Abstract

Proportional to considerable progress in protein–drug conjugations, attention to the efficient peptide coupling reagents is being increased. Hence, in this study, a versatile heterogeneous nanoscale reagent is presented for chemical, biological, and medical purposes. A combination of silver and silica-coated iron oxide nanoparticles (Ag/Fe3O4) has been well functionalized with isothiazolone rings via a silver-modified Heck mechanism. An appropriate condition is provided for peptide bond formation through the surface interplay between silanol groups and the loaded isothiazolone rings. A logical mechanism including a series of successive covalent bonds onto the surface of Ag/Fe3O4 nanocomposites is suggested for this catalyzed peptide bond formation. Accurate comparisons have been made to obtain the optimum value of the nanocatalyst and suitable conditions. As an additional application, the biological activity of the desired product has also been investigated through antibacterial assay tests. The results showed that our desired product could also be used as an effective heterogeneous nanoscale antibacterial agent for different purposes. In this regard, all of the essential structural and practical analyses have been carried out and precisely interpreted.
1. Introduction
Peptides have always been one of the most important materials in chemistry, biochemistry, and pharmaceutical research. In recent years, attention to conjugated drugs as a new generation of the high-tech pharmacy with high efficiency has been increased.1−4 In the field of peptide–drug conjugation, we have to deal with amide bond formation between chemical compounds and biological structures that are mostly made of protein strands and amino acid units.5,6 As one of the most important medicinal scopes, we could refer to antibody–drug conjugates as a new generation of anticancer drugs with high efficiencies.7,8 Therefore, preparation of efficient amide/peptide coupling reagents that could assist the amide/peptide bond formation has always been considered as a significant research field. For instance, the efficiency of the surface catalytic sites has been demonstrated using TiO2 anatase for peptide bond formation by Pantaleone et al.9 Additionally, (1-cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylaminomorpholinocarbenium hexafluorophosphate salt has been introduced as an effective coupling reagent for peptide bond formation in the aqueous phase under mild condition by Gabriel et al.10 In this regard, a substantial procedure has also been reported for peptide bond formation without the need of coupling reagents or protecting groups, using N-carboxyanhydrides and polyethylene glycol resins in water, by Gentilucci et al.11
On the other hand, nanomaterials with notable features have attracted so much attention as instrumental species for various purposes like catalysts and coupling reagents in chemical reactions. First, they have a large surface area for catalyzing a chemical reaction through their nanoscale. Therefore, a partial amount of nanocatalyst would be enough for a carrying out a reaction. Second, they are easily separable from the reaction mixture through their heterogeneity and magnetic traits.12,13 Additionally, their surfaces could be modified with various organic compounds or amorphous structures like silica network, as a secondary shell.14,15 Moreover, the use of water absorbents or reductive agents like active phosphorus-containing molecular sieves in amide/peptide dehydration condensations is necessary.16,17 Silica networks have been reported as great molecular sieves due to the presence of an amorphous and mesoporous structure and also effective interactions between silicon and oxygen atoms.18,19 In addition, the presence of an extreme number of silanol groups makes them an appropriate substrate for peptide bond formation.20 Hence, in this study, we have used silica-network-coated magnetic nanoparticles (MNPs) as dehydrating agents and also as a helpful assistant in peptide bond formation. An isothiazolone (IT) ring was also used as another assistant for peptide bond formation.32 The IT ring was loaded onto the surface of MNPs through covalent bonding via a modified Heck reaction approach. The final product was used in the peptide bond formation as a heterogeneous, recyclable, and nanoscale coupling reagent instead of homogeneous and expensive reagents. Since we deal with biosynthetic protein structures in many peptide conjugates, it is essential to prepare and use the tools with biological properties such as antimicrobial and antibacterial activities.21 Therefore, silver NPs were used for both modification of the Heck mechanism and endowment of antibacterial activity to the designed nanoscale product.22,23 However, this efficient product in the large-scale fabrications prevents the use of expensive and hazardous materials. In addition, in the industrial production and scale up, the recycled nanoscale reagent could be isolated with more convenience through the magnetic property. Overall, in this work, we report a nanoscale amide/peptide coupling reagent that could be a substantial alternative for conventional peptide coupling reagents such as O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate (TBTU) and O-benzotriazol-1-yl-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU).24 Our novel reagent is constructed of IT-functionalized silica-coated silver/iron oxide (Ag/Fe3O4@SiO2-IT) core/shell nanostructures and takes advantage of an independent strategy to assist amide/peptide binding that does not require any additional reagent such as phosphorous-containing molecular sieves or redox agents (Figure 1).
Figure 1.
Peptide bond formation using recyclable Ag/Fe3O4@SiO2-IT nanocomposites.
2. Results and Discussion
2.1. Preparation of Ag/Fe3O4@SiO2-IT Nanocomposites
In the first stage, Fe3O4 MNPs were obtained through co-deposition of iron(II) and iron(III) chloride salts in basic condition (pH ∼ 12), according to the method introduced in the literature.26 The generated dark precipitations were collected by magnetic separation, washed with deionized water and ethanol, and dried at 50 °C. To increase hydroxyl groups onto the surface of MNPs, they were coated with a SiO2 network using tetraethyl orthosilicate (TEOS). Next, the surfaces of MNPs were modified with vinyl groups using trimethoxy(vinyl)silane.27 In the last step, produced Fe3O4@SiO2@C=C MNPs were functionalized with isothiazolone rings by performing a Heck reaction on the surface of MNPs using palladium(II) chloride and other moieties of Heck reaction. According to the mechanism of Heck reaction, alkyl iodides and alkyl bromides are preferred for this approach due to having more suitable leaving groups.28 In this work, it has been shown that alkyl chlorides could also be used in the Heck reactions using partial amounts of silver nitrate as a modifier. Through using AgNO3, we could either modify the Heck reaction between 5-chloro-2-methyl-4-isothiazolin-3-one (CMI) and surface vinyl groups to raise the yield of the reaction and also produce Ag/Fe3O4@SiO2-IT nanocomposites as the desired nanoscale product (Scheme 1).
Scheme 1. Synthetic Pathway of Ag/Fe3O4@SiO2-IT Nanocomposites.
(a) Fe3O4 MNPs, (b) Fe3O4@SiO2 core–shell MNPs, (c) Fe3O4@SiO2@vinyl MNPs, (d) Fe3O4@IT MNPs, and (e) Ag/Fe3O4@SiO2-IT nanocomposites.
2.2. Optimization of the Ag/Fe3O4@SiO2-IT Nanocomposite Synthesis
The optimization reactions were carried out in different solvents, at different temperatures, and with different amounts of silver nitrate by performing various methods. The results were monitored using Fourier transform infrared spectroscopy (FT-IR) and especially energy-dispersive X-ray (EDX) analysis. The obtained results have been reported in Table 1. As a criterion of the loading ratio for each product, we considered the weight percentage of a sulfur atom in the EDX spectra. The maximum ratio of CMI loading was obtained under reflux condition in dimethylformamide (DMF) at 110 °C using 0.1 g of AgNO3. It has been distinguished by the superscript letter “a” in the table. The results of EDX analysis have been reported in the Supporting Information.
Table 1. Obtained Results from the Optimization of Heck Coupling Reaction onto the Surface of Fe3O4@SiO2@Vinyl MNPs.
| entry | method | solvent | AgNO3 (mg) | time (h) | sulfur (wt %) |
|---|---|---|---|---|---|
| 1 | reflux | W/P | 24 | 0.7 | |
| 2 | reflux | W/P | 25 | 24 | 1.47 |
| 3 | reflux | W/P | 50 | 24 | 1.79 |
| 4 | reflux | DMF | 25 | 12 | 3.4 |
| 5 | reflux | DMF | 50 | 12 | 3.46a |
| 6 | reflux | W/E | 50 | 12 | 1.77 |
| 7 | autoclave | DMF | 50 | 12 | 1.65 |
| 8 | autoclave | DMF | 50 | 24 | 3.43 |
| 9 | ultrasound | W/P | 50 | 1 | 0.96 |
| 10 | ultrasound | W/P | 50 | 3 | 1.24 |
Optimum condition, W/P: water–PEG-200 [1:1], W/E: water–ethanol [1:1].
2.3. Characterization of Ag/Fe3O4@SiO2-IT Nanocomposites
The identification and characterization of Ag/Fe3O4@SiO2-IT nanocomposites were performed using various equipment and methods. FT-IR spectroscopy was used to investigate surface functional groups that were loaded onto the MNPs during each step. The presence of new elements after coating reactions was confirmed by EDX analysis. Scanning electron microscope (SEM) imaging for the size and morphology of MNPs and transmission electron microscope (TEM) imaging for investigation of core–shell and composite structures were used. A vibrating sample magnetometer (VSM) was used to measure the magnetic properties of the desired nanoscale product. Thermogravimetric analysis (TGA) and X-ray diffraction (XRD) were used for structural studies. CHNS analysis was used to obtain more confirmation about the existence of carbon, nitrogen, and sulfur atoms in the structure of the final product.
2.3.1. Structural Studies
The infrared spectrum of (c) Ag/Fe3O4@SiO2-IT was compared with the spectra of (b) vinyl-modified Fe3O4@SiO2 and (a) Fe3O4@SiO2 MNPs. As it can be seen in Figure 2, the absorption peaks at 582, 800, 941, and 1089–1100 cm–1, observed in all samples, can be assigned to Fe–O, Si–O, Si–OH, and Si–O–Si stretching vibrations, respectively. For Fe3O4@SiO2@vinyl, the peak at 1654 cm–1 ascribed to C=C stretching and the signal related to sp2 C–H bonds were covered by the broad peak of surface −OH groups at 3000–3100 cm–1. In the spectrum (c), new absorption peaks appeared at 1635, 2925, and 3326 cm–1, which are related to C=O amide, sp3 C–H stretching, and hydroxyl groups, respectively. According to the structure of the desired product (Scheme 1d), the carbonyl group has an intense resonance with nitrogen and π-bond in a conjugated system. That is why the absorption peak of C=O appeared at 1635 cm–1. Figure 3 shows the results of EDX analysis on (a) Fe3O4@SiO2 and (b) Fe3O4@SiO2@vinyl MNPs. The spectra revealed the existence of Fe, Si, O, and C elements after performing the coating reactions on the surface of iron oxide MNPs. The figure also compares the results after executing Ag-modified Heck reaction on the surface of Fe3O4@SiO2@vinyl MNPs, with a typical Heck reaction (Figure 3c,d). According to the figure, we observe more loading ratio when AgNO3 is used. We considered the intensity of the sulfur signal as a criterion of loading ratio. To obtain more confirmation about the final product, CHNS analysis was also carried out, and related results are reported in the Supporting Information of this article.
Figure 2.
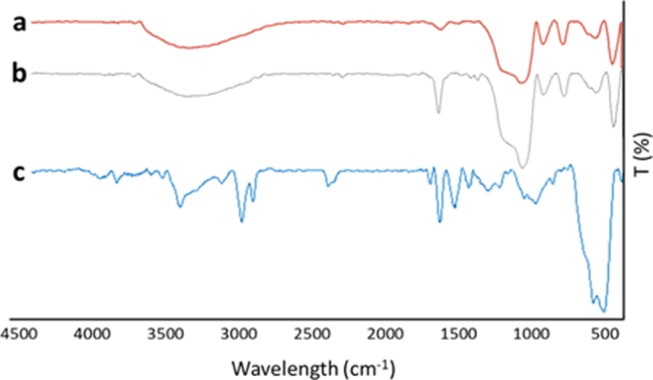
Fourier transform infrared (FT-IR) spectra of desired nanoscale products: (a) Fe3O4@SiO2, (b) Fe3O4@SiO2@C=C, and (c) Ag–Fe3O4@IT NCs.
Figure 3.
EDX spectra of (a) Fe3O4@SiO2 MNPs, (b) Fe3O4@SiO2@C=C MNPs, (C) Fe3O4@SiO2@IT MNPs, and (d) Ag/Fe3O4@SiO2-IT nanocomposites. Y-axis: number of counts (intensity), X-axis: energy (keV).
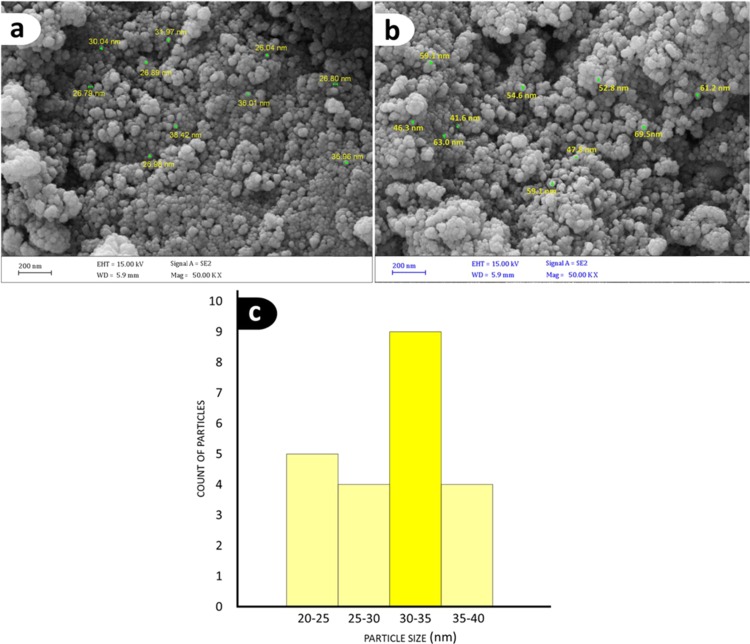
Microscopic imaging methods have always been as an instrumental tool for monitoring morphologies, real structures, sizes, and other properties of the micro- and nanoparticles. Therefore, we used these techniques to determine the physical properties of our nanoscale products. As we can see in Figure 4a,b, the morphology of IT-coated MNPs exhibits highly dispersed particles with spherical morphology. The SEM images of (a) Fe3O4@SiO2 and (b) Ag/Fe3O4@SiO2-IT nanocomposites also revealed the particle size. Statistical data of particle sizes (for 22 particles) revealed no remarkable differences between the particles. According to Figure 4c, the mean size was also reported as 31 nm for Fe3O4@SiO2 MNPs and then it increased to 66 nm for the final product. This means that a new layer has been loaded onto the surface by Heck reaction. The core–shell structure of Fe3O4@SiO2@IT MNPs, which have been placed on the surface of Ag nanoparticles, was proven by TEM imaging (Figure 5a–d). As it can be seen in the figure (c), the dark areas in the structure of Fe3O4@SiO2@IT MNPs belong to the Fe3O4 MNPs present as a core and bright areas belong to SiO2@IT that have coated the core. In the figure (b, d), Ag and Fe3O4 MNPs appear in black and gray, respectively, because silver has a higher electron density in comparison with iron oxide NPs, so fewer electrons can transmit through the Ag nanoparticles.
Figure 4.
SEM images of (a) Fe3O4@SiO2@vinyl MNPs, (b) Ag/Fe3O4@SiO2-IT nanocomposites, and (c) particle size distribution diagram of Fe3O4@SiO2@vinyl MNPs.
Figure 5.
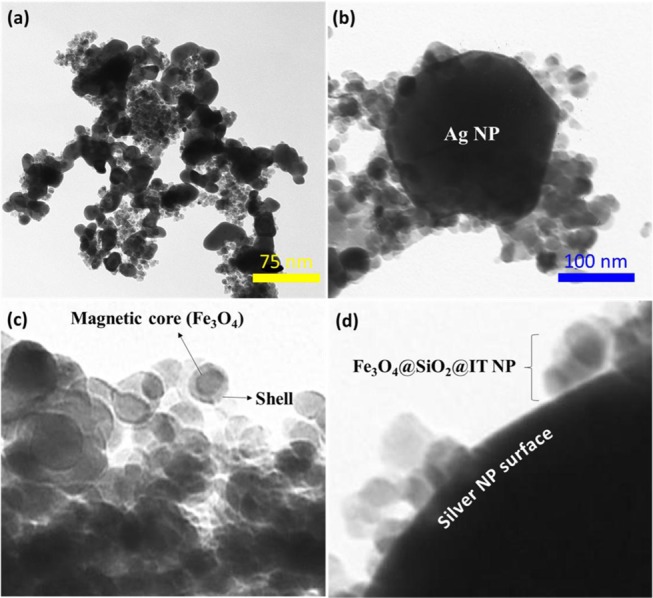
TEM images of (a) Ag/Fe3O4@SiO2-IT nanocomposites (zoom out), (b) Ag/Fe3O4@SiO2-IT nanocomposites (zoom in), (c) core–shell structure of Fe3O4@SiO2 MNPs, and (d) Fe3O4@SiO2 MNPs that have been placed onto the surface of Ag nanoparticles.
Figure 6 shows the XRD patterns of (a) Fe3O4 MNPs, (b) Ag nanoparticles, and (c) Ag/Fe3O4@SiO2-IT nanocomposites. The XRD pattern of Fe3O4 MNPs and Ag NPs have been reported according to the database of JCPDS (PDF#99-0073) and (PDF#87-0597), respectively. As the figure shows, the peaks that appeared at 30.72, 36.22, 43.83, 57.73, and 63.43° correspond to the diffraction pattern of Fe3O4 MNPs. They are marked by their Miller indices of (2 2 0), (3 1 1), (4 0 0), (5 1 1), and (4 4 0), respectively. In addition, the peaks that appeared at 38.07, 44.26, 64.35, and 77.28° correspond to the diffraction pattern of Ag nanoparticles. They are marked by their Miller indices of (1 1 1), (2 0 0), (2 2 0), and (3 1 1), respectively. As compared with the two reference patterns (a, b), eight new peaks at 9.13, 29.33, 31.78, 32.48, 34.00, 34.59, 36.92, and 37.51°, which have been marked with the stars, were observed in the spectrum of Ag/Fe3O4@SiO2-IT nanocomposites. These new peaks are attributed to the new layer created by the IT coating. The average size of Ag/Fe3O4@SiO2-IT nanocomposites was also calculated as 66 nm by Highscore Plus software and Scherrer formula (see the Supporting Information). It can be deduced that this increase in the particle size is due to coating of the core with the organic layer.
Figure 6.

XRD spectra of (a) Fe3O4 MNPs, (b) Ag nanoparticles, and (c) Ag/Fe3O4@SiO2-IT nanocomposites. (*): new peak.
2.3.2. Thermal Resistance Behavior
The thermogravimetric analysis (TGA) of the Ag/Fe3O4@SiO2-IT nanocomposite is shown in Figure 7. As we can see in the figure, at the beginning of the study, a partial increase in the weight (1%) was observed at the first stage (a). This increase in weight was due to the absorbance of the moisture. According to the curve, with the increasing temperature, the weight loss trend was descending till around 580 °C (b). At this stage, almost 7% of the weight was lost due to the removal of absorbed physical and chemical water. It can be deduced that water molecules were trapped in the silica network and they were removed by the increasing temperature. Then, the second shoulder in the curve appeared at 625 °C (c) and most likely belonged to the decomposition of the loaded IT ring. Next, the vinyl layer was probably separated and the main structure of the silica network and Fe3O4 MNPs was collapsed at higher temperatures. Through this remarkable thermal resistance, Ag/Fe3O4@SiO2-IT nanocomposites could also be used for local heating in the field of photodynamic therapy (PDT).
Figure 7.
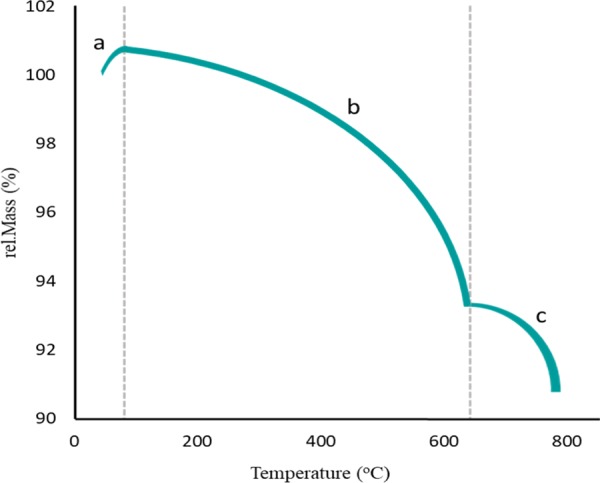
Thermogravimetric analysis of the Ag/Fe3O4@SiO2-IT nanocomposite. (a) Partial rise in the weight through the absorption of the moisture, (b) first shoulder created through the losing water, and (c) second shoulder showing the main decomposition of nanocomposites.
2.3.3. Magnetic Property Investigation
Among various species of chemical structures that play a catalytic role in the chemical and biochemical reactions through relatively the same mechanism, the Ag/Fe3O4@SiO2-IT nanocomposite has several noticeable advantages. First, it is a magnetic nanoscale catalyst, which is separable from the reaction mixture with more convenience in comparison with other organocatalysts such as benzoisothiazolone or diselenide derivatives.25,29 Using an external magnet would be adequate to separate the catalyst from the reaction mixture due to the existence of iron element in the core of NPs. The magnetic trait of our nanoscale product was investigated using VSM analysis (Figure 8a). The Ag/Fe3O4@SiO2-IT nanocomposite exhibits a typical superparamagnetic behavior, as presented in the figure. Obviously, the magnetic feature would be reduced proportional to coating of the core with more layers. The images of the collection of catalyst particles using an external magnet have been shown in Figure 8b.
Figure 8.
(a) Room-temperature M–H curve of (1) Fe3O4 MNPs and (2) Ag/Fe3O4@SiO2-IT nanocomposites; (b) images of the collection of the catalyst particles using an external magnet.
2.4. Catalytic Application in Peptide Construction
In recent decade, significant progress in the fabrication of bio-conjugated protein medications such as liraglutide (Victoza) and antibody-drug antibody−drug conjugates has led researchers to design and introduce novel amide/peptide coupling reagents that are more instrumental for protein−drug conjugation purposes. However, to form an amide bond via traditional methods, first, the carboxylic acid group is activated by wasteful, expensive, or hazardous activators.30 Then, the workup process is needed to extract the desired product from the reaction mixture. Concisely, a multistep process is required to make amide/peptide bonds in the solution phase. In this study, we present a convenient strategy for peptide bond formation using Ag/Fe3O4@SiO2-IT as a recyclable and easily separable nanoscale coupling reagent. In this regard, Fmoc-Phe-OH, Fmoc-Ala-OH, and glycine methyl ester were used to form the peptide bonds. To investigate the efficiency of the fabricated nanocomposite, first, a simple amidation reaction between benzoic acid and ethylamine was carried out. The progress rate of catalyzed reactions was monitored by thin-layer chromatography (TLC). The reaction times were reduced and in contrast the yields of peptide-binding reactions were increased when we used Ag/Fe3O4@SiO2-IT as the coupling reagent. As a limitation of this catalyst, it should be noted that the presented strategy could not be implemented for all of the amino acids. For instance, cysteine-containing couplings are not executed via this method due to the existence of a thiol site in their structure and S–S bond formation. However, the resulted dipeptide products have been identified by FT-IR and 1H NMR spectroscopies (see the Supporting Information). In the related FT-IR spectra of both (A) Fmoc-Ala-Gly-OMe and (B) Fmoc-Phe-Gly-OMe, the peak at 1541.02 cm–1 is ascribed to the stretching vibration of the carbonyl group of the Fmoc protector group. The peaks appearing at 1650–1700 cm–1 belong to the peptide bond carbonyl group, and 1730 and 1749 cm–1 peaks are related to C=O of glycine methyl ester. In the 1H NMR spectra, as they were predicted by Chemdraw software, in the spectrum of A, a doublet signal at 1.26 ppm and a quartet of doublet signal at 3.85 ppm appeared, which are related to the methyl group and α proton of alanine, respectively. In the spectrum B, diastereotopic protons of the benzyl group belonging to phenylalanine appeared at 2.81 and 3.06 ppm as doublet and triplet signals, respectively. In both spectra, a singlet signal appearing at 3.65 ppm was attributed to the methoxy group of glycine methyl ester and broad base signals at 8.31 and 8.54 ppm were related to −NHCO of peptide bonds.
2.4.1. Optimization of Catalytic Values in Peptide Coupling Reactions
The control experiments were carried out using TBTU/HOBT as a conventional amide/peptide coupling reagent,24 P(OEt)3 as an additional molecular sieve,25 and Ag/Fe3O4@SiO2-IT nanocomposites (Figure 9). The progression of the reactions was controlled by TLC and ninhydrin spray after every 30 min of reactions. The conditions and the results of control tests are reported in the Supporting Information in more detail. The separation of the catalyst from the reaction mixture was carried out using an external magnet without the need of more purifications. To confirm carbocation formation in step 2, the 2,4-dinitrophenylhydrazine test was performed. The images of the reaction mixture before and after addition of 2,4-dinitrophenylhydrazine solution instead of glycine methyl ester revealed that the carboxylic acid group of N-protected amino acid has been activated according to the mechanism (see the Supporting Information). These control experiments revealed that the catalyzed peptide binding does not require any additional auxiliaries and works as an independent driving force for amide/peptide bond formation. In the structure of desired final product, the silica network around the magnetic core works as a good molecular sieve and plays an important role in the dehydrative amide condensations. The presented core/shell nanoscale system provides a suitable substrate for amide/peptide bond formation through the surface hydroxyl groups in the vicinity of isothiazolone rings (see the suggested mechanism).
Figure 9.
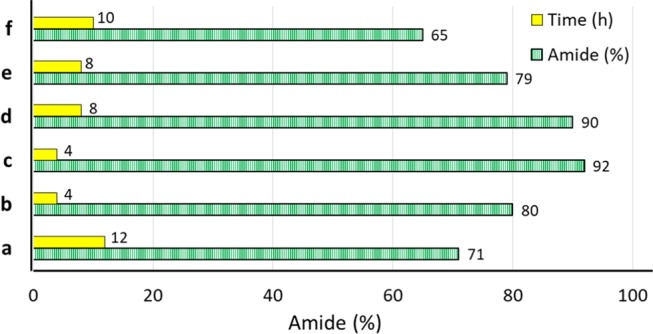
Ag/Fe3O4@SiO2-IT screening and the comparative values of Fmoc-Ala-Gly-OMe coupling reaction times (h) and yields (%) in the presence of TBTU and various amounts of Ag/Fe3O4@SiO2-IT nanocomposites: (a) TBTU/0.64 g, (b) Ag/Fe3O4@SiO2-IT 0.2 g/P(EtO)3, (c) Ag/Fe3O4@SiO2-IT 0.2 g, (d) Ag/Fe3O4@SiO2-IT 0.2 g, (e) Ag/Fe3O4@SiO2-IT 0.15 g, and (f) Ag/Fe3O4@SiO2-IT 0.1 g.
The catalytic amounts of Ag/Fe3O4@SiO2-IT also affected reaction times and yields. Figure 9 also shows the comparative values of reduced reaction times and reaction yields when various amounts of the catalyst were used for peptide coupling of equivalent quantities (2 mmol) of Fmoc-Ala-OH and glycine methyl ester. As can be seen in the graph, the optimum condition for peptide coupling reaction was obtained when 0.2 g of the catalyst was used in 4 h.
2.4.2. Catalyst Recyclability
To investigate the reusability of the desired product, it was magnetically isolated after each peptide coupling reaction, washed, dried, and used again in further reaction. For this purpose, first, the catalyst MNPs were collected using an external magnet and then washed with deionized water three times. Next, they were well dispersed in deionized water via ultrasonication. Next, MNPs were washed with ethanol and acetone three additional times and collected again. Ultimately, they were well dried at 60 °C. It should be noted that, after several uses (five times), the light-brown color of the catalyst was converted to dark brown. Most likely, it originates from a damage to the silica network that coated iron oxide MNPs during washing and ultrasonication. This is proven by successive reductions in the peptide coupling reaction yields between Fmoc-Phe-OH and glycine methyl ester (Figure 10). After five-time recycling and reusing, partial yields (under 30%) were obtained, which confirm the color change and the structural damage that were mentioned earlier. The structure of Ag/Fe3O4@SiO2-IT was preserved after using it in the peptide forming reactions, and it is confirmed by EDX analysis after recycling (see the SI file).
Figure 10.
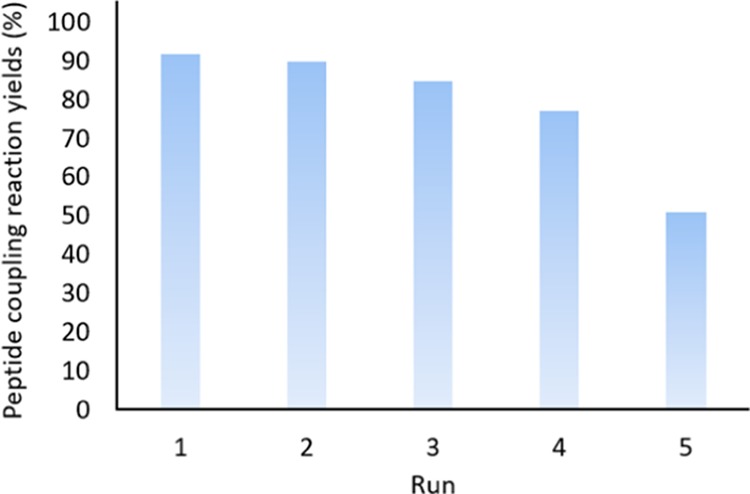
Recyclability investigation of Ag/Fe3O4@SiO2-IT in catalyzed peptide coupling reactions. The results were obtained from the coupling reaction between Fmoc-Phe-OH and methyl glycinate, per 0.2 g of the catalyst at room temperature.
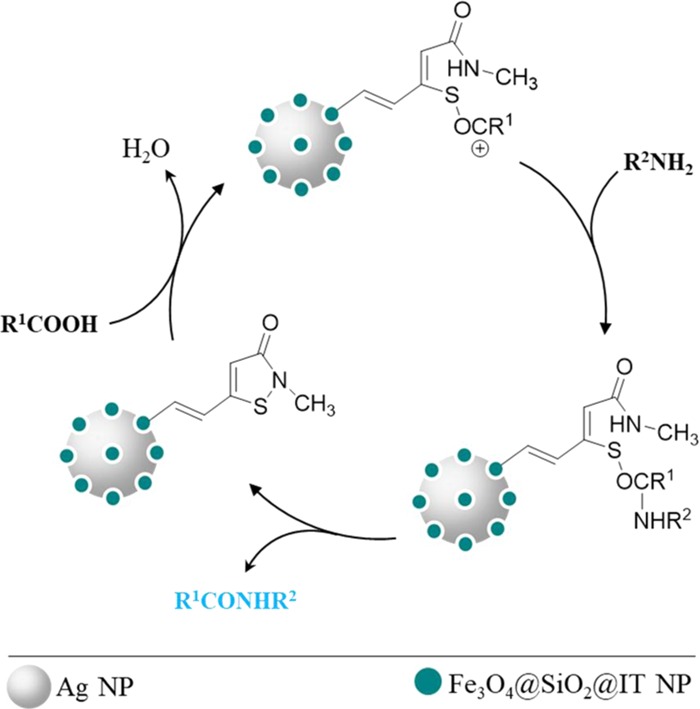
2.4.3. Suggested Mechanism
The mechanism of assisted amide/peptide bond formation on the surface of Ag/Fe3O4@SiO2-IT nanocomposites has been shown in Figure 11. As it can be observed, a four-step process occurred after addition of N-protected amino acids and then Ag/Fe3O4@SiO2-IT was recycled during the reaction. Before initiating the mechanistic study, it should be mentioned that some of the hydroxyl groups, which are present in the vicinity of isothiazolone rings on the surface, will participate in the surface functionalization reaction with trimethoxy(vinyl)silane and the rest will remain intact. Therefore, in the first stage, a nucleophilic attack by the hydroxyl group in the vicinity of the vinyl group present on the SiO2 network around the core occurs on the carboxyl group, which leads to dehydration condensation. Additionally, it should be noted that the protected amino acids should be in their canonical state (nonprotonated state) via controlling the pH (isoelectric point). In stage 2, by transferring the electron charge from oxygen to sulfur atoms, the S–N bond is broken and the IT ring is opened. It is so crucial to use a dry solvent and a neutral atmosphere as reaction conditions due to the formation of carbocations. Next, in stage 3, the amine group of glycine methyl ester attacks carbocations, created by the transfer of electrons of the carbonyl. Ultimately, in the fourth stage, by an electron-transferring process, which is started from the nitrogen atom and ends at the oxygen atom, the IT ring is closed and the catalyst is recycled again and an amide/peptide bond is formed.
Figure 11.
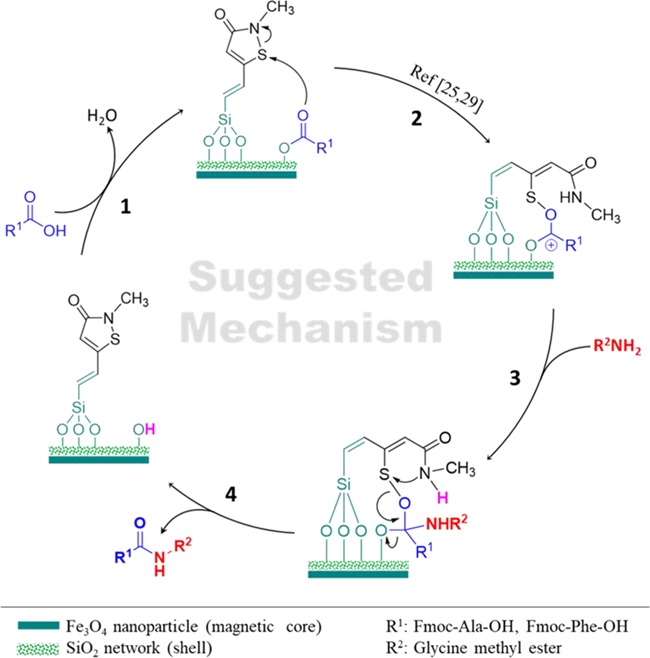
Catalytic process of assisted-peptide bond formation by Ag/Fe3O4@SiO2-IT.
2.5. Biological Applications
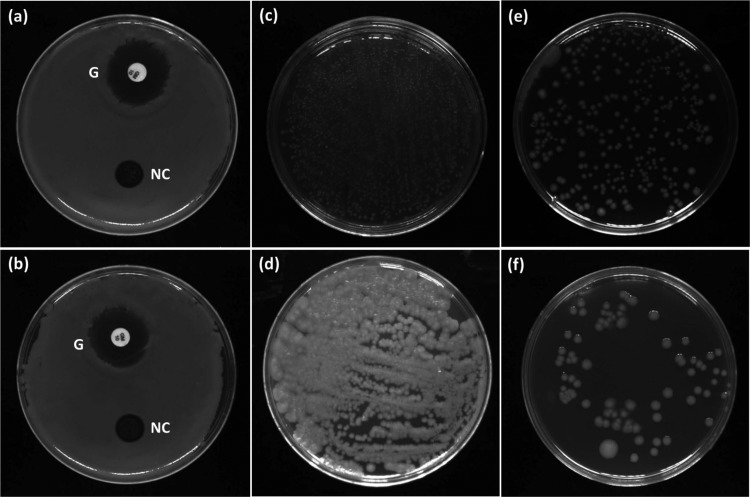
The fast progress in catalytic chemistry has been stimulating research in bioinorganic and bioorganometallic chemistry, with the discovery of the biological relevance of organometallic catalysts. Since for protein conjugations we deal with biosynthetic structures, it is essential to prepare and use the tools with biological properties such as antimicrobial and antibacterial activities. In addition to the catalytic activity, our nanoscale product could be used for biological purposes as an additional application. The IT ring is individually used in cosmetic substances as an antimicrobial agent.31 This property was enhanced through combination with silver nanoparticles. Accordingly, we evaluated the antibacterial activity of our desired product by carrying out the in vitro biological tests. The antibacterial properties of the samples toward Gram-positive Staphylococcus aureus (ATCC 12600) and Gram-negative Escherichia coli (ATCC 9637) bacteria were evaluated according to the disc diffusion antibiotic sensitivity test. The samples were subjected to the nutrient agar plates consisting of bacterial cells, and then the plates were incubated at 37 °C for 24 h. The zone of inhibition (ZOI), which shows the inhibition of microbial growth around the discs, was measured to determine the relative antibacterial effects of Ag/Fe3O4@SiO2-IT nanocomposites on E.coli and S. aureus (Figure 12a,b). The antibiotic gentamicin was employed as a positive control. Disk diffusion test results also indicated that the Ag/Fe3O4@SiO2 MNPs show a smaller inhibition zone for E. coli (1.86 ± 0.27 mm) and S. aureus (2.25 ± 0.31 mm), as reported in Table 2. This reduced effect is due to the lack of the IT ring. The colony-forming unit (CFU) test has been carried out to monitor the antibacterial activity of Ag/Fe3O4@SiO2-IT using E. coli and S. aureus strains. As can be seen in Figure 12c–f, a great number of colonies of E. coli and S. aureus (c, d) were reduced by our nanoscale composite product after 24 h. The obtained results from the plate count technique have been reported in Table 2.
Figure 12.
Inhibition zones of gentamicin (G) and Ag/Fe3O4@SiO2-IT against (a) E. coli and (b) S. aureus bacteria for 24 h; antibacterial experiment photograph of the (c) E. coli control and (d) S. aureus control and Ag/Fe3O4@SiO2-IT against (e) E. coli and (f) S. aureus for 24 h.
Table 2. Obtained Results from Disk Diffusion and CFU Tests of Ag/Fe3O4@SiO2-IT Nanocomposites.
| sample | ZOI E. coli (mm) | ZOI S. aureus (mm) | P.C (E. coli) | P.C (S. aureus) |
|---|---|---|---|---|
| gentamicina | 6 ± 0.7 | 7.5 ± 0.8 | 768 ± 71 | 644 ± 59 |
| Ag/Fe3O4@SiO2 | 1.86 ± 0.27 | 2.25 ± 0.31 | 287 ± 45 | 67 ± 5 |
| Ag/Fe3O4@SiO2-IT | 2.13 ± 0.31 | 2.98 ± 0.22 | 255 ± 34 | 52 ± 3 |
Control+, mm: clear area in millimeters, P.C: plate count; results were obtained after 24 h.
3. Conclusions
Nowadays, protein–drug conjugates as the next generation of the pharmaceutical compounds have led researchers to design novel and more efficient coupling reagents that are easily isolated from the reaction mixture and also conveniently recycled. In this work, a novel nanoscale peptide coupling reagent has been synthesized and its application has been investigated in peptide bond formation. Silver–iron oxide nanocomposites have been prepared by the execution of Heck reaction between vinyl-modified core–shell silica-coated magnetic nanoparticles and isothiazolone rings. In this regard, we have made an effort to design a heterogeneous, magnetic, and recyclable nanocomposite functionalized with isothiazolone and implement a green and self-running mechanism in the amide/peptide bond formation. The EDX analysis has confirmed the main structure of our produced nanoscale product and also the maintenance of the structure after several uses in peptide coupling reactions. The coupling reaction time is reduced to about 4 h using this nanoscale product. As another substantial feature, a self-running process occurred that did not require any extra reagent as a starting reductant or terminal oxidant. In other words, the peptide-binding catalysis process is completely green using the presented product. The morphology and particle sizes have also been monitored by SEM and TEM analyses (Figures 4 and 5), and the mean sizes of particles were determined as 31 and 66 nm for Fe3O4@SiO2@vinyl magnetic nanoparticles and Ag/Fe3O4@SiO2-IT nanocomposites, respectively. Finally, a partial amount of the produced catalyst was used to carry out the peptide coupling reaction. As an additional application, the biological activity of the desired product was investigated through antimicrobial assay tests. The results showed that the Ag/Fe3O4@SiO2-IT nanocomposite could also be used as an effective heterogeneous nanoscale antibacterial agent for different purposes. The functionalization of iron oxide nanoparticles with isothiazolone rings was confirmed by FT-IR, EDX, XRD, and CHNS. The thermal resistance of the desired product was studied using TGA analysis. It was deduced that Ag/Fe3O4@SiO2-IT nanocomposites could be used for local heating in the field of photodynamic therapy (PDT) because of having a substantial thermal resistance.
4. . Experimental Section
4.1. Materials and Equipment
All solvents, chemicals, and reagents were purchased from Merck, Fluka, and Sigma-Aldrich, except CMI, which was purchased from Santacruz (CAS Number: 26172-55-4). Melting points were measured on an Electrothermal 9100 apparatus and were uncorrected. SEM images were taken with a Zeiss-DSM 960A microscope with an attached camera. EDX spectra were recorded on Numerix DXP–X10P. FT-IR spectra were recorded on a Shimadzu IR-470 spectrometer. Transmission electron microscopy was carried out on a Philips CM200. XRD measurements were carried out using an X’Pert Pro X-ray diffractometer operating at 40 mA and 40 kV. 1H NMR spectra were recorded with Varian Unity Inova 500 MHz. Thermal analysis (TGA) was carried out by Bahr-STA 504 instrument under an argon atmosphere. The magnetic properties of samples were analyzed at room temperature using a VSM (Meghnatis Kavir Kashan Co., Kashan, Iran). An incubator (Sh, Noor Sanat Ferdos) was used for biological tests. Elemental analysis (CHNS) was carried out using an elemental combustion system, Costech 4010. The statistical data of particle sizes from SEM imaging and XRD analysis were obtained by Digimizer and Highscore plus, respectively.
4.2. Methodology
4.2.1. Preparation of Fe3O4 MNPs
In a three-necked round-bottom flask (250 mL), a mixture of FeCl3·6H2O (10 mmol), FeCl2·4H2O (10 mmol), and deionized water (100 mL) was stirred and heated to about 45 °C. The stirring was continued for 30 min until the iron salts were dissolved in the water. Then, the temperature was increased to 85 °C under a N2 atmosphere with rapid stirring for additional 2 h. A solution of concentrated aqueous ammonia (15 mL, 25 wt %) was added dropwise to the solution for 1 h. Then, the reaction mixture was cooled to room temperature and the resulting magnetic Fe3O4 NPs were collected with an external magnet. Dark magnetic NPs were washed with water and ethanol several times. Finally, drying was carried out at 70 °C.
4.2.2. Preparation of the Core/Shell Fe3O4@SiO2 MNPs
The prepared Fe3O4 NPs (1.0 g) were initially dispersed in water (10 mL), ethanol (5 mL), PEG-300 (5 mL), and concentrated aqueous ammonia (1 mL, 28 wt %), using an ultrasonic bath. Then, a solution of TEOS (2 mL) in ethanol (10 mL) was loaded dropwise into the dispersion under stirring conditions. The vigorous stirring was continued for 12 h at room temperature. The resulting product was collected by magnetic separation and washed with ethanol. The light-brown Fe3O4@SiO2 NPs were dried at 70 °C.
4.2.3. Preparation of Fe3O4@SiO2@Vinyl MNPs
Initially, in a three-necked flask (100 mL) containing dry chloroform (70 mL), Fe3O4@SiO2 NPs (10 g) were charged. Then, trimethoxy(vinyl)silane (3.54 g, 0.02 mol) was added to the reaction mixture dropwise over a period of 10 min at room temperature. After completion of addition, the mixture was stirred for 24 h at the refluxing temperature of chloroform. Ultimately, the magnetic Fe3O4@SiO2@vinyl NPs were collected using an external magnet and also washed with deionized water and ethanol several times. Relatively rough particles were dried in a vacuum at 50 °C.
4.2.4. Preparation of Ag/Fe3O4@SiO2-IT Nanocomposites in Optimum Conditions
Mixture A: In a round-bottom flask (25 mL), Fe3O4@SiO2@vinyl NPs (1.0 g) were completely dispersed by ultrasound in DMF (7 mL) for 10 min.
Mixture B: In a beaker (10 mL), PdCl2 (35.4 mg, 0.2 mmol) and PPh3 (130 mg, 0.5 mmol) were dissolved in 0.5 M HCl (5 mL) by stirring at 60 °C for 2 h. The color of the mixture was changed to olive after 2 h.
Then, mixture B was added to the flask of the mixture A, and the contents of the flask were mixed using magnetic stirring. In the next step, TEA (0.1 mL), AgNO3 (50 mg, 0.3 mmol), NaOAc (0.01 g, 0.12 mmol), and CMI (1 mL, 8.35 mol) were added to the flask and the contents were stirred under reflux condition for 12 h. After completion of stirring, the desired nanoparticles were magnetically collected and washed with distilled water and ethanol several times. The dark Ag/Fe3O4@SiO2-IT particles were dried at 50 °C.
4.2.5. General Procedure for Catalyzed Dipeptide Construction in the Solution Phase
Initially, Ag/Fe3O4@SiO2-IT particles (0.05 g) were dispersed in dry DCM (5.0 mL) using an ultrasonic bath for an adequate time. Next, N-protected amino acids (2 mmol) were added into the flask. The mixture was stirred for 30 min under a N2 atmosphere. Then, acid-protected amino acids (2 mmol) were added and the mixture was stirred for 3 h under a N2 atmosphere at room temperature. After completion of the reaction, magnetic NPs were simply separated from the reaction mixture using an external magnet, washed with methanol, and then dried in an oven at 60 °C. The extraction process was carried out by addition of excess dry DCM to the mixture. Then, the DCM phase was evaporated by a rotary evaporator. The desired product was obtained as a white powder and dried at room temperature.
4.2.5.1. Fmoc-Ala-Gly-OMe
1H NMR (500 MHz, DMSO): δ = 1.26 (d, 3H, J = 7 Hz, CHCH3), 3.64 (s, 3H, COOCH3), 3.80–3.95 (qd, 1H, J = 16.5 Hz, J = 6.5 Hz, NHCHCH3), 4.12 (t, 1H, J = 6.5 Hz, CHCH2O), 4.23 (d, 2H, J = 6.5 Hz, CHCH2O), 4.27 (s, 2H, NHCH2CO), 7.34 (t, 2H, J = 7 Hz, Ar), 7.43 (t, 2H, J = 7 Hz, Ar), 7.58 (d, 1H, J = 7.5 Hz, OCONHCH), 7.73–7.76 (t, 2H, J = 7.5 Hz, Ar), 7.90 (d, 2H, J = 7.5 Hz, Ar), 8.31 (s, 1H, CONHCH2).
4.2.5.2. Fmoc-Phe-Gly-OMe
1H NMR (500 MHz, DMSO): δ = 2.81 (t, 1H, J = 11 Hz, PhCH2), 3.06 (d, 1H, J = 15 Hz, PhCH2), 3.65 (s, 3H, COOCH3), 3.89–3.93 (m, 2H, NHCH2COOCH3), 4.10–4.18 (m, 3H, CHCH2OCONH), 4.29–4.34 (td, 1H, J = 3.5 Hz, J = 12.5 Hz, NHCHCH2Ph), 7.18–7.2 (t, 1H, J = 5, CONHCH2COOCH3), 7.25–7.35 (m, 7H, Ar), 7.39–7.43 (m, 2H, Ar), 7.63–7.71 (m, 2H, Ar), 7.88 (d, 2H, J = 7.5 Hz, Ar), 8.54 (d, 1H, J = 5.5 Hz, OCONHCH).
Acknowledgments
The authors gratefully acknowledge the partial support from the Research Council of the Iran University of Science and Technology and the Iran National Science Foundation (INSF) grant no. 96002280. We are also thankful to Prof. Dr. Lanny Liebeskind (Emory University, USA) for editing our manuscript and Siavash Salek Soltani (University of Tehran, Iran) for his help in analyses section.
Glossary
Abbreviations
- MNPs
magnetic nanoparticles
- IT
isothiazolone
- TEOS
tetraethyl orthosilicate
- CMI
5-chloro-2-methyl-4-isothiazolin-3-one
- FT-IR
Fourier transform infrared
- EDX
energy-dispersive X-ray analysis
- SEM
scanning electron microscope
- TEM
transmission electron microscope
- nm
nanometer
- Fmoc
fluorenylmethyloxycarbonyl
- Fmoc-Ph
Fmoc-l-phenylalanine-OH
- Fmoc-Ala
Fmoc-l-alanine-OH
- Gly-OMe
glycine methyl ester
- ZOI
zone of inhibition
- CFU
colony-forming unit
- TLC
thin-layer chromatography
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b00986.
FT-IR and EDX spectra and elemental percentages of all nanoscale products; original results of XRD, TGA, and CHNS analyses of the desired nanoscale product; VSM graphs of Fe3O4 NPs and Ag–Fe3O4@IT NCs; FT-IR and 1H NMR spectra of Fmoc-Ala-Gly-OMe and Fmoc-Phe-Gly-OMe; Digimizer and Highscore Plus data and EDX table of NC after recycling (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Fotticchia T.; Vecchione R.; Scognamiglio P. L.; Guarnieri D.; Calcagno V.; Natale C. D.; Attanasio C.; Gregorio M. D.; Cicco C. D.; Quagliariello V.; Maurea N.; Barbieri A.; Arra C.; Raiola L.; Iaffaioli R. V.; Netti P. A. Enhanced Drug Delivery into Cell Cytosol via Glycoprotein H-Derived Peptide Conjugated Nanoemulsions. ACS Nano 2017, 11, 9802–9813. 10.1021/acsnano.7b03058. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Hong S.; Cai Q.; Zhang M.; Chen J.; Zhang X.; Icon O.; Xu J. C. Transcriptional control of the MUC16 promoter facilitates follicle-stimulating hormone peptide-conjugated shRNA nanoparticle-mediated inhibition of ovarian carcinoma in vivo. Drug Delivery 2018, 25, 797–806. 10.1080/10717544.2018.1451934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E.; Joseph C.; Peterson H.; Bouwman R.; Tang S.; Cannon J.; Sinniah K.; Choi S. K. Measuring the Adhesion Forces for the Multivalent Binding of Vancomycin-conjugated Dendrimer to Bacterial Cell-Wall Peptide. Langmuir 2018, 34, 7135–7146. 10.1021/acs.langmuir.8b01137. [DOI] [PubMed] [Google Scholar]
- Lee S. G.; Kim C. H.; Sung S. W.; Lee E. S.; Goh M. S.; Yoon H. Y.; Kang M. J.; Lee S.; Choi1 Y. W. RIPL peptide-conjugated nanostructured lipid carriers for enhanced intracellular drug delivery to hepsin-expressing cancer cells. Int. J. Nanomed. 2018, 13, 3263–3278. 10.2147/IJN.S166021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos S.; Hernández J. L.; Otero A.; Calvis C.; Adan J.; Mitjans F.; Vila-Perelló M. Site-Specific Antibody Drug Conjugates Using Streamlined Expressed Protein Ligation. Bioconjugate Chem. 2018, 29, 3503–3508. 10.1021/acs.bioconjchem.8b00630. [DOI] [PubMed] [Google Scholar]
- Blaskovich M. A. T.; Hansford K. A.; Cooper M. A.; et al. Protein-inspired antibiotics active against vancomycin-and daptomycin-resistant bacteria. Nat. Commun. 2018, 9, 22. 10.1038/s41467-017-02123-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh A. N.; McLaughlin C. K.; Duan D.; Shoichet B. K.; Shoichet M. S. A New Spin on Antibody–Drug Conjugates: Trastuzumab-Fulvestrant Colloidal Drug Aggregates Target HER2-Positive Cells. ACS Appl. Mater. Interfaces 2017, 9, 12195–12202. 10.1021/acsami.6b15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard H.; Viskov C.; Garcia-Echeverria C. Antibody–drug conjugates—a new wave of cancer drugs. Bioorg. Med. Chem. Lett. 2014, 24, 5357–5363. 10.1016/j.bmcl.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Pantaleone S.; Ugliengo P.; Sodupe M.; Rimola A. When the Surface Matters: Prebiotic Peptide-Bond Formation on the TiO2 (101) Anatase Surface through Periodic DFT-D2 Simulations. Chem. - Eur. J. 2018, 24, 16292–16301. 10.1002/chem.201803263. [DOI] [PubMed] [Google Scholar]
- Gabriel C. M.; Keener M.; Gallou F.; Lipshutz B. H. Amide and Peptide Bond Formation in Water at Room Temperature. Org. Lett. 2015, 17, 3968–3971. 10.1021/acs.orglett.5b01812. [DOI] [PubMed] [Google Scholar]
- Gentilucci L.; Marco R. D.; Tolomelli A.; Greco A. Controlled Solid Phase Peptide Bond Formation Using N-Carboxyanhydrides and PEG Resins in Water. ACS Sustainable Chem. Eng. 2013, 1, 566–569. 10.1021/sc400058r. [DOI] [Google Scholar]
- Hosseinifar A.; Shariaty-Niassar M.; Seyyed Ebrahimi S. A.; Moshref-Javadi M. Synthesis, Characterization, and Application of Partially Blocked Amine-Functionalized Magnetic Nanoparticles. Langmuir 2017, 33, 14728–14737. 10.1021/acs.langmuir.7b02093. [DOI] [PubMed] [Google Scholar]
- Iwasaki S.; Kawasaki H.; Iwasaki Y. Label-Free Specific Detection and Collection of C-Reactive Protein Using Zwitterionic Phosphorylcholine-Polymer-Protected Magnetic Nanoparticles. Langmuir 2019, 35, 1749–1755. 10.1021/acs.langmuir.8b01007. [DOI] [PubMed] [Google Scholar]
- Haldar K. K.; Kundu S.; Patra A. Core-size-dependent catalytic properties of bimetallic Au/Ag core–shell nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 21946–21953. 10.1021/am507391d. [DOI] [PubMed] [Google Scholar]
- Qu H.; Tong S.; Song K.; Ma H.; Bao G.; Pincus S.; Zhou W.; O’Connor C. Controllable in Situ Synthesis of Magnetite Coated Silica-Core Water-Dispersible Hybrid Nanomaterials. Langmuir 2013, 29, 10573–10578. 10.1021/la4022867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L.; Zhao J. Ynamide: A New Coupling Reagent for Amide and Peptide Synthesis. Synlett 2017, 28, 1663–1670. 10.1055/s-0036-1588860. [DOI] [Google Scholar]
- Georgelin T.; Akouche M.; Jaber M.; Sakhno Y.; Matheron L.; Fournier F.; Méthivier C.; Martra G.; Lambert J. F. Iron(III) Oxide Nanoparticles as Catalysts for the Formation of Linear Glycine Peptides. Eur. J. Inorg. Chem. 2017, 2017, 198–211. 10.1002/ejic.201601296. [DOI] [Google Scholar]
- ALOthman Z. A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. 10.3390/ma5122874. [DOI] [Google Scholar]
- Meléndez-Ortiz H. I.; García-Cerda L. A.; Olivares-Maldonado Y.; Castruita G.; Mercado-Silva J. A.; Perera-Mercado Y. A. Preparation of spherical MCM-41 molecular sieve at room temperature: Influence of the synthesis conditions in the structural properties. Ceram. Int. 2012, 38, 6353–6358. 10.1016/j.ceramint.2012.05.007. [DOI] [Google Scholar]
- Rimola A.; Sodupe M.; Ugliengo P. Amide and Peptide Bond Formation: Interplay between Strained Ring Defects and Silanol Groups at Amorphous Silica Surfaces. J. Phys. Chem. C 2016, 120, 24817–24826. 10.1021/acs.jpcc.6b07945. [DOI] [Google Scholar]
- Song Y.; Jiang H.; Wang B.; Kong Y.; Chen J. Silver-Incorporated Mussel-Inspired Polydopamine Coatings on Mesoporous Silica as an Efficient Nanocatalyst and Antimicrobial Agent. ACS Appl. Mater. Interfaces 2018, 10, 1792–1801. 10.1021/acsami.7b18136. [DOI] [PubMed] [Google Scholar]
- Ouay B. L.; Stellacci F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. 10.1016/j.nantod.2015.04.002. [DOI] [Google Scholar]
- Arpana P.; Ruchi K.; Niranjan K. Antibacterial activity of silver nanoparticles. Int. J. Pharm. Res. Bio-Sci. 2013, 2, 358–371. [Google Scholar]
- Carpino L. A. 1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive. J. Am. Chem. Soc. 1993, 115, 4397–4398. 10.1021/ja00063a082. [DOI] [Google Scholar]
- Liebeskind L. S.; Gangireddy P.; Lindale M. G. Benzoisothiazolone Organo/Copper-Cocatalyzed Redox Dehydrative Construction of Amides and Peptides from Carboxylic Acids using (EtO) 3P as the Reductant and O2 in Air as the Terminal Oxidant. J. Am. Chem. Soc. 2016, 138, 6715–6718. 10.1021/jacs.6b03168. [DOI] [PubMed] [Google Scholar]
- Maleki A. Fe3O4/SiO2 nanoparticles: an efficient and magnetically recoverable nanocatalyst for the one-pot multicomponent synthesis of diazepines. Tetrahedron 2012, 68, 7827–7833. 10.1016/j.tet.2012.07.034. [DOI] [Google Scholar]
- Maleki A.; Taheri-Ledari R.; Soroushnejad M. Surface functionalization of magnetic nanoparticles via palladium-catalyzed Diels-Alder approach. ChemistrySelect 2018, 3, 13057–13062. 10.1002/slct.201803001. [DOI] [Google Scholar]
- Yang J.; Zhao H.; He J.; Zhang C. Pd-Catalyzed Mizoroki-Heck Reactions Using Fluorine-Containing Agents as the Cross-Coupling Partners. Catalysts 2018, 8, 23. 10.3390/catal8010023. [DOI] [Google Scholar]
- Akondi S. M.; Gangireddy P.; Pickel T. C.; Liebeskind L. S. Aerobic, Diselenide-Catalyzed Redox Dehydration: Amides and Peptides. Org. Lett. 2018, 20, 538–541. 10.1021/acs.orglett.7b03620. [DOI] [PubMed] [Google Scholar]
- Valeur E.; Bradley M. Amide bond formation: beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. 10.1039/B701677H. [DOI] [PubMed] [Google Scholar]
- Pucci M. J.; Cheng J.; Podos S. D.; Thoma C. L.; Thanassi J. A.; Buechter D. D.; Mushtaq G.; Vigliotti G. A.; Bradbury B. J.; Deshpande M. In Vitro and In Vivo Antibacterial Activities of Heteroaryl Isothiazolones against Resistant Gram-Positive Pathogens. Antimicrob. Agents Chemother. 2007, 51, 1259–1267. 10.1128/AAC.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimola A.; Fabbiani M.; Sodupe M.; Ugliengo P.; Martra G. How Does Silica Catalyze the Amide Bond Formation under Dry Conditions? Role of Specific Surface Silanol Pairs. ACS Catal. 2018, 8, 4558–4568. 10.1021/acscatal.7b03961. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.