ABSTRACT
Background
Patients with atrial fibrillation (AF) in Germany are often managed jointly by primary‐care physicians in cooperation with cardiologists. We aimed to investigate the management and 1‐year outcomes of AF patients in this setting.
Hypothesis
We set out to describe the current management of AF patients in primary care settings in Germany.
Methods
Observational registry with 1‐year follow‐up, performed by a representative, randomly selected sample of 781 primary‐care physicians in Germany.
Results
Of 3781 patients with electrocardiographically documented AF, 3163 patients (age 71.9 ± 9.2 years, 57.9% males) were followed for 1 year; 28.4% had paroxysmal, 27.0% persistent, and 43.3% permanent AF. Comorbid conditions were common (mean CHA2DS2‐VASc score 3. 8 ± 1.7). Rhythm‐control therapy was used in 16.4%. Although oral anticoagulation was often used (82.7% at baseline), stroke rate during follow‐up was high (2.7% stroke, 3.0% transient ischemic attack). Despite a long duration of AF (mean duration 61 months at enrollment), 18.5% of patients were hospitalized during the 1‐year follow‐up.
Conclusions
In this unselected group of patients with long‐standing AF managed in primary care, hospitalizations and cardiovascular complications including strokes are frequent, illustrating the need to improve management of AF patients.
Introduction
Atrial fibrillation (AF) is a common arrhythmia, affecting 1% to 2% of the population.1 The condition leads to increased mortality,2 strokes,3, 4 and frequent hospitalizations,5, 6 causing a considerable burden to society. In affected patients, AF can present with a broad range of symptoms, from clinically silent to severe impairment of normal daily activities.
The management of AF patients may be complex, as they often require anticoagulant therapy,1, 7, 8 rate‐control therapy to improve left‐ventricular function and symptoms, and/or rhythm‐control therapy to prevent recurrent symptomatic AF.1, 8
Most of the currently available surveys and registries reporting outcomes and management of AF patients have been restricted to patients treated by cardiologists and/or hospital‐based physicians5, 9, 10, 11, 12 or were performed on population‐wide samples.3, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 Even the large AF registry managed by Competence Network on Atrial Fibrillation (AFNET), which enrolled almost 10 000 patients from different levels of care, shows a slight under‐representation of patients managed in primary care (9%).23, 24 Given the fact that family physicians in most healthcare systems, including Germany's, serve as gatekeepers and are responsible for the long‐term management of their patients, there is an obvious need to obtain data from the primary‐care sector concerning AF.
Unlike the majority of other AF registries, the prospective German Outpatient Registry Upon Morbidity of Atrial Fibrillation (ATRIUM) study investigated patients primarily treated by primary‐care physicians.6 Here, we report the 1‐year follow‐up data of this cohort, including management and outcomes.
Methods
Design
The design of the ATRIUM registry has previously been described.6 In short, this prospective, multicenter, epidemiological, noninterventional cohort study was conducted in 781 primary‐care practices in Germany between 2009 and 2012. The study was approved by the ethics committee of the Technical University of Dresden, and all patients provided written informed consent prior to enrollment. Participating centers were selected based on a multistep procedure, with random sampling of centers throughout Germany to obtain a representative physician sample. Patients were eligible for consecutive enrollment if they had AF documented by electrocardiography (ECG) in the 12 months prior to enrollment. No exclusion criteria were defined, to minimize selection bias. All data were recorded during an inclusion visit (baseline) and at an end‐of‐study visit after 1 year.
Parameters
At baseline, patient demographics (sex, age), basic data and vital signs, cardiac risk factors, cardiac history, and concomitant diseases were noted. Regarding AF, the following information was documented: month of first diagnosis, AF type (paroxysmal, defined as self‐terminating within 7 days of recognized onset; persistent, defined as not self‐terminating within 7 days or terminated electrically or pharmacologically; or permanent, defined as lasting for >1 year when cardioversion failed or was not attempted), diagnostic procedures, suspected cause, predisposing factors, treatment within the last 12 months, hospitalizations due to AF, current therapy for the prevention of thromboembolic complications, and management of AF in the last 12 months or after referral by another office‐based physician. At the follow‐up visit, we collected information on AF treatments including antithrombotic medication, rate‐control therapy, and rhythm‐control interventions (eg, cardioversions, antiarrhythmic drugs, catheter ablation). We counted transient ischemic attacks (TIA), strokes, myocardial infarctions, and acute heart failure events. Furthermore, we specifically asked physicians whether rate control, rhythm control, or both were followed as therapeutic strategy. Rate control was defined as accepting AF and controlling the ventricular rate, and rhythm control as aiming to restore sinus rhythm by cardioversion, antiarrhythmic drugs, or ablation procedures. After study closure, physicians were contacted again by the study center in case there was follow‐up information missing on ≥1 of their patients at study end.
Data Entry and Analysis
Data were collected using paper‐pencil case‐record forms. Duplicate data entry was performed by the contract research organization (Dr. Schauerte Studien und Marketing in der Medizin, Oberhaching, Germany) and plausibility checks were executed using a validation plan. Current analyses are based on the per‐protocol sample, comprising all patients for whom information at both visits was available.
All analyses were predefined and exploratory. Continuous variables are reported as mean ± standard deviation, categorical variables as percentage of patient population. Due to incomplete answers and multiple answering options, observed numbers and percentages do not always add up to 100%. Deaths were not accounted for, as they were not part of the per‐protocol population with documented follow‐up visit. The CHADS2 score (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus [DM], and prior stroke, TIA, or thromboembolism) and the CHA2DS2‐VASc score (CHADS2 + vascular disease, age 65 to 74 years, and sex category)—which was recently proposed as a refinement of the CHADS2 score1—were computed using the available information.
Data were analyzed using SAS statistical package version 9.2 (SAS Institute Inc., Cary, NC).
Results
Baseline Data
From the patient population analyzed at baseline, 611 patients without a follow‐up examination were excluded, and the remaining 3163 formed the per‐protocol group (100%) reported here. Mean age in the total cohort was 71.9 ± 9.2 years, mean body mass index was 28.6 ± 4.8 kg/m2, and 57.9% of patients were male.
Atrial Fibrillation Characteristics and Symptoms
As displayed in the Table 1, 28.4% of patients had paroxysmal AF, 27.0% had persistent AF, and 43.3% had permanent AF (1.2% not reported). Patients with permanent AF were older (73.7 years) than those with persistent AF (71.4 years) or paroxysmal AF (69.7 years). In 78.5% of patients, the AF diagnosis had been made on the basis of long‐term Holter ECG monitoring. Atrial fibrillation was noted in 95.9% and atrial flutter in 4.8% (missing information in 0.7%). At enrollment, mean duration of AF since first diagnosis was 60.6 ± 65.2 months (median, 42 months; interquartile range, 13–87 months). Family physicians suspected the diagnosis based on initial ECG recordings and had it confirmed by Holter ECG performed by cardiologists. Initial suspicion for AF was raised by the family physician in 63.2% of cases, by an office‐based cardiologist in 13.2%, and by hospital‐based physicians in 23.9%.
Table 1.
Patient Characteristics at Baseline
| Total, N = 3163 | Paroxysmal AF, n = 898 | Persistent AF, n = 855 | Permanent AF, n = 137f1 | Rate Control, n = 2381 | Rhythm Control, n = 168 | Rate and Rhythm Control, n = 359 | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age, y | 71.9 ± 9.2 | 69.7 ± 10.0 | 71.4 ± 9.1 | 73.7 ± 8.3 | 72.5 ± 8.9 | 68.5 ± 10.3 | 69.0 ± 9.5 |
| >65 years, % | 79.9 | 72.8 | 77.8 | 86.1 | 81.9 | 64.9 | 68.8 |
| Male sex, % | 57.9 | 57.0 | 59.2 | 57.7 | 56.8 | 58.9 | 56.0 |
| BMI, kg/m2 | 28.6± 4.8 | 28.5 ± 4.7 | 28.8 ± 4.9 | 28.6 ± 4.8 | 28.7 ± 4.8 | 27.6 ± 4.3 | 28.8 ± 4.8 |
| Risk factors, % | |||||||
| Arterial hypertension | 83.6 | 82.5 | 84.7 | 83.7 | 85.6 | 73.2 | 83.8 |
| Hyperlipidemia | 60.0 | 61.1 | 60.8 | 59.4 | 60.0 | 59.5 | 66.9 |
| CHF | 41.7 | 28.4 | 40.9 | 50.8 | 43.2 | 26.8 | 44.6 |
| CHD | 34.3 | 29.1 | 33.5 | 38.1 | 34.5 | 29.8 | 35.7 |
| DM | 35.1 | 29.8 | 34.4 | 39.1 | 36.5 | 19.6 | 35.1 |
| CKD | 19.0 | 15.6 | 17.9 | 21.7 | 19.2 | 14.3 | 20.9 |
| Smoking status | |||||||
| Never | 55.6 | 56.6 | 55.3 | 55.5 | 56.2 | 54.2 | 54.9 |
| Previously | 38.5 | 37.8 | 38.6 | 38.8 | 38.1 | 39.9 | 39.0 |
| Currently | 5.8 | 5.7 | 6.1 | 5.7 | 5.8 | 6.0 | 6.1 |
| Hyperthyreosis | 5.9 | 5.6 | 5.6 | 6.3 | 5.6 | 6.0 | 8.1 |
| Alcohol abuse | 4.0 | 3.1 | 5.1 | 4.0 | 4.0 | 3.6 | 3.3 |
| CHADS2 score | 2.2 ± 1.3 | 1.9 ± 1.3 | 2.1 ± 1.2 | 2.4 ± 1.3 | 2.2 ± 1.2 | 1.6 ± 1.4 | 2.0 ± 1.2 |
| CHA2DS2‐VASc score | 3.8 ± 1.7 | 3.4 ± 1.8 | 3.7 ± 1.6 | 4.1 ± 1.7 | 3.9 ± 1.7 | 3.1 ± 1.9 | 3.6 ± 1.7 |
aAbbreviations: BMI, body mass index; CHADS2, congestive heart failure, hypertension, age ≥75 years, DM, and prior stroke, TIA, or thromboembolism; CHA2DS2‐VASc, CHADS2 + vascular disease, age 65 to 74 y, and sex category; CHD, coronary heart disease; CHF, heart failure; CKD, chronic kidney disease; DM, diabetes mellitus; SD, standard deviation; TIA, transient ischemic attack.
bValues for age, BMI, and CHADS2/CHA2DS2‐VASc score are presented as mean ± SD.
The therapeutic strategy was predominantly rate control (75.3%), whereas rhythm control (5.3%) and the combination of both (11.3%) were less frequent (not reported in 8.1%).
Risk Factors and Comorbidities
Cardiac risk factors and comorbidities were frequent, irrespective of AF type or treatment strategy (Table 1), in particular arterial hypertension (2645 of 3163 patients, 83.6% of cases), dyslipidemia (1898 patients, 60.0%), chronic heart failure (1320 patients, 41.7%), DM (1109 patients, 35.1%), chronic kidney disease (600 patients, 19.0%), hyperthyreosis (187 patients, 5.9%), and pathological alcohol consumption (128 patients, 4.0%). Compared with the other AF groups, patients with permanent AF more often tended to have chronic heart failure, DM, or thyroid disease. Patients on rate control were older and tended to have higher rates of comorbidities.
The mean CHADS2 score was 2.2± 1.3 at baseline and 2.3± 1.3 at follow‐up. The mean CHA2DS2‐VASc score was 3.8 ± 1.7 at baseline and 4.0 ± 1.7 at follow‐up.
Drug Therapy
Therapy was usually continued as recommended by the treating cardiologist (at baseline, 41.7%; at follow‐up, 29.5%) or a hospital‐based physician (at baseline, 38.0%; at follow‐up, 18.6%).
Details about antiarrhythmic medication use at baseline and at follow‐up are shown in Figure 1. By decreasing frequency, β‐blockers were given in 2360 patients (74.6%) at baseline and in 2083 patients (65.9%) after 1 year (follow‐up); digitalis in 936 patients (29.6%) at baseline and 772 patients (24.4%) at 1 year; calcium channel antagonists in 483 patients (15.3%) at baseline and 402 patients (12.7%) at 1 year; and potassium channel blockers (such as amiodarone or dronedarone) in 340 patients (10.7%) at baseline and 308 patients (9.7%) at 1 year. Sodium channel blockers of the type IA (usually quinidine) were given in 35 patients (1.1%) at baseline and 19 patients (0.6%) at 1 year, and those of type IC (usually flecainide or propafenone) were given in 172 patients (5.4%) at baseline and in 127 patients (4.0%) at 1 year.
Figure 1.
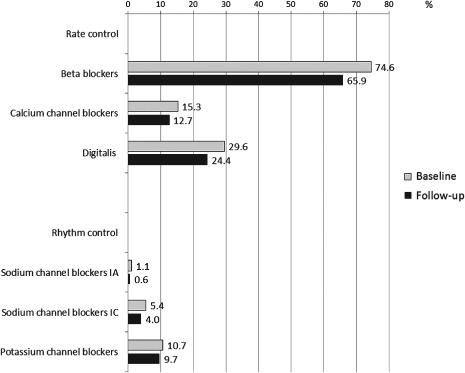
Medications for rate and rhythm control of atrial fibrillation at baseline and at 1‐year follow‐up.
Thus, all drug classes were given less frequently at follow‐up compared with baseline.
Anticoagulation
The use of any antithrombotic medication was reported by 2938 patients (92.9%) at baseline and by 2919 patients (92.3%) at follow‐up (no anticoagulation in 171 patients [5.4%] at baseline and 185 patients [5.8%] at 1 year; missing information in 54 patients at baseline [1.7%] and 59 patients [1.9%] at 1 year). Contraindications for oral anticoagulants (OAC) were reported in 200 patients (6.3%) at baseline and in 238 patients (7.5%) at 1 year, which were specified at follow‐up as previous bleeding in 74 patients (2.3% of the analyzed cohort), malignancies in 40 patients (1.3%), other contraindications in 144 patients (4.6%), and missing reasons in 2 patients. The pattern of OAC medication use is displayed by eligibility status according to CHA2DS2‐VASc score in Figure 2. Sixty‐eight patients (2.1%) had 0 points and thus were not eligible, and 208 patients (6.6%) had 1 point and were possibly eligible for anticoagulation therapy1 at baseline; this pattern hardly changed at follow‐up. Regarding the subgroups, no antithrombotic therapy was given in 15 patients (22.1%) with 0 points and 18 patients (8.7%) with 1 point at baseline, and in 16 patients (24.2%) and 21 patients (11.6%) at follow‐up. Of the patients eligible for antithrombotic therapy (≥2 points), only 62 (2.2%) at baseline and 90 (3.1%) at follow‐up did not receive any therapy.
Figure 2.
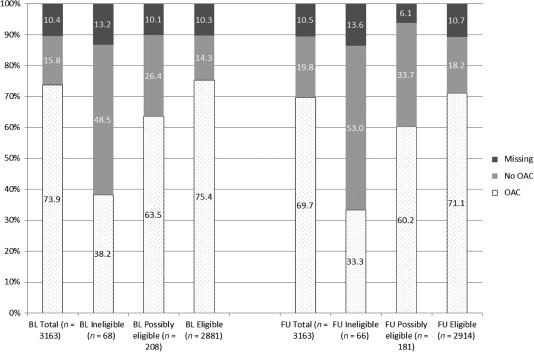
Anticoagulation, by eligibility status according to CHA2DS2‐VASc score. Eligible for antithrombotic therapy is defined as 0 points according to CHA2DS2‐VASc score, possibly eligible as 1 point, eligible as 2+ points. OAC indicates patients receiving vitamin K antagonists alone or in any combination with antiplatelets and/or LMWH. Abbreviations: BL, baseline; CHF, congestive heart failure; DM, diabetes mellitus; FU, follow‐up at 1 year; LMWH, low‐molecular‐weight heparin; OAC, oral anticoagulation; TIA, transient ischemic attack. The CHA2DS2‐VASc score refines the original CHADS2 score (CHF, hypertension, age ≥75 years, DM, and prior stroke, TIA, or thromboembolism) by adding vascular disease, age 65 to 74 years, and sex category.
Oral anticoagulants at baseline were given in the total cohort in 82.7%, in patients with stroke occurring during follow‐up in 82.1%, and in patients with TIA occurring during follow‐up in 79.8%.
At baseline, 1827 patients (57.8%) had an international normalized ratio value between 2 and 3 in >65% of measurements in the last 3 months, consistent with effective anticoagulant therapy. At follow‐up, the number was 1823 patients (57.6%).
As the registry was initiated before the introduction of the novel OAC agents, only 1 patient each received dabigatran or rivaroxaban during follow‐up.
Interventions
Pharmacological cardioversion was reported in 725 patients (22.9%) prior to study entry (baseline) and in 223 patients (7.1%) during follow‐up; electrical cardioversion was reported in 716 patients (22.6%) at baseline and in 182 patients (5.8%) at follow‐up. Catheter ablation had been used in 175 patients (5.5%) at baseline and in 98 patients (3.1%) at follow‐up; implantable devices (pacemakers or defibrillators) had been used in 338 patients (10.7%) at baseline and in 150 patients (4.7%) at follow‐up.
Events
Despite a good adherence to recommendations for anticoagulation, the rate of stroke (2.7%) and TIA (3.0%) was high during the 1‐year follow‐up. Furthermore, myocardial infarctions (3.3%) and coronary revascularization procedures (4.8%) were common. Overall, event rates did not differ much between the various AF types and treatment strategies (data not shown). At baseline, 1401 out of 3163 patients (44.3%) had been hospitalized at least once in the 12 months before enrollment and 584 (18.5%) were hospitalized during the follow‐up period (mean, 0.4 ± 1.5; Figure 3). The main causes of hospitalization were electrical and pharmacological cardioversion, placement of an implantable cardioverter‐defibrillator (ICD), AF, and catheter ablation (Figure 4). Patients who were hospitalized due to AF‐related events including stroke received OAC less frequently compared with patients without such events (63.9% vs 71.4%).
Figure 3.
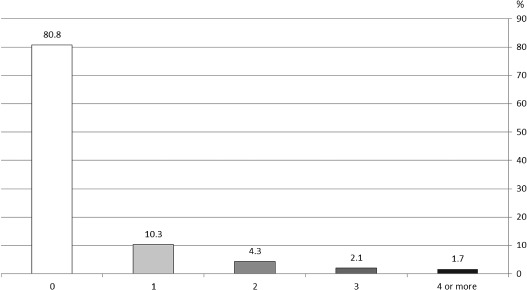
Frequency of hospitalizations per patient during follow‐up, regardless of underlying cause.
Figure 4.
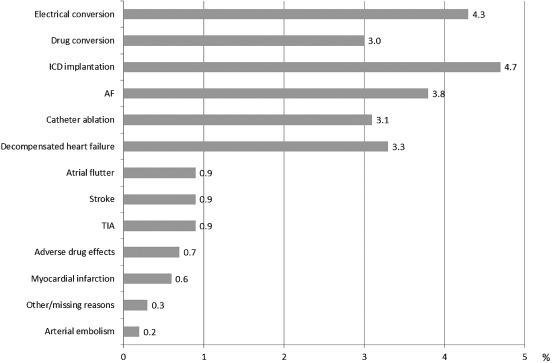
Reasons for hospitalizations during follow‐up. Abbreviations: AF, atrial fibrillation; ICD, implantable cardioverter‐defibrillator; TIA, transient ischemic attack.
Discussion
Primary Findings
The ATRIUM registry shows that in an elderly cohort of patients with a long history of arrhythmia who are considered to be suitable for management in primary care, AF is associated with high incidence of complications during follow‐up. Specialized cardiologic care was required in many patients, related to AF (eg, cardioversion, catheter ablation, antiarrhythmic drug therapy) or related to other cardiovascular diseases (eg, percutaneous coronary intervention, ICD implantations). Despite high anticoagulation usage, strokes and TIAs were common. Most prominently, many patients with AF were hospitalized. These insights urge an effort to find new and better ways to manage AF patients, possibly including earlier therapy to prevent the arrhythmia in the first place. A medical need for comprehensive cardiovascular‐risk reduction, as specified in the third AFNET/European Heart Rhythm Association consensus conference,25 is evident in arrhythmia patients, even in those with long‐standing AF.
Clinical Characteristics of the Study Cohort
Although many patients with AF are managed as outpatients by primary‐care physicians, most registry data available so far report on patients enrolled by cardiologists who are often hospital‐based (eg, Euro Heart Survey or AFNET registry). Therefore, ATRIUM provides much‐needed information on the management and outcome of patients with AF in the primary‐care setting.26, 27, 28
Patients enrolled in ATRIUM were older than those enrolled in the Euro Heart Survey (69 ±10 years) or the AFNET registry (67 ± 13 years), similar to the age profile in recent large controlled trials.29, 30, 31, 32 Reflective of the long duration of AF and of the enrollment in primary‐care outpatient settings, a high proportion of the ATRIUM patients had permanent AF (43%), more than in the Euro Heart Survey (29%) or AFNET (33%) and consistent with the AFNET dataset showing a higher proportion of patients with permanent AF in outpatient centers.5, 24 Characterization of symptoms in ATRIUM was similar to the AFNET report.24
Rhythm control was one of the treatment modalities in only 17% of patients, whereas the great majority were on rate control. The use of antiarrhythmic drugs declined during follow‐up in ATRIUM. Reasons for this are speculative and may include the fact that more patients passed the transition to permanent AF, which is common in AF patients. The type of rate‐control therapy was in line with findings from other trials and registries, with the exception that there was slightly lower use of digitalis glycosides, and probably reflects the growing experience that these agents control heart rate well in sedentary patients.24, 33
Stroke Prevention and Anticoagulant Therapy Use
Antithrombotic medication was used in 93% of patients at baseline and 92% at follow‐up. Given the fact that 7.5% of patients at follow‐up had contraindications to OAC, such as malignancies or previous bleeding episodes, the rates seem very satisfactory at first glance. A small number of patients received antiplatelet therapy despite recommendations1 and clear evidence34, 35 that OAC prevents strokes better than antiplatelet agents. Consistent with other registries,24, 36 a substantial portion of patients potentially ineligible for OAC received such therapy, possibly owing to an anticipation of more recent guidelines,8 based on data that were available at the time of enrollment into ATRIUM.30, 34 It was also remarkable that a high proportion did not achieve satisfactory international normalized ratio values during the 1‐year follow‐up, illustrating the difficulties in achieving good anticoagulation with vitamin K antagonists in these patients.37
The mean CHA2DS2‐VASc score at enrollment was 3.8 ± 1.7, which would translate into an annual stroke rate of about 3.8% in the absence of anticoagulation.7 However, because not all components of the score were specifically asked for on the case‐report form (eg, peripheral arterial disease, which occurs in about 20% of the elderly),38 the calculated scores will underestimate the true risk. Nonetheless, the observed stroke rate (2.7%; 3% for TIA) in this study—against the background of anticoagulation in the great majority of patients—was higher than expected.
Although electrical cardioversions were reported in 22.6% of patients at baseline, during follow‐up only 5.8% received this intervention; the rates for pharmacological conversion also were very similar at baseline (22.9%) and follow‐up (7.1%). For comparison, in the Health‐Related Quality of Life of Patients With Atrial Fibrillation Managed by Cardiologists (MOVE) study, which collected data from German cardiologists, the rate of pharmacological cardioversion was 18%33; in the AFNET registry it was 3% to 16%,24 and in the Euro Heart Survey it was 3% to 14% each, depending on AF type.5 Electrical cardioversion was reported in AFNET at 7% to 23% depending on AF type, in MOVE at 18%, and in the Euro Heart Survey at 3% to 24%.24, 33 The high rate of ablation procedures during follow‐up is notable, as in the great majority of patients AF was persistent or permanent.
The hospitalization rate of about 20% in ATRIUM is lower than the 30% in the placebo arm of the Efficacy of Dronedarone for the Prevention of Cardiovascular Hospitalization or Death From Any Cause in Patients With Atrial Fibrillation/Atrial Flutter (ATHENA) study, but at the same level as the 20% rate in large AF trials such as the European Trial in Atrial Fibrillation or Flutter Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm (EURIDIS) and the American‐Australian‐African Trial With Dronedarone in Atrial Fibrillation or Flutter Patients for the Maintenance of Sinus Rhythm (ADONIS),39 or Atrial Fibrillation Follow‐up Investigation of Rhythm Management AFFIRM.40 The high rates illustrate the substantial burden of disease related to AF in these elderly patients.
Study Limitations
The current analysis might be prone to bias due to the absence of randomization and to selection processes. It is conceivable that centers with low interest and/or expertise in AF refused participation. Underreporting of events or missing information could compromise findings in the absence of source data verification. The events were not adjudicated, and they cannot be causally be linked to AF (in particular because many patients had other forms of cardiac disease). Nonetheless, the diagnosis‐related group‐based healthcare system ensures good medical review of all stroke or TIA events; there is an incentive for hospitals to code them, and an equally strong incentive for payors to audit all events to avoid paying an excess diagnosis‐related group fee.
Conclusion
Treatment of AF in primary care is mostly guided by cardiologists' recommendations to individual patients. Although antithrombotic treatment rates are among the highest reported to date, the rate of stroke during follow‐up is unexpectedly high. Furthermore, frequent AF‐related hospitalizations indicate unsolved problems in the management of patients and challenges in the treatment of AF in these multimorbid patients.
Acknowledgments
The authors thank Dr. Hechenbichler (from the Dr. Schauerte contract research organization) for the statistical analyses and a thorough review of the article.
The ATRIUM registry was performed by Sanofi‐Aventis Germany in cooperation with the German Atrial Fibrillation Network (AFNET).
Drs. Schmalowsky and Rosin are full‐time employees of Sanofi‐Aventis, and the other authors have received consulting honoraria from sanofi‐aventis for research and advice. Dr. Kirchhof has served as an advisor or consultant for 3M Medica, AstraZeneca Pharmaceuticals LP, Bayer HealthCare Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals, Inc., Bristol‐Myers Squibb, Daiichi‐Sankyo, Inc., Meda Pharmaceuticals, Inc., Medtronic, Inc., Merck & Co., Inc., Merck Sharp & Dohme Corp., Otsuka Pharmaceutical Co., Ltd., Pfizer Inc., sanofi‐aventis, Servier, Siemens AG, and Takeda Pharmaceuticals North America, Inc. and has received grants for clinical research from 3M Medica, CV Therapeutics, Meda Pharmaceuticals, Inc., Medtronic, Inc., Omron Corp., sanofi‐aventis, and St. Jude Medical.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 3. Vidaillet H, Granada JF, Chyou PH, et al. A population‐based study of mortality among patients with atrial fibrillation or flutter. Am J Med. 2002;113:365–370. [DOI] [PubMed] [Google Scholar]
- 4. Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. [DOI] [PubMed] [Google Scholar]
- 5. Nieuwlaat R, Capucci A, Camm AJ, et al. Atrial fibrillation management: a prospective survey in ESC Member Countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26:2422–2434. [DOI] [PubMed] [Google Scholar]
- 6. Meinertz T, Kirch W, Rosin L, et al. Management of atrial fibrillation by primary care physicians in Germany: baseline results of the ATRIUM registry. Clin Res Cardiol. 2011;100:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 8. Camm A, Lip G, Atar D, et al. 2012 Focused Update of the ESC Guidelines on the Management of Atrial Fibrillation. Eur Heart J. 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 9. Carlsson J, Tebbe U, Rox J, et al; ALKK‐Study Group . Cardioversion of atrial fibrillation in the elderly. Am J Cardiol. 1996;78:1380–1384. [DOI] [PubMed] [Google Scholar]
- 10. Santini M, De Ferrari GM, Pandozi C, et al. Atrial fibrillation requiring urgent medical care: approach and outcome in the various departments of admission. Data from the Atrial Fibrillation/Flutter Italian Registry (FIRE). Ital Heart J. 2004;5:205–213. [PubMed] [Google Scholar]
- 11. Frykman V, Beerman B, Rydén L, et al. Management of atrial fibrillation: discrepancy between guideline recommendations and actual practice exposes patients to risk for complications. Eur Heart J. 2001;22:1954–1959. [DOI] [PubMed] [Google Scholar]
- 12. Le Heuzey J, Breithardt G, Camm J, et al. The RecordAF Study: design, baseline data, and profile of patients according to chosen treatment strategy for atrial fibrillation. Am J Cardiol. 2010;105:687–693. [DOI] [PubMed] [Google Scholar]
- 13. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 14. Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study. Am J Med. 1995;98:476–484. [DOI] [PubMed] [Google Scholar]
- 15. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population‐based estimates. Am J Cardiol. 1998; 82:2N–9N. [DOI] [PubMed] [Google Scholar]
- 16. Kerr CR, Boone J, Connolly SJ, et al. The Canadian Registry of Atrial Fibrillation: a noninterventional follow‐up of patients after the first diagnosis of atrial fibrillation. Am J Cardiol. 1998;82:82N–85N. [DOI] [PubMed] [Google Scholar]
- 17. Goudevenos JA, Vakalis JN, Giogiakas V, et al. An epidemiological study of symptomatic paroxysmal atrial fibrillation in northwest Greece. Europace. 1999;1:226–233. [DOI] [PubMed] [Google Scholar]
- 18. Granada J, Uribe W, Chyou PH, et al. Incidence and predictors of atrial flutter in the general population. J Am Coll Cardiol. 2000;36:2242–2246. [DOI] [PubMed] [Google Scholar]
- 19. Stewart S, Hart CL, Hole DJ, et al. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart. 2001;86:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wandell PE. A survey of subjects with present or previous atrial fibrillation in a Swedish community. Scand J Prim Health Care. 2001;19:20–24. [PubMed] [Google Scholar]
- 21. Friberg J, Scharling H, Gadsboll N, et al. Sex‐specific increase in the prevalence of atrial fibrillation (the Copenhagen City Heart Study). Am J Cardiol. 2003;92:1419–1423. [DOI] [PubMed] [Google Scholar]
- 22. Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation. 2003;108:711–716. [DOI] [PubMed] [Google Scholar]
- 23.Kompentenznetz Vorhofflimmern (AFNET). http://www.kompetenznetz‐vorhofflimmern.de/de/afnet. Accessed October 24, 2011.
- 24. Nabauer M, Gerth A, Limbourg T, et al. The Registry of the German Competence Network on Atrial Fibrillation: patient characteristics and initial management. Europace. 2009;11:423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kirchhof P, Lip GY, Van Gelder IC, et al. Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options. Executive summary of the report from the 3rd AFNET/EHRA consensus conference. Thromb Haemost. 2011;106:1012–1019. [DOI] [PubMed] [Google Scholar]
- 26. Lip GY, Golding DJ, Nazir M, et al. A survey of atrial fibrillation in general practice: the West Birmingham Atrial Fibrillation Project. Br J Gen Pract. 1997;47:285–289. [PMC free article] [PubMed] [Google Scholar]
- 27. Levy S, Maarek M, Coumel P, et al; the College of French Cardiologists . Characterization of different subsets of atrial fibrillation in general practice in France: the ALFA study. Circulation. 1999;99:3028–3035. [DOI] [PubMed] [Google Scholar]
- 28. Majeed A, Moser K, Carroll K. Trends in the prevalence and management of atrial fibrillation in general practice in England and Wales, 1994–1998: analysis of data from the general practice research database. Heart. 2001;86:284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hohnloser SH, Crijns HJGM, van Eickels M, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation [published corrections appear in N Engl J Med. 2009;360:2487 and N Engl J Med. 2011;364:1481]. N Engl J Med. 2009;360:668–678. [DOI] [PubMed] [Google Scholar]
- 30. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 31. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 32. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 33. Kirch W, Pittrow D, Bosch R, et al. Health‐related quality of life of patients with atrial fibrillation managed by cardiologists: MOVE study [article in German]. Dtsch Med Wochenschr. 2010;135(suppl 2):S26–S32. [DOI] [PubMed] [Google Scholar]
- 34. Diener HC, Eikelboom J, Connolly SJ, et al. Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a predefined subgroup analysis from AVERROES, a randomised trial. Lancet Neurol. 2012;11:225–231. [DOI] [PubMed] [Google Scholar]
- 35. Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–1912. [DOI] [PubMed] [Google Scholar]
- 36. Nieuwlaat R, Olsson SB, Lip GY, et al. Guideline‐adherent antithrombotic treatment is associated with improved outcomes compared with undertreatment in high‐risk patients with atrial fibrillation: the Euro Heart Survey on Atrial Fibrillation. Am Heart J. 2007;153:1006–1012. [DOI] [PubMed] [Google Scholar]
- 37. Ansell J, Hollowell J, Pengo V, et al. Descriptive analysis of the process and quality of oral anticoagulation management in real‐life practice in patients with chronic non‐valvular atrial fibrillation: the International Study of Anticoagulation Management (ISAM). J Thromb Thrombolysis. 2007;23:83–91. [DOI] [PubMed] [Google Scholar]
- 38. Diehm C, Allenberg JR, Pittrow D, et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120:2053–2061. [DOI] [PubMed] [Google Scholar]
- 39. Singh BN, Connolly SJ, Crijns HJ, et al. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007;357:987–999. [DOI] [PubMed] [Google Scholar]
- 40. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]


