricated from dopamine-melamine via fast mechanochemical copolymerization for highly selective separation of CO2 from CO2/N2. Chem. Eng. J. 2018, 349, 92–100. 10.1016/j.cej.2018.05.072. [DOI] [Google Scholar]
- McCann N.; Maeder M.; Attalla M. Simulation of enthalpy and capacity of CO2 absorption by aqueous amine systems. Ind. Eng. Chem. Res. 2008, 47, 2002–2009. 10.1021/ie070619a. [DOI] [Google Scholar]
- Gupta M.; da Silva E. F.; Hartono A.; Svendsen H. F. Theoretical study of differential enthalpy of absorption of CO2 with MEA and MDEA as a function of temperature. J. Phys. Chem. B 2013, 117, 9457–9468. 10.1021/jp404356e. [DOI] [PubMed] [Google Scholar]
- Ebune G. E.Carbon Dioxide Capture from Power Plant Flue Gas Using Regenerable Activated Carbon Powder Impregnated with Potassium Carbonate. M.S. Thesis, 2008. [Google Scholar]
- DeCoste J. B.; Peterson G. W.; Jasuja H.; Glover T. G.; Huang Y.-g.; Walton K. S. Stability and degradation mechanisms of metal–organic frameworks containing the Zr6O4(OH)4 secondary building unit. J. Mater. Chem. A 2013, 1, 5642–5650. 10.1039/c3ta10662d. [DOI] [Google Scholar]
- Goeppert A.; Czaun M.; May R. B.; Prakash G. K.; Olah G. A.; Narayanan S. R. Carbon dioxide capture from the air using a polyamine based regenerable solid adsorbent. J. Am. Chem. Soc. 2011, 133, 20164–20167. 10.1021/ja2100005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.