Abstract
The present work envisaged an adherent luliconazole-loaded bilayer nail lacquer (BNL) with significant transungual activity. The locally applied sustained-release BNL formulation was designed for an improved retention, payload, and final dermatokinetic disposition. A primary step in the fabrication of a BNL included overcoming of physical barriers like α-keratin (also α-keratin), a protein present in human nails, and then allowing the drug molecule to permeate at the site of action. Although luliconazole is an established antifungal agent, has limited clinical exploitation for its use in treating onychomycosis. An in silico study elucidating its interaction with lanosterol-14-α demethylase, an enzyme which is the key region of drug action mechanism, was highly supportive of its imminent clinical potential. Optimization of prepared BNL formulations via response surface modeling (Box–Behnken Design-Expert 10.0.6) logically ascertained the effect of selected independent variables and showcased its effect via dependent responses. Surface morphology of the prepared BNL films was well corroborated for the presence of two distinct polymeric layers through scanning electron microscopy imaging. Nail permeation studies revealed a cumulative drug release of 71.25 ± 0.11% through bovine hooves up to 24 h. Luliconazole while exposing antifungal activity against clinical isolates of Trichophyton rubrum in agar cup-plate method disclosed a 38 mm diameter zone of inhibition. Further, the optimized BNL exhibited a bioadhesive force of 1.9 ± 0.11 N, which assured its retention on the nail surface for prolonged duration of time. In Conclusion, it is deduced that the conventional treatment modalities for onychomycosis require circumvention of certain pharmacotechnical caveats. Therefore, in the present study, a multipronged BNL system was proposed, which negates the need of frequent drug application, improvises cosmetic appearance, yields fruitful therapeutic outcomes, and has a clinical supremacy over the available therapeutics.
Introduction
Onychomycosis is a locoregional fungal infection of human toes and finger nails resulting in highly mutilated nails, which often appear severely disfigured and brittle. The disease is degenerative in nature and warrants medical intervention possibly also salvaging the mutilated nail surface by providing a suitable aesthetic appeal. This is highly relevant as diseased, ill appearing nails lead to discomfort and poor quality of life.1 Onychomycosis is prominently caused by Trichophyton rubrum (dermatophyte), which is responsible for causing 60% of such infections. Nondermatophytes species such as Candida spp., Aspergillus spp., and Fusarium spp. are also considered as a causative agent for the aforementioned infection.2
Owing to its adversity, onychomycosis often requires both systemic as well as frequent topical application for prolonged duration. To circumvent such challenges, researchers have reported transungual route, i.e., drug administration through the nail route as a promising option for the delivery and maintenance of an active medicament on and across the nail. This targeted drug delivery is achieved with simple formulation, improved bioadhesion, and locoregional action. It also has attributes of voluntary removal, reduced drug interactions, low systemic adverse effects, and avoidance of first-pass metabolism.3−7
However, low or insufficient permeation/penetration of the drug on and across highly keratinized nail barrier, composed of α-keratin having strong disulfide linkages, serves as a major barrier in the delivery of a therapeutically active formulation via transungual route.8 Recent studies have highlighted the growing concern of a drug inhibition/resistance development by a fungal cell enzyme, lanosterol-14-α-demethylase, which further adds onto the aforesaid challenges.9
To countervail the aforementioned impediments, the present research study proposes fabrication of a locally active, aesthetic, antifungal-based, transungual bilayer nail lacquer (BNL), which is basically an amalgamation of two nail lacquer layers, i.e., drug-loaded hydrophilic nail lacquer (primary layer) and drug-free hydrophobic nail lacquer (secondary layer).10 The purported BNL system is a novel approach wherein the primary layer efficiently covers the nail plate aiding substantial permeation of a drug, whereas secondary layer supports hydrophobicity and BNL integrity. Also, primary layer ensures longer stay on the nail enamel due to its uneven surface supported by polymeric mucoadhesion. The attached BNL acts as a drug reservoir, which is essentially formed after all of the volatile solvent (methanol) have evaporated.3 Owing to its topical application, the BNL system considerably reduces systemic side effects and adverse drug interactions generally observed in oral preparations, thus improving therapeutic efficacy.9,10
The major active constituents of a prepared BNL is an antifungal drug (luliconazole), film-forming polymers (hydroxypropyl methyl cellulose (HPMC) and cellulose acetate phthalate, CAP), and a permeation enhancer (thioglycolic acid, TGA).
Luliconazole, a newer class of imidazole antifungal agent, is reported to have a broad spectrum of antifungal activity against various species of fungi, majorly dermatophytes. It has got a limited molecular reporting, and therefore the scope of understanding its mechanism is still pending.11−14 Henceforth, the current study emphasized upon carrying out an in silico molecular docking study to check the suitability of luliconazole and to get better comprehension of the α-keratin (protein present in human nails)15,16 and lanosterol-14-α demethylase (an enzyme present in fungal cell walls)17 inhibitory potency at molecular level and also to shed light on the interactions in the active sites of α-keratin and lanosterol-14-α demethylase. The resultant protein interaction and enzyme inhibition by luliconazole and its comparison with the standard of care, ciclopirox, revealed superimposable three-dimensional (3D) images, which envisaged it to be a drug of choice. The combinatorial effect of a mucoadhesive BNL along with the certitude of a drug delivery followed by its interaction at the drug-target site ensures its suitability for treatment of onychomycosis.
Additionally, thioglycolic acid has been added in primary layer of a BNL with a sole purpose of enhancing drug penetration on and across highly keratinized nail barrier by cleaving the α-keratin disulfide linkages, making surface of human nail more porous due to the formation of new pores for better drug permeation and retention.18−20
The incorporation of film-forming polymers was done to provide a bioadhesive and pliable lacquer film over the surface of a highly dysmorphic nail.21
Further, the histopathological assessment of a BNL-treated Wistar rat skin has revealed a low skin irritation profile as it shows an excellent local tolerability with very minor application-site skin irritations or reactions.11
Results and Discussion
In Silico
Therapeutic success of any delivery system heavily relies on the suitable drug choice and its optimal binding at the drug action site.3 Molecular docking has emerged as a dependable tool for in silico drug selection,22 which was suitably employed to shortlist luliconazole as a drug of choice for the current study. It further revealed that luliconazole binds well with the α keratin of the nail and also engages at molecular level with lanosterol-14-α demethylase.17
Luliconazole−α-Keratin Interaction
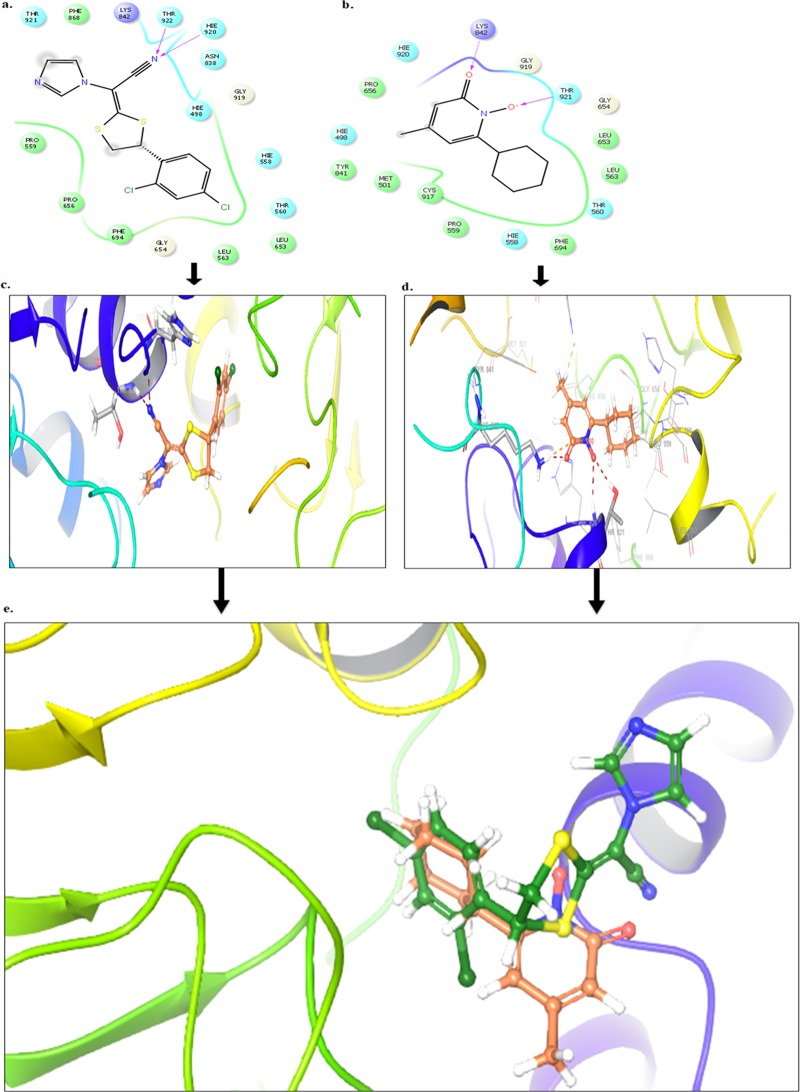
Docking studies were performed using Glide module of the Schrodinger 9.6 software on the α-keratin receptor (PDB ID: 4XIF). Docking results reveal that the compound luliconazole bound tightly in the active site of α-keratin, as it was well occupied in the receptor cavity and makes hydrogen bond with Thr 922 and His 920 as presented in Figure 1a,c. The redocking of reference ligand (ciclopirox) into active site of α-keratin reveals that it occupies the same binding pocket as presented in Figure 1b,d with a root-mean-square deviation (RMSD) of 0.38 A, which further validates the present docking protocol. Further docking analysis of compound luliconazole with co-crystallized ligand (ciclopirox) by superimposition gives the same interaction pattern of binding with catalytic domain of α-keratin, as represented in Figure 1e. The docking scores of standard ciclopirox and luliconazole were compared and found to be same.
Figure 1.
(a–c) Binding interaction of compound luliconazole against α-keratin (PDB ID 4XIF). (b–d) Binding interaction of standard drug ciclopirox against α-keratin (PDB ID 4XIF). (e) Superimposition of compound luliconazole (green) with ciclopirox (brown) against α-keratin.
Luliconazole–Lanosterol-14-α Demethylase Interaction
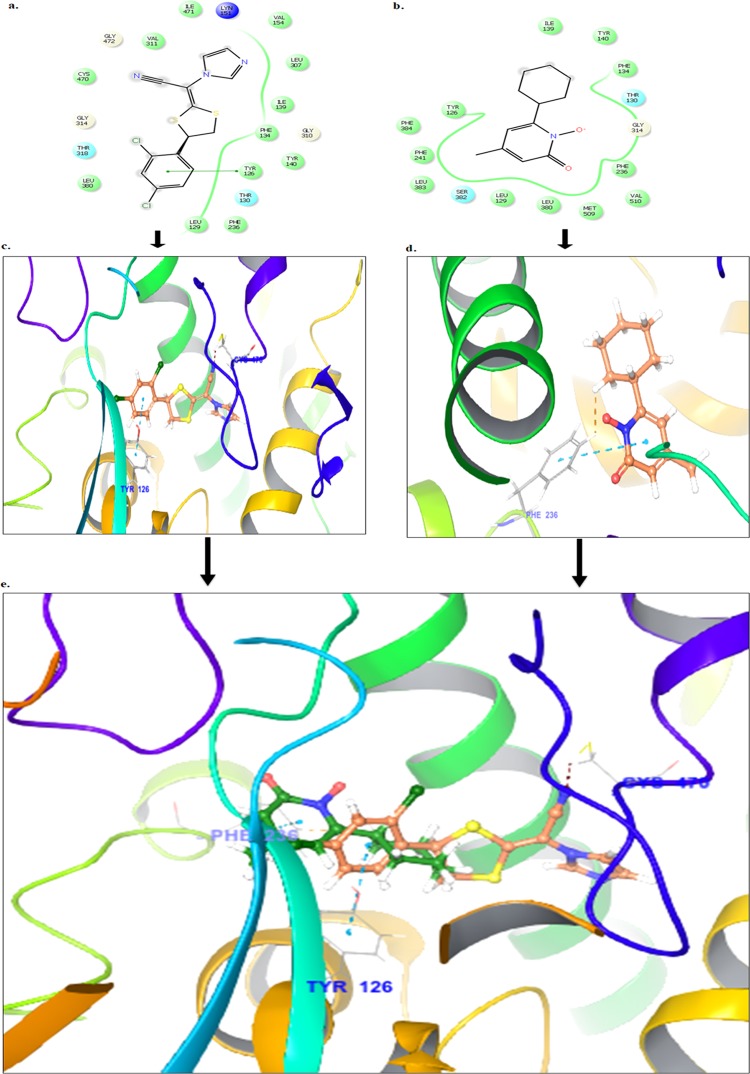
Docking studies were performed using the Glide module of the Schrodinger 9.6 software on the lanosterol-14-α demethylase receptor (PDB ID: 5HS1). Docking results reveal that the compound luliconazole bound tightly in the active site of lanosterol-14-α demethylase as it was well occupied in the receptor cavity thereby forming hydrogen bond with Cys 470 and π–π stacking with Tyr 126, as presented in Figure 2a,c. The redocking of reference ligand (ciclopirox) into the active site of lanosterol-14-α demethylase enzyme reveals that it occupies the same binding pocket as presented in Figure 2b,d, with a root-mean-square deviation (RMSD) of 0.42 A, which further validates the present docking protocol. Further docking analysis of compound luliconazole with co-crystallized ligand (ciclopirox) by superimposition gives the same interaction pattern of binding with catalytic domain of lanosterol-14-α demethylase enzyme as represented in Figure 2e. The docking score of standard ciclopirox and luliconazole were compared and found to be the same.
Figure 2.
(a–c) Binding interaction of compound luliconazole against lanosterol-14-α demethylase (PDB ID 5HS1). (b–d) Binding interaction of standard drug ciclopirox against lanosterol-14-α demethylase (PDB ID 5HS1). (e) Superimposition of compound luliconazole (green) with ciclopirox (brown) against lanosterol-14-α demethylase.
The resultant protein interaction and enzyme inhibition by luliconazole and its comparison with the standard of care, ciclopirox, revealed superimposable 3D images, which reiterated it to be an effective drug choice. The combinatorial effect of a mucoadhesive BNL and the certainty of drug delivery across the nail with adequate binding at the active drug action site ensures its suitability for the treatment of difficult-to-treat disease onychomycosis.11
Formulation Development
Optimization
The ultimate success of any formulation design both in vitro and in vivo heavily relies on its optimization, which assures its optimum or the best possible performance. Therefore, to successfully fabricate a bilayer nail lacquer (BNL), parameters such as drying time and nonvolatile content were taken into consideration as they are cited as crucial parameters in formulation, design, and optimization of a nail lacquer, listed by Bureau of Indian Standards (BIS), IS 9245:1994. Henceforth, the sequential optimization was focused at matching these formulation requirements using Design of Expert (DoE) software, a Quality by Design (QbD) tool, which uses certain predecided independent variables, and the proposed dependent variables often lead to successful designing of optimized formulation. In the current study, response surface modeling via Box–Behnken design (BBD) logically ascertains the effect of selected independent variables and showcases its formulation design outcomes via dependent responses (drying time and nonvolatile content).23
Drying Time
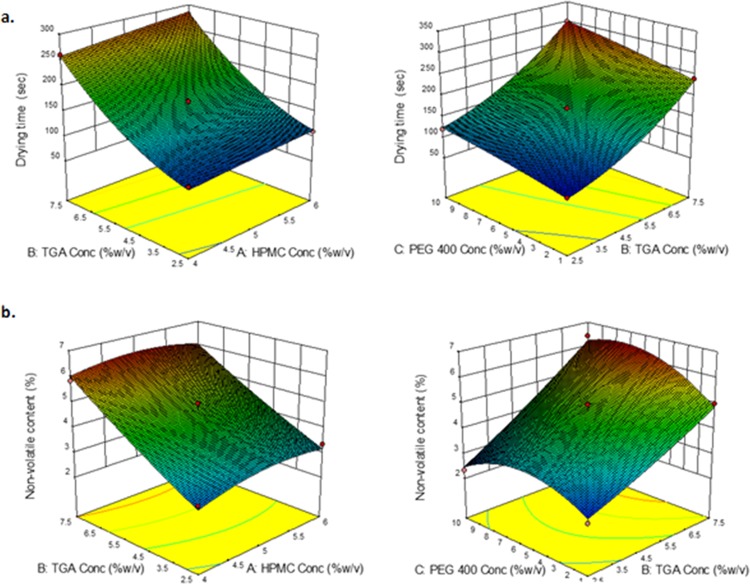
The prepared BNL is proposed to be a fast-drying formulation, which will dictate an often-acceptable drug release while remaining adhered to the nail surface;24 therefore, drying time, which was indicated as a primary dependent response, was evidently affected by the concentration of a thioglycolic acid (independent variable) as it is liquid and nonvolatile. Thus, with increase in thioglycolic acid concentration, drying time increases since more of the nonvolatile component leads to higher dying time, as presented in Figure 1a. The concentrations of hydroxypropyl methyl cellulose (HPMC) and poly(ethylene glycol)-400 (PEG 400) (independent variable) have a low or negligible effect on drying time (Figure 3a).
Figure 3.
BBD quadratic model graphs showcasing the effect of independent variables (HPMC, PEG, and thioglycolic acid) on (a) drying time and (b) nonvolatile content.
Nonvolatile content represents the amount of a nonvolatile matrix left after the evaporation of volatile organic solvents. These components are generally responsible for the formation of a film over the surface of a nail plate, thus providing an aesthetical covering over a mutilated nail surface with enhanced drug delivery and cosmetic quotient.25 Therefore, the optimum balance of polymer, i.e., film former and nonvolatile solvents, shall be a key to formulation design, as higher the concentration of polymer (film former) or nonvolatile solvents used, higher will be the nonvolatile content or vice versa, which is evident from a significant rise in nonvolatile content (dependent response 2) after increasing the HPMC concentration (independent variable),26 as highlighted in Figure 3b. Thioglycolic acid being a highly nonvolatile compound from the liquid surface increases the nonvolatile content with increase in its concentration. Poly(ethylene glycol)-400 (independent variable) have however shown a minor effect on nonvolatile content (Figure 3b) and eventual film formation.
QbD Optimization of BNL
Further, the obtained BBD graphs suggested response surface quadratic models as shown in Figure 3a,c for both the dependent responses, i.e., drying time and nonvolatile content, as evident from the adjusted and predicted R2 values of 0.9245 and 0.8219, respectively. An analysis of variance revealed that the predecided independent factors have positively influenced and favored the dependent responses presenting significant probability values (p value) of less than 0.0001 and 0.0002 and nonsignificant “lack of fit” values of 0.73 and 0.7129 for dependent responses drying time and nonvolatile content, respectively, both of which successfully reflect a highly acceptable quadratic model.
The following equation in terms of code factors can be used to make predictions about the response for given levels of each factor:
Equation obtained via BBD for drying time in terms of coded factor = +164.00 + 11.25 × A + 83.75 × B + 27.50 × C + 2.50 × AB – 5.00 × AC + 5.00 × BC – 0.75 × A2 + 24.25 × B2 – 3.25 × C2
Equation obtained via BBD for nonvolatile content in terms of coded factor = +4.71 + 0.11 × A + 1.54 × B + 0.34 × C – 0.095 × AB – 0.075 × AC + 0.35 × BC – 0.31 × A2 + 0.10 × B2 – 0.80 × C2
Accordingly, the BBD has yielded 17 experimental runs predicting the limits of drying time and nonvolatile content. Formulation coded F17 having concentrations of HPMC, PEG-400, and thioglycolic acid as 5, 2.5, and 1 (% w/v), respectively, offers the lowest value for both drying time, i.e., 80 ± 0.10 s, and nonvolatile content, i.e., 2.3 ± 0.10%, and therefore, it has been chosen as an optimized formulation, as highlighted in Table 1.
Table 1. Summary of Experimental Runs as Given by DoE/BBD Showing the Effect of Independent Variables on the Dependent Responses.
| factor 1 | factor 2 | factor 3 | response 1 | response 2 | |
|---|---|---|---|---|---|
| run | A: HPMCa concentration (% w/v) | B: TGAa concentration (% w/v) | C: PEG 400a concentration (% w/v) | drying time (s) | nonvolatile content (%) |
| 1 | 4 | 7.5 | 5.5 | 260 ± 0.21 | 5.84 ± 0.31 |
| 2 | 6 | 7.5 | 5.5 | 280 ± 0.21 | 5.9 ± 0.24 |
| 3 | 4 | 5 | 1 | 110 ± 0.11 | 3.11 ± 0.67 |
| 4 | 4 | 5 | 10 | 180 ± 0.11 | 3.89 ± 0.49 |
| 5 | 5 | 7.5 | 10 | 300 ± 0.23 | 6.42 ± 0.42 |
| 6 | 6 | 5 | 10 | 200 ± 0.23 | 3.93 ± 0.12 |
| 7 | 5 | 2.5 | 10 | 120 ± 0.12 | 2.33 ± 0.15 |
| 8 | 6 | 5 | 1 | 150 ± 0.12 | 3.45 ± 0.16 |
| 9 | 5 | 5 | 5.5 | 170 ± 0.12 | 4.96 ± 0.13 |
| 10 | 5 | 5 | 5.5 | 160 ± 0.11 | 4.52 ± 0.18 |
| 11 | 5 | 5 | 5.5 | 170 ± 0.10 | 4.98 ± 0.28 |
| 12 | 5 | 5 | 5.5 | 170 ± 0.21 | 4.96 ± 0.25 |
| 13 | 6 | 2.5 | 5.5 | 110 ± 0.11 | 3.34 ± 0.30 |
| 14 | 5 | 7.5 | 1 | 240 ± 0.33 | 5 ± 0.21 |
| 15 | 5 | 5 | 5.5 | 150 ± 0.10 | 4.11 ± 0.14 |
| 16 | 4 | 2.5 | 5.5 | 100 ± 0.14 | 2.9 ± 0.10 |
| 17 | 5 | 2.5 | 1 | 80 ± 0.10 | 2.3 ± 0.10 |
HPMC: hydroxypropyl methyl cellulose; TGA: thioglycolic acid; PEG-400: polyethylene glycol-400.
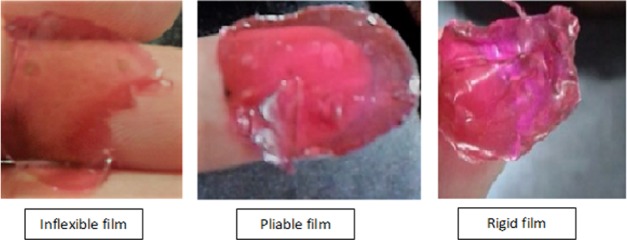
Further, secondary nail lacquer formulations have been visually optimized based on the degree of pliability of various prepared films, as presented in Table 2. Visual observation has revealed formulation coded as F4, consisting of 4% (w/v) of cellulose acetate phthalate (CAP) and 2% (w/v) of poloxamer F-127 as an optimized formulation, as represented in Figure 4.
Table 2. Various Secondary Nail Lacquer Formulation Concentrations and the Quality of Films Obtained.
| formulation code | CAPa (% w/v) | poloxamer F-127 (% w/v) | acetone (mL) | quality of film |
|---|---|---|---|---|
| F1 | 1 | 0.5 | 10 | very poor and peeling off |
| F2 | 2 | 1 | 10 | poor and peeling off |
| F3 | 3 | 1.5 | 10 | poor and inflexible |
| F4 | 4 | 2 | 10 | excellent and pliable |
| F5 | 5 | 2.5 | 10 | good and rigid |
CAP: cellulose acetate phthalate.
Figure 4.
Visual optimization of secondary nail lacquer formulations highlighting various qualities of films obtained.
Evaluation
Determination of Drying Time
Drying time has emerged as a major process parameter for nail lacquer evaluation. It significantly affects nail lacquer application and performance properties as low and high drying times result in gloomy (not clear) and rigid nail lacquer films, respectively. Henceforth, for the formation of a pliable and nonsticky lacquer film, a moderate drying time is required,24 and all of the BNL formulations prepared using BBD fell in an acceptable range of drying time, i.e., 60–360 s, which is in concordance with the previously mentioned BSI limits. Formulation coded as F5 exhibited the highest drying time value of 300 ± 0.23 s, whereas formulation coded as F17 took the minimum time of 80 ± 0.10 s to dry. The drying times for different formulations are presented in Table 1.
Nonvolatile Content Determination
The amount of a nonvolatile matrix left after the evaporation of a volatile organic solvents is usually responsible for the formation of a film over the surface of a nail plate, thus ensuring complete coverage of a mutilated nail surface.25,26 BNL formulations prepared via BBD show an acceptable range of nonvolatile content, i.e., 2.3–6.42%, which is in agreement with the previously mentioned BSI limits. Among the various prepared formulations, F5 exhibited the highest value of nonvolatile content, i.e., 6.42 ± 0.42%, whereas formulation F17 consisted of a least amount of nonvolatile content, i.e., 2.3 ± 0.10%, Table 1 displays the nonvolatile content for different formulations.
Characterization
Determination of the Physical Properties
X-ray Diffraction (XRD)
XRD profiling provides a commentary upon the magnitude and outline of trivial crystalline areas and alignment of single crystal as well.
XRD analysis of BNL formulation showed a smaller crystalline area compared to pure drug, which has elicited a larger crystalline area, as presented in Figure 5a.
Figure 5.
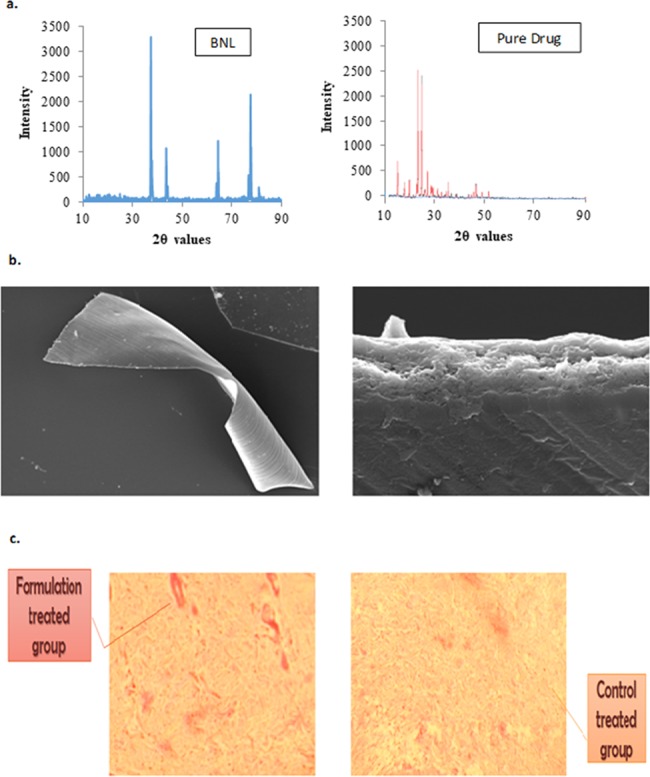
(a) X-ray diffractograms of an optimized BNL formulation and a pure drug. (b) Scanning electron microscopy (SEM) image showcasing BNL structure. (c) Histological assessment of a Wistar rat skin sections.
SEM
Surface morphology of the prepared BNL films was well corroborated for the presence of two distinct polymeric layers through SEM imaging with smooth surface morphology, confirming a bilayer structure10 as represented in Figure 5b.
Histological Assessment
The histological assessment of rat skin has indicated only slight application-site reactions or irritations in the formulation-treated group, where due to bursting of surface capillaries, some disruptions in rat skin layer was observed, as highlighted in Figure 5c. On the contrary, in the control-treated group, no such irritation was observed, as the surface capillaries of rat skin are intact without any disruptions (Figure 5c).33
Luliconazole offers a wide window of skin safety and tolerability;13 henceforth, the disruptions of a formulation-treated group were not significant enough compared to the control-treated group, as evident in Figure 5c.
Characterization of BNL
Skin Irritation
Luliconazole has been widely exploited for topical application and has shown a high local tolerability and a skin-friendly profile,11 which is also evident from the skin irritation studies that showcase BNL formulation as nonirritant to skin with only trivial signs of erythema and edema (<2).32 Further, evidences of any undesirable skin changes such as change in color and morphology were also not observed in skin irritation profile of a BNL formulation, as presented in Table 3.
Table 3. Skin Irritation Profile of a BNL Formulation.
Erythema scale: 0 = none, 1 = slight, 2 = well defined, 3 = moderate, and 4 = scar formation.
Edema scale: 0 = none, 1 = slight, 2 = well defined, 3 = moderate, and 4 = severe.
In Vitro Release
In vitro release data significantly showcase a cumulative drug release of 47.5 ± 0.11 and 69.07 ± 0.14% for single layer (primary) and BNL, respectively, across a dialysis membrane for a period of 24 h. To further explicate the in vitro release mechanism, various release models were employed and the data were best fitted in the zeroth-order model (R2 = 0.9748), as presented in Figure 6a, suggesting drug release to be diffusion-controlled.27
Figure 6.
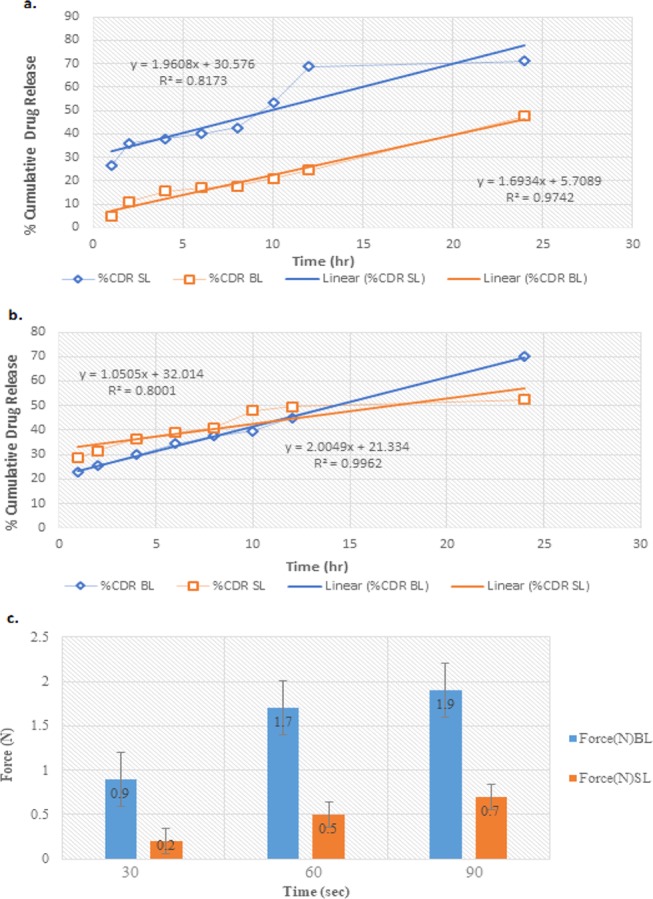
(a) In vitro release profile of optimized single-layer (SL) and bilayer (BL) nail lacquer formulations. (b) Nail permeation profile of optimized single-layer (SL) and bilayer (BL) nail lacquer formulations. (c) Bioadhesive force of a single-layer nail lacquer and BNL at different contact times measured via texture analyzer using bovine hooves as a substrate.
Nail Permeation
Nail permeation data of a single layer (primary) reveal a cumulative drug release of 52.5 ± 0.12%, and for BNL, it was found to be 71.25 ± 0.11% through bovine hooves up to 24 h. A high cumulative drug release of 71.25 ± 0.11% (nail permeation studies) justifies the use of thioglycolic acid as permeation enhancer, since it is responsible for breaking disulfide linkages present in bovine hooves leading to enhanced porosity and the resultant drug permeation.28 In vitro release kinetic modeling suggested it as a best fit for zeroth-order model (R2 = 0.9962), as presented in Figure 6b, thus indicating a diffusion-controlled drug release.27
Bioadhesion
One of the salient features of the current formulation design was to ascertain adequate bioadhesion which is solely responsible for a significant retention of a supersaturated lacquer film onto the nail for a prolonged time duration.29−31 Single layer (primary) revealed bioadhesive forces of 0.2 ± 0.11, 0.5 ± 0.11, and 0.7 ± 0.13 N at times 30, 60, and 90 s respectively, whereas BNL unveils bioadhesive forces of 0.9 ± 0.11, 1.7 ± 0.12, and 1.9 ± 0.11 N at 30, 60, and 90 s, respectively. As evident from the obtained data, the degree of bioadhesive force increases with an increase in the contact time intervals,31 as presented in Figure 6c; a bioadhesive force of 1.9 ± 0.11 N (optimized formulation) provided substantial bioadhesion and ensured sufficient drug availability for delivery across the nail.
Evaluation of BNL
Microbiological Evaluation—In Vitro Antifungal Activity
Microbiological evaluation of the formulation supported the choice of a luliconazole,17 which developed an active zone of inhibition diameter (38 mm) against T. rubrum, which is considered as a major causative fungal agent for onychomycosis2,11,33 (Table 4).
Table 4. In Vitro Antifungal Activity of an Optimized BNL Formulation (F17).
| antifungal activity | drug solution
diameter of zone of inhibition (mm) |
optimized BNL formulation diameter of zone of inhibition
(mm) |
blank (methanol solution) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| time | 24 h | 48 h | 120 h | 24 h | 48 h | 120 h | 24 h | 48 h | 120 h |
| T. rubrum | 5.1 ± 0.1 | 13 ± 0.1 | 40 ± 0.1 | 4.9 ± 0.1 | 13.2 ± 0.1 | 38 ± 0.1 | 0.8 ± 0.1 | 0.87 ± 0.1 | 0.9 ± 0.1 |
| Candida albicans | 1.2 ± 0.1 | 1.9 ± 0.2 | 2.5 ± 0.2 | 1.5 ± 0.1 | 2 ± 0.1 | 2.9 ± 0.2 | 0.8 ± 0.1 | 0.85 ± 0.1 | 0.9 ± 0.1 |
Stability Studies
The obtained ranges of drying time and nonvolatile content for an optimized nail lacquer formulation, i.e., (F17), were found to be 110 ± 0.3 and 8 ± 0.1 s and 4.96 ± 0.5 and 1.3 ± 0.1%, respectively, for a storage period of 1 month at a temperature of 4–25 °C, which are in concordance with the previously mentioned BIS limits.
Conclusions
The stability of the BNL system for a period of 1 month and its highly relevant pharmacotechnical attributes like low drying time, appreciable nonvolatile content, and minimal skin irritation with suitable aesthetic coverage indicates its potential to address the caveats associated with the treatment of a challenging disease like onychomycosis and offers a novel drug-delivery platform for its successful treatment.
Experimental Section
Materials
Luliconazole was procured as gift sample from Cadila Pharmaceuticals Limited (Ahmedabad, Gujarat, India); hydroxypropyl methyl cellulose (HPMC) and cellulose acetate phthalate (CAP) were procured from Jubilant Life Sciences Limited. All other chemicals and reagents were of analytical grade obtained from Merck, India.
Methods
In Silico
Molecular docking simulations of luliconazole at the α-keratin and lanosterol-14-α demethylase receptor catalytic ligand binding site, PDB ID: 4XIF and PDB ID: 5HS1, respectively, were carried out to study the binding mode of luliconazole with α-keratin and lanosterol-14-α demethylase at the molecular level. The docking of drug compounds was performed using Maestro, version 9.6, implemented from Schrodinger software suite. The ligand was sketched in 3D format using build panel and were prepared for docking using LigPrep application. The protein for docking study was taken from Protein Data Bank (PDB ID: 4XIF; PDB ID: 5HS1) and prepared by removing solvent, adding hydrogen, and further minimization in the presence of bound ligand; H4V and VOR, respectively, using protein preparation wizard. Grids for molecular docking were generated with bound co-crystallized ligand. For the validation of docking parameters, the standard ligand (ciclopirox) was redocked at the catalytic site of protein, and the RMSDs between co-crystal and redocked pose was found to be 0.38 and 0.42 A, respectively. Luliconazole was docked using Glide extraprecision mode, with up to three poses saved per molecule.
Fabrication of a BNL System
The two prominent layers of a BNL system (primary and secondary) were prepared via simple mixing.
For primary layer, API/luliconazole (1% w/v) was dissolved in 5 mL of methanol and placed on a magnetic stirrer at 150 rpm and room temperature. To the resulting drug–solution mixture, HPMC, poly(ethylene glycol)-400 (PEG-400), glycerol, and thioglycolic acid were sequentially added with continuous stirring.3 A total of 17 formulations were prepared as presented in Table 1 and optimized further.
For the secondary layer, Poloxamer F-127 and cellulose acetate phthalate (CAP) were dissolved in acetone on a vortex mixer (50 rpm) at room temperature. A total of four formulations were prepared as presented in Table 2 and optimized further.
Optimization
The Box–Behnken design (BBD), Design-Expert 10.0.6, was employed for the optimization of prepared nail lacquer (primary) formulations. For exploring the experimental design selection of independent variables such as concentrations of HPMC, thioglycolic acid and PEG-400 were done on the basis of their ability to affect a formulation and also to analyze their effects on dependent variables such as drying time and nonvolatile content, which were selected as crucial parameters in determining formulation attributes.
The basic optimization criterion for secondary nail lacquer formulations was the degree of pliability of various prepared films.
Evaluation
The evaluation parameters listed by Bureau of Indian Standards (BIS), IS 9245:1994, were exploited for the evaluation of the prepared nail lacquers.
Determination of Drying Time
In the present study, the uniformly thin nail lacquer layer (primary) was applied onto a clean aluminum plate using a lacquer brush and observed further (For BNL, the primary layer was initially applied and dried, followed by an application of a secondary layer.) It was allowed to dry for 60 s, this being the minimum time needed for drying of a lacquer, and subsequently after an interval of 10 s, the applied lacquer film was slightly touched using the tip of a clean glass rod. This exercise was repeated until there was no resultant transfer of any matter on the tip of a glass rod or up to 360 s (maximum drying time range for a nail lacquer). The lacquer formulations falling in the range of 60–360 s were considered to have passed the test for drying time.
Nonvolatile Content Determination
A small amount of sample (1.0 ± 0.2 g) of each layer was weighed accurately in a tin plate and further subjected to drying in a hot-air oven at a temperature of 105 ± 2 °C for 1 h. The tin plate was then removed, cooled, and reweighed by using the following formula
Equation for the determination of a nonvolatile content
where
M = mass in g of the material (nail lacquer) taken
M1 = mass in g of the dry and empty tin plate
M2 = mass in g of the tin plate and dried material (nail lacquer)
According to BIS, the acceptable range of nonvolatile content for nail lacquers is up to 20%.
Characterization
Determination of the Physical Properties
Characterization of an optimized BNL formulation was done by exploiting the following parameters.
X-ray Diffraction (XRD)
PANalytical X’Pert3 powder was employed for recording the diffractograms of pure drug and optimized BNL formulation (sample amount, 2 mg). The obtained diffractograms were further traced via an X-ray diffractometer (Ultima IV) exploiting monochromatic Ni-filtered Cu K radiation, with a 40 kV voltage and 30 mA current radiations scattered in the crystalline regions of the sample, which were measured with a vertical goniometer. XRD patterns of the aforesaid samples were obtained by using a step width of 0.04° with a detector resolution in 2θ (diffraction angle) between 10 and 90° at ambient temperature.
Scanning Electron Microscopy (SEM)
The optimized BNL samples were mounted on a metal stub with gold coated under vacuum and then examined on Zeiss EVO40, Carl Zeiss NTS (North America).
Skin Irritation Studies
The protocol approved by the Institutional Animal Ethics Committee was followed for the aforesaid studies. An optimized BNL formulation (F17) was applied on the dorsal side (2 cm2) of properly shaved skin of Wistar strain rats (180–200 g) and was subsequently compared to a control group (no formulation applied) of Wistar strain rats. Further, to scrutinize any signs of erythema and edema, the applied formulation was removed after 24 h and evaluated on an observation scale of 0–4.33
Histological Assessment
After a 24 h observation period of skin irritation studies, the rats were sacrificed and the excised skin samples were immediately immersed in 10% (v/v) formalin solution buffered with phosphate-buffered saline (pH 7.4). Subsequently, the samples were dehydrated in graded concentrations of ethanol and then immersed in xylene, to prepare paraffin blocks. Skin sections of thickness 5 μm were cut using a microtome and stained with hematoxylin and eosin. The samples were then observed for gross histopathology under a microscope (Motic, Japan ix71, Olympus Corporation, Japan) at 10× magnifications.32
In Vitro Release Studies
A formulation sample of about 100 μL (primary layer) was applied over a dialysis membrane (1 mm thickness), which was then adjusted over a glass vial in such a manner that membrane only touches the surface of the release media. Phosphate-buffered saline (5 mL) was filled in a 5 mL glass or receptor vial, which was placed over a magnetic stirrer at 37 °C with a stirring speed of 600 rpm. Appropriate amounts of samples (3 mL) were collected over a predetermined time interval and replaced with an equal amount of fresh dissolution medium. Luliconazole in the sample solution was analyzed by the UV method at λmax = 296 nm. The samples of BNL were subjected to similar protocols.
Nail Permeation Studies
Owing to their keratinized structure similar to human nails, bovine hooves were taken as a sample model to determine permeation of a nail lacquer via nails.,834
The lower part of the bovine hooves was placed in a microtome for cutting a 1 mm thick section, which could be further mounted in between the two compartments (donor and receptor) of the Franz diffusion cell with an effective surface area and receptor cell volume of 1.23 cm2 and 10 mL, respectively. A formulation sample of about 100 μL (primary layer) was applied carefully over a bovine hoof membrane placed in donor compartment of the Franz diffusion cell. The receptor compartment was filled with solvent system (phosphate-buffered saline/methanol, 7:3), and the whole assembly was maintained at 37 °C with a constant stirring speed of 100 rpm for 24 h. The samples (2 mL) were collected through a sampling port over a predetermined time interval upholding sink condition and were analyzed by the UV method at λmax = 296 nm. A graph was constructed between % cumulative drug permeated and time.3,8 The sample of BNL was subjected to a similar protocol.
Bioadhesion Studies
The degree of bioadhesion for nail lacquer samples was estimated using bovine hooves model via a texture analyzer fitted with a 5 kg load cell (TA. XT2i, Technologies Corp., Scarsdale, NY/STable Micro Systems, Godalming, Surrey, U.K.).
The bovine hooves samples were cut into 6 mm diameter plates, which were further placed onto the mucoadhesion rig and a formulation sample of about 100 μL (primary layer) was applied over it. As a standard procedure, the test probe was adjusted to a height of 20 mm and then lowered at a speed of 10 mm/s with a force of 3 N. As the probe slightly touched the surface of the lacquer film, a trigger force of 0.3 N was applied for 30, 60, or 90 s. After elapsing of the predetermined time, the probe was withdrawn from the surface of the sample at a preset speed of 0.5 mm/s. The force that was needed to withdraw the probe from the lacquer film was recorded as bioadhesive force.8 The sample of BNL was subjected to a similar protocol.
Microbiological Evaluation—In Vitro Antifungal Activity
To examine the antifungal activity of luliconazole against C. albicans and T. rubrum, the agar cup-plate method was exploited. A nutrient agar medium (30 mL) was prepared and sterilized via autoclaving at 120 ± 1.5 °C and 15 pounds pressure for 15 min and inoculated with C. albicans and T. rubrum fungal strains (2 mL of inoculum to 100 mL of nutrient agar media). The concentration of inoculum was adjusted with 0.5 McFarlands standard (0.5 McFarlands = 1.5 × 108 CFU/mL). The inoculated nutrient agar medium was allowed to solidify in two sterilized Petri plates, and in each Petri plate, wells were prepared, having an effective diameter of 5 mm via sterile borer.8 A stock solution of 0.25 μg/mL of luliconazole was prepared in methanol. The stock solution (0.1 mL) was pipetted onto the fungal wells (T. rubrum and C. albicans).34 For blank, methanol solution of same concentration as stock was taken. The prepared Petri plates were incubated at 28 °C and zone diameters were measured after 24, 48, and 120 h to estimate the marked zone of inhibition using (Antibiotic Zone Reader, HICON, New Delhi). The sample of BNL was subjected to a similar protocol.
Stability Studies
The optimized formulation was stored at a temperature of 4 ± 25 °C for 1 month and was subsequently evaluated for its drying time and nonvolatile content.
Acknowledgments
The authors acknowledge the Department of Microbiology, Hamdard Institute of Medical Sciences and Research (HIMSR), Jamia Hamdard.
The authors declare no competing financial interest.
References
- Murdan S.; Hinsu D.; Guimier M. A few aspects of transonychial water loss (TOWL): inter-individual, and intra-individual inter-finger, inter-hand and inter-day variabilities, and the influence of nail plate hydration, filing and varnish. Eur. J. Pharm. Biopharm. 2008, 70, 684–689. 10.1016/j.ejpb.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Elewski B. E. Onychomycosis: pathogenesis, diagnosis, and management. Clin. Microbiol. Rev. 1998, 11, 415–429. 10.1128/CMR.11.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar N.; Sharma H.; Pathak K. Onychomycosis: potential of nail lacquers in transungual delivery of antifungals. Scientifica 2016, 1387936 10.1155/2016/1387936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar H. N.; Juluri A.; Desai B. G.; Murthy S. N. Ungual and transungual drug delivery. Drug Dev. Ind. Pharm. 2012, 38, 901–911. 10.3109/03639045.2011.637931. [DOI] [PubMed] [Google Scholar]
- Firoz S.; Sirisha M. N.; Rajalakshmi R. Transungual drug delivery system: a review. Int. J. Innovative Drug Discovery 2011, 1, 9–14. [Google Scholar]
- Hussan D.; Choudhary S. R.; Sharma D.; Bhandari V.; Singh M. Transungual drug delivery-a novel approach of unique features. Indo Am. J. Pharm. Res. 2013, 3, 4460–4470. [Google Scholar]
- Kumar P. T.; Narayana R. P. Transungual drug delivery: a promising route to treat nail disorders. Int. J. Pharma Res. Rev. 2013, 2, 22–33. [Google Scholar]
- Akhtar N.; Sahu S.; Pathak K. Antifungal potential of tolnaftate against Candida albicans in the treatment of onychomycosis: development of nail lacquer and ex vivo characterization. Pharm. Biomed. Res. 2016, 2, 1–2. 10.18869/acadpub.pbr.2.3.1. [DOI] [Google Scholar]
- Hussan S. D.; Choudhary S. R.; Sharma D.; Bhandari V.; Singh M. Transungual drug delivery-a novel approach of unique features. Indo Am. J. Pharm. Res. 2013, 3 (6), 4460–9. [Google Scholar]
- Shivakumar H. N.; Vaka S. R.; Madhav N. V.; Chandra H.; Murthy S. N. Bilayered nail lacquer of terbinafine hydrochloride for treatment of onychomycosis. J. Pharm. Sci. 2010, 99, 4267–4276. 10.1002/jps.22150. [DOI] [PubMed] [Google Scholar]
- Scher R. K.; Nakamura N.; Tavakkol A. Luliconazole: A review of a new antifungal agent for the topical treatment of onychomycosis. Mycoses 2014, 57, 389–393. 10.1111/myc.12168. [DOI] [PubMed] [Google Scholar]
- Watanabe S.; Takahashi H.; Nishikawa T.; Takiuchi I.; Higashi N.; Nishimoto K.; Kagawa S.; Yamaguchi H.; Ogawa H. Dose-finding comparative study of 2 weeks of luliconazole cream treatment for tinea pedis–comparison between three groups (1%, 0.5%, 0.1%) by a multi-center randomised double-blind study. Mycoses 2007, 50, 35–40. 10.1111/j.1439-0507.2006.01305.x. [DOI] [PubMed] [Google Scholar]
- Jones T.; Tavakkol A. Safety and tolerability of luliconazole solution 10-percent in patients with moderate to severe distal subungual onychomycosis. Antimicrob. Agents Chemother. 2013, 57, 2684–2689. 10.1128/AAC.02370-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura T.; Miyamae A.; Imai A.; Hirayanagi K.; Iwanaga T.; Kubota N.; et al. Comparison of characteristics of two topical therapeutic agents for onychomycosis. Med. Mycol. J. 2016, 57, J141–J147. 10.3314/mmj.16-00020. [DOI] [PubMed] [Google Scholar]
- Baswan S.; Kasting G. B.; Li S. K.; Wickett R.; Adams B.; Eurich S.; Schamper R. Understanding the formidable nail barrier: A review of the nail microstructure, composition and diseases. Mycoses 2017, 60, 284–295. 10.1111/myc.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasuko M.; Shiomi R.; Takahashi Y.; Motoba K.; Hirano H.; Tsuboi R.; Inagaki K. Affinity of Luliconazole for Human Nail Derived Keratin. Med. Mycol. J. 2017, 58, J113–J119. 10.3314/mmj.17-00009. [DOI] [PubMed] [Google Scholar]
- Koga H.; Nanjoh Y.; Makimura K.; Tsuboi R. In vitro antifungal activities of luliconazole, a new topical imidazole. Med. Mycol. 2009, 47, 640–647. 10.1080/13693780802541518. [DOI] [PubMed] [Google Scholar]
- Dessai A. S.; Shripathi D.; Shabaraya A. R. Formulation and in vitro characterization of nail lacquer containing fluconazole for preungual drug delivery system. Int. J. Pharma Bio Sci. 2014, 3, 200–214. [Google Scholar]
- Nogueiras-Nieto L. N.; Begoña Delgado-Charro M.; Otero-Espinar J. F. Thermogelling hydrogels of cyclodextrin/poloxamer polypseudorotaxanes as aqueous-based nail lacquers: Application to the delivery of triamcinolone acetonide and ciclopirox olamine. Eur. J. Pharm. Biopharm. 2013, 83, 370–377. 10.1016/j.ejpb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Kiran R. S.; Shekar C. B.; Vishnu P.; Prasad M. V. V. Ungual drug delivery system of ketoconazole nail lacquer. Int. J. Appl. Pharm. 2010, 2, 17–19. [Google Scholar]
- Bednarek M. B.; Scopazzi C. inventors; EI du Pont de Nemours and Co, assignee Nail polish compositions containing acrylic polymers. U.S. Patent US6,254,878, 2001.
- Mirza M. A.; Panda A. K.; Asif S.; Verma D.; Talegaonkar S.; Manzoor N.; Khan A.; Ahmed F. J.; Dudeja M.; Iqbal Z. A vaginal drug delivery model. Drug Delivery 2016, 23, 3123–3134. 10.3109/10717544.2016.1153749. [DOI] [PubMed] [Google Scholar]
- Shah V. V.; Sharma M.; Gandhi K.; Suthar V.; Parikh R. K. Quality by Design (QbD) Approach for Optimization of Microemulsion based Topical Gel. Marmara Pharm. J. 2016, 20, 415–424. 10.12991/mpj.20162084614. [DOI] [Google Scholar]
- Chandra R.; Kumar S.; Aggarwal A. Evaluation of nail lacquer. Indo Global J. Pharm. Sci. 2012, 2, 379–382. [Google Scholar]
- Dessai A. S.; Shripathi D.; Shabaraya A. R. Formulation and in vitro characterization of nail lacquer containing fluconazole for preungual drug delivery system. Int. J. Pharma Bio Sci. 2014, 3, 200–214. [Google Scholar]
- Trey S. M.; Wicks D. A.; Mididoddi P. K.; Repka M. A. Delivery of itraconazole from extruded HPC films. Drug Dev. Ind. Pharm. 2007, 33, 727–735. 10.1080/03639040701199225. [DOI] [PubMed] [Google Scholar]
- Kiran R. S.; Chandrashekhar B.; Vishnu P.; Prasad M. Ungual drug delivery systems of ketoconazole nail lacquer. Int. J. Appl. Pharm. 2010, 4, 17–19. [Google Scholar]
- Coviello T.; Trotta A.; Marianecci C.; Carafa M.; Di Marzio L.; Rinaldi F.; et al. Gel-embedded niosomes: preparation, characterization and release studies of a new system for topical drug delivery. Colloids Surf., B 2015, 125, 291–299. 10.1016/j.colsurfb.2014.10.060. [DOI] [PubMed] [Google Scholar]
- Rai V. K.; Yadav N. P.; Sinha P.; Mishra N.; Luqman S.; Dwivedi H.; et al. Development of cellulosic polymer based gel of novel ternary mixture of miconazole nitrate for buccal delivery. Carbohydr. Polym. 2014, 103, 126–133. 10.1016/j.carbpol.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Tan Y. T.; Peh K. K.; Al-Hanbali O. Effect of Carbopol and polyvinylpyrrolidone on the mechanical, rheological, and release properties of bioadhesive polyethylene glycol gels. AAPS PharmSciTech 2000, 1, 69–78. 10.1208/pt010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khullar R.; Kumar D.; Seth N.; Saini S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm. J. 2012, 20, 63–67. 10.1016/j.jsps.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W.; Guo C.; Yu A.; Gao Y.; Cao F.; Zhai G. Micro emulsion based hydrogel formulation of penciclovir for topical delivery. Int. J. Pharm. 2009, 378, 152–158. 10.1016/j.ijpharm.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Mahtab A.; Anwar M.; Mallick N.; Naz Z.; Jain G. K.; Ahmad F. J. Transungual Delivery of Ketoconazole Nanoemulgel for the Effective Management of Onychomycosis. AAPS PharmSciTech 2016, 17, 1477–1490. 10.1208/s12249-016-0488-0. [DOI] [PubMed] [Google Scholar]