ABSTRACT
Background
Elevated cardiac troponin I (cTnI) occurs in acute coronary syndrome (ACS) as well as various scenarios not associated with ACS.
Hypothesis
Simple clinical criteria can reliably exclude ACS among hospitalized patients with elevated cTnI.
Methods
Records for patients hospitalized from January to April 2011 with elevated cTnI (>0.29 ng/dL) and an available echocardiogram were retrospectively reviewed. Patients with ST‐segment elevation myocardial infarction were excluded. Based on available clinical data, patients were classified as having ACS or elevation of cTnI unrelated to ACS (non‐ACS). Median follow‐up was 365 days.
Results
Of 265 records meeting inclusion criteria, 82 (31%) had ACS and 183 (69%) had non‐ACS. In multivariable analysis, odds ratios for non‐ACS were 7.6 (95% confidence interval [CI]: 3.8‐15.3) for peak cTnI <2 ng/dL, 6.3 (95% CI: 3.1‐13.0) for absent wall‐motion abnormality, and 4.4 (95% CI: 2.2‐8.6) for no prior coronary artery disease history. The area under the receiver operating curve for a model using these 3 variables was 0.86, with a 98% negative predictive value for excluding ACS. Patients who met these 3 criteria had no ACS‐related deaths over 1‐year follow‐up.
Conclusions
Hospitalized patients with peak Tn level <2 ng/dL, no prior history of coronary artery disease, and no new echocardiographic wall‐motion abnormality appear to have a very low likelihood of ACS. Prospective validation of these results is needed to determine whether additional diagnostic testing could be safely avoided in hospitalized patients meeting these simple clinical criteria.
Introduction
Cardiac troponin I (cTnI) is a sensitive and specific marker of myonecrosis, but it is not by itself diagnostic of acute coronary syndrome (ACS) or myocardial infarction.1 The European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation (ESC/ACC/AHA/WHF) Task Force statement differentiates between myocyte damage caused by acute coronary arterial plaque rupture (type 1 acute myocardial infarction [AMI], or ACS) and myocyte injury from increased myocardial oxygen demand with inadequate supply causing elevated serum cTnI (type 2 AMI).2, 3 A variety of clinical conditions cause cTnI elevation, including hypotension, anemia, decompensated heart failure, myo‐ or pericarditis, pulmonary embolism, sepsis, and cardiac defibrillator discharge.4, 5, 6 Hospitalized patients are frequently found to have elevated cTnI of unclear significance. In the absence of typical angina or ischemic electrocardiographic (ECG) changes, this common scenario presents a diagnostic dilemma.
Echocardiography aids in the diagnosis of myocardial ischemia and assessment of risk among patients presenting with chest pain7, 8, 9, 10, 11, 12; however, the utility of echocardiographic imaging is less well‐defined among the broader population of hospitalized patients with elevated cTnI.
There is substantial clinical importance in discriminating ACS (type 1 AMI) from other causes of elevated cTnI. The aim of this study was to determine whether simple clinical data, potentially including the absence of a new regional wall‐motion abnormality (WMA) on echocardiography, can reliably exclude the diagnosis of ACS among hospitalized patients with elevated cTnI.
Methods
Patients
Consecutive adult patients hospitalized at the University of Michigan Health Systems from January through April 2011 were retrospectively reviewed. Inpatients with elevated cTnI (>0.29 ng/dL) and an echocardiogram performed during the same hospitalization were identified using an electronic search algorithm. Patients were included if the echocardiogram was sufficient for analysis and was performed within 48 hours following the initial elevated cTnI. Patients were excluded from analysis in the setting of ST‐segment elevation myocardial infarction (STEMI), elevated cTnI following coronary artery bypass surgery (CABG) or percutaneous coronary intervention (PCI), or if admission followed initial management at another hospital. The University of Michigan Institutional Review Board approved this study.
Biochemical Analysis
Blood samples were analyzed for cTnI per standard laboratory protocol using the ADVIA Centaur immunoanalyzer (Siemens Healthcare Diagnostics Inc., Tarrytown, NY). In our hospital, cTnI >0.29 ng/dL is considered significant and reliable based on the 10% coefficient of variation of the analyzers in our laboratory. No stored blood samples were used.
Clinical Data
Demographic data and past medical history were obtained from the admission history and physical examination. A history of coronary artery disease (CAD) was defined as a stated or retrievable history of myocardial infarction (MI), PCI, or CABG, or documentation of a significant stenosis (>70%) on prior coronary angiography. Histories of hypertension, hyperlipidemia, diabetes mellitus, atrial fibrillation, peripheral vascular occlusive disease, cerebrovascular accident, tobacco use, and family history of CAD were based on the available documented past medical history. End‐stage renal disease was defined as requirement for dialysis during the index hospitalization. History of aortic stenosis or left ventricular (LV) systolic dysfunction was based on either documentation in the past medical history or on prior echocardiogram.
The clinical presentation and symptoms that prompted the clinician to order cTnI were analyzed by reviewing the admission history and physical examination, daily progress notes, and cardiology consultation notes, if applicable. Symptoms were characterized as typical in nature if recorded as substernal chest pain or pressure with exertion or at rest, and not associated with a documented noncardiac cause such as musculoskeletal pain.
Electrocardiograms from the time period of the elevated cTnI were reviewed. Interpretations were based on the final analysis of the interpreting clinical cardiologist. Analyzed laboratory data included cTnI and serum creatinine. The change in cTnI (ΔcTnI) was defined as the absolute difference between the highest and initial cTnI values. Serum creatinine level was measured at the same time as the initial cTnI.
Echocardiographic findings were taken from the clinical analysis performed at the time of hospitalization. Analyzed data included left ventricular ejection fraction (LVEF), right ventricular function, moderate or severe pulmonary hypertension, moderate or severe aortic stenosis, and other severe valvular disease, all defined by overall impression in the clinical echocardiography/Doppler report. Any regional LV WMA was recorded as described in the final clinical report. If a WMA was described as old, scarred, or consistent with a prior MI, it was classified as an old WMA rather than a new WMA. A new regional WMA was defined as hypokinesis, akinesis, or dyskinesis of ≥1 LV wall segments, not documented by a previous echocardiogram in the University of Michigan Health System.
Follow‐up occurred 18 to 22 months after index hospitalization. All records (including hospitalizations, outpatient visits, and phone notes) were reviewed (A.D.). Mortality outcomes were classified as related to ACS or not.
Definition of Acute Coronary Syndrome
Each patient was classified as having had either ACS (type 1 AMI) or non‐ACS using the established criteria from the ESC/ACCF/AHA/WHF Universal Definition.3 Classification was based on detailed chart review by 2 authors (M.D., A.S.), which included the clinical scenario, discharge diagnosis, and impressions of the treating physicians and the consulting cardiologist, when applicable. In the event of any uncertainty, the records were reviewed by a third cardiologist (D.B.), and a diagnosis was determined by consensus. The term “ACS” was used to characterize patients believed to have had an AMI related to a thrombotic or thromboembolic occlusion of coronary blood flow (ie, type 1 AMI). All other clinical scenarios were categorized as non‐ACS.
Statistical Analysis
Standard statistical methods were used to compare the characteristics of patients diagnosed with ACS to those without ACS. Laboratory data were evaluated as continuous or dichotomous variables, as appropriate. For clinical utility, dichotomization of peak serum Tn level occurred at 2 ng/dL. Between‐group comparisons were performed using either the Student t test or the Mann‐Whitney U test for parametric and nonparametric data, respectively. Continuous variables were summarized using mean ± standard deviation (SD) and/or median ± interquartile range if the variables showed a large amount of skewness. Categorical data were compared with the likelihood ratio χ2 test. Univariable logistic regression analyses were performed. Variables were chosen based on their known clinical significance and ease of use. Low level of peak Tn <2 ng/dL, absence of a new WMA on echocardiogram, and past history of CAD were entered into a multivariable logistic regression model and odds ratios (OR) were generated. A receiver operating characteristic (ROC) curve was created, and the area under the curve (AUC) was calculated. The logistic‐regression model was validated using bootstrap resampling to ensure that the model could be applied to new patients.13 Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC), validation was performed using R‐2.15.1, and follow‐up analysis used SPSS version 20.0.0.1 (IBM Corp., Armonk, NY).
Results
Of 265 records meeting inclusion criteria among 260 patients, 82 (31%) had cTnI elevation related to ACS and 183 (69%) had cTnI elevation from a non‐ACS cause. Demographic data are shown in Table 1. Patients diagnosed with ACS were older. There were no significant differences between groups in gender, body mass index, or race. Patients classified as non‐ACS were further categorized by the clinical diagnosis presumed responsible for the elevated cTnI (Figure 1).
Table 1.
Clinical Demographics and the Diagnosis of ACS
| Total, n = 265 | ACS, n = 82 | Non‐ACS, n = 183 | P Valuea | |
|---|---|---|---|---|
| Age, y, mean (SD)/median | 63 (17)/64 | 67.8 (13.7)/70 | 61.4 (18.1)/63 | 0.0047 |
| Male sex, n (%) | 151 (57) | 49 (60) | 102 (56) | 0.51 |
| BMI, kg/m2, mean (SD)/median | 29.9 (9.2)/28.4 | 30.0 (8.1)/28.6 | 29.9 (9.7)/28.2 | 0.93 |
| Race, n (%) | ||||
| Caucasian | 215 (81) | 70 (85) | 145 (80) | 0.23 |
| African American | 32 (12) | 7 (9) | 25 (14) | 0.24 |
| Other | 15 (6) | 4 (5) | 11 (6) | 0.71 |
| Hypertension, n (%) | 177 (66) | 61 (74) | 116 (63) | 0.066 |
| Diabetes mellitus, n (%) | 89 (33) | 37 (45) | 52 (28) | 0.008 |
| Hyperlipidemia, n (%) | 125 (47) | 50 (61) | 75 (41) | 0.002 |
| Family history, n (%) | 77 (29) | 31 (38) | 46 (25) | 0.036 |
| Tobacco history, n (%) | 146 (55) | 52 (63) | 94 (51) | 0.061 |
| PVOD, n (%) | 32 (12) | 16 (20) | 16 (9) | 0.016 |
| CVA, n (%) | 19 (7) | 5 (6) | 14 (8) | 0.65 |
| ESRD, n (%) | 22 (8) | 7 (8) | 15 (8) | 0.92 |
| CAD history, n (%) | 107 (40) | 51 (62) | 56 (30) | <0.0001 |
| PCI/CABG, n (%) | 75 (28) | 38 (46) | 37 (20) | <0.0001 |
| LV dysfunction, LVEF <55%, n (%) | 32 (12) | 12 (15) | 20 (11) | 0.39 |
| Atrial fibrillation, n (%) | 49 (18) | 6 (7) | 43 (23) | 0.0008 |
| Aortic stenosis (moderate/severe), n (%) | 14 (5) | 4 (4) | 10 (5) | 0.85 |
Abbreviations: BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CVA, cerebrovascular accident; ESRD, end‐stage renal disease; LV, left ventricular; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; PVOD, peripheral vascular occlusive disease; SD, standard deviation.
P value for between‐group comparisons.
Figure 1.
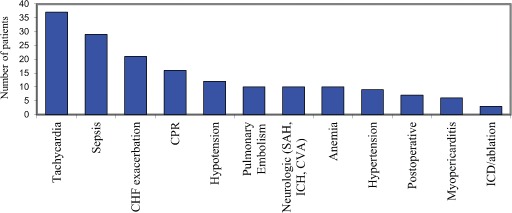
Diagnoses of elevated troponin in patients with non‐ACS. Abbreviations: ACS, acute coronary syndrome; CHF, congestive heart failure; CPR, cardiopulmonary resuscitation; SAH, subarachnoid hemorrhage; ICH, intracranial hemorrhage; CVA, cerebrovascular accident; ICD, implantable cardioverter defibrillator.
Clinical data are shown in Table 2. Typical angina was present in 10% of the total population with elevated cTnI vs 28% of patients with ACS. The majority of ECG findings were nonspecific or inconclusive. Among patients diagnosed with ACS, 51% had ST‐segment or T‐wave changes consistent with regional ischemia, and 12% had no evidence of ischemia by ECG. A normal ECG was not predictive of either ACS or non‐ACS. Mean serum Tn levels (initial, peak, and Δ) were significantly lower in the non‐ACS group (Table 2, Figure 2).
Table 2.
Clinical Presentation, Laboratory, Electrocardiographic, and Echocardiographic Data
| Total, n = 265 | ACS, n = 82 | Non‐ACS, n = 183 | P Valuea | |
|---|---|---|---|---|
| Symptoms, n (%) | ||||
| Typical angina | 27 (10) | 23 (28.0) | 4 (2.2) | <0.0001 |
| Intubated/sedated | 44 (16.6) | 3 (3.7) | 41 (22.4) | 0.0002 |
| Atypical | 41 (15.5) | 21 (25.6) | 20 (10.9) | 0.0023 |
| Dyspnea | 43 (16.2) | 8 (9.8) | 35 (19.1) | 0.056 |
| Asymptomatic | 15 (5.7) | 5 (6.1) | 10 (5.5) | 0.84 |
| Mental status change | 15 (5.7) | 5 (6.1) | 10 (5.5) | 0.84 |
| Tachycardia | 7 (2.6) | 1 (1.2) | 6 (2.3) | 0.33 |
| Cardiac arrest | 8 (3.0) | 1 (1.2) | 7 (3.8) | 0.25 |
| Syncope | 6 (2.3) | 3 (3.7) | 3 (1.6) | 0.31 |
| Stroke | 4 (1.5) | 2 (2.4) | 2 (1.1) | 0.41 |
| Tn level, ng/dL | ||||
| Initial Tn, median (IQR) | 0.9 (0.4–3.0) | 3.0 (0.9–10.8) | 0.6 (0.4–1.6) | <0.0001 |
| Peak Tn, median (IQR) | 1.6 (0.6–8.1) | 11.0 (3.1–35.2) | 1.0 (0.5–2.6) | <0.0001 |
| ΔTn, median (IQR) | 0.1 (0–2.3) | 2.6 (0.4–16.1) | 0 (0–0.5) | <0.0001 |
| Creatinine, mg/dL, mean (SD) | 1.9 (1.8) | 1.8 (1.8) | 2.0 (1.8) | 0.28 |
| Electrocardiography (ECG), n (%) | ||||
| No ischemic changes | 40 (15) | 10 (12.2) | 30 (16.3) | 0.38 |
| Regional ST‐T wave changes | 71 (27) | 42 (51.2) | 29 (15.8) | <0.0001 |
| Echocardiography, normal, n (%) | 84 (32) | 15 (18.3) | 69 (37.5) | 0.0013 |
| Mean LVEF, %, (SD)/median | 49.5 (18.7)/55 | 45.2 (16.9)/50 | 51.4 (19.2)/60 | 0.018 |
| LV dysfunction, n (%) | 73 (27) | 26 (31.7) | 47 (25.5) | 0.3 |
| RV dysfunction, n (%) | 54 (20) | 9 (11) | 45 (24.5) | 0.0086 |
| Pulmonary hypertension, n (%) | 30 (11) | 3 (3.7) | 27 (14.7) | 0.0087 |
| Aortic stenosis, moderate/severe, n (%) | 21 (8) | 7 (8.5) | 14 (7.6) | 0.797 |
| Other severe valve disease, n (%) | 18 (7) | 2 (2.4) | 16 (8.7) | 0.0409 |
| Old WMA, n (%) | 17 (6) | 11 (13.4) | 6 (3.3) | 0.003 |
| WMA, n (%) | 69 (26) | 43 (52.4) | 25 (13.6) | <0.0001 |
| Anterior | 21 (8) | 17 (20.7) | 4 (2.2) | <0.0001 |
| Lateral | 15 (6) | 12 (14.6) | 3 (1.6) | <0.0001 |
| Posterior | 26 (10) | 20 (24.4) | 6 (3.3) | <0.0001 |
| Inferior | 40 (15) | 26 (31.7) | 14 (7.6) | <0.0001 |
| Septal | 33 (12) | 21 (25.6) | 12 (6.5) | <0.0001 |
| Apical | 29 (11) | 18 (22.0) | 11 (6.0) | 0.0002 |
| Takotsubo | 1 (0.4) | 0 (0) | 1 (0.5) | NS |
Abbreviations: ACS, acute coronary syndrome; IQR, interquartile range; LV, left ventricular; LVEF, left ventricular ejection fraction; NS, not significant; RV, right ventricular; SD, standard deviation; Tn, troponin; WMA, wall‐motion abnormality.
P value for between‐group comparisons.
Figure 2.
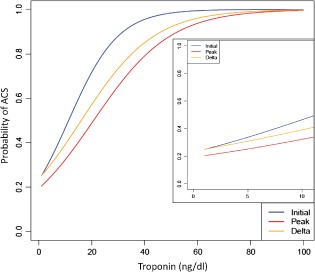
Probability of ACS (type 1 myocardial infarction) with increasing level of troponin. Abbreviations: ACS, acute coronary syndrome.
Echocardiographic data revealed that a new WMA was significantly more common among patients diagnosed with ACS (52%) compared with non‐ACS patients (14%; P < 0.0001) (Table 2). The presence of moderate or severe pulmonary hypertension or right ventricular (RV) systolic dysfunction was more common among patients with non‐ACS (P = 0.009).
Relatively few patients underwent stress testing (31 [12%]) or cardiac catheterization (58 [22%]) during hospitalization. Among those who underwent catheterization, 45 (78%) had ACS and 13 (22%) had non‐ACS. Revascularization (PCI or CABG referral) occurred in 34 (59%) of the ACS patients and in 2 (3%) of those with non‐ACS.
Predictors of Acute Coronary Syndrome
A new WMA on echocardiography had a negative predictive value of 80% and positive predictive value of 63% for the identification of ACS. Variables entered into multivariable logistic regression analysis included: (1) peak cTnI <2 ng/dL, (2) absent new WMA by echocardiogram, and (3) no prior history of CAD. The ORs for non‐ACS were 7.6 (95% confidence interval [CI]: 3.8‐15.3) for peak cTnI <2 ng/dL, 6.3 (95% CI: 3.1‐13.0) for absent WMA, and 4.4 (95% CI: 2.2‐8.6) for no prior CAD history. The area under the ROC curve (ROC AUC) for a model using these 3 variables was 0.86 (Figure 3), with a 98% negative predictive value for excluding ACS.
Figure 3.
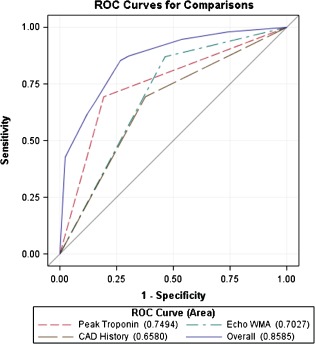
Receiver operator curves for individual variables (peak cTn <2 ng/dL, no new echocardiographic WMA, no history of CAD) and combined variables in the discrimination of ACS among hospitalized patients with elevated cTn. Abbreviations: ACS, acute coronary syndrome; CAD, coronary artery disease; cTn, cardiac troponin; WMA, wall‐motion abnormality.
Records of the 2 patients who met all 3 criteria but had a diagnosis of ACS were reviewed further. Both patients were being treated for active hematologic malignancies; one had undergone bone marrow transplant and the other had received chemotherapy at the time of cTnI elevation. Both were medically managed and had no further cardiovascular complications. Neither patient had additional cardiology follow‐up or stress testing, and both patients died later that year from oncologic causes.
Follow‐up
All‐cause mortality for the whole cohort during median follow‐up of 365 days was 30.6%. Among the 81 patients who met our proposed 3 criteria (ie, cTnI <2 ng/dL, no WMA, no history of CAD), there were no cardiac deaths attributed to ACS during the follow‐up period (0% vs 2.2% of the remaining cohort, P = 0.007).
Discussion
This study presents a simple derived model to reliably exclude ACS among hospitalized patients with elevated cTnI. Patients meeting the 3 criteria of (1) no prior history of CAD, (2) no WMA on echocardiogram, and (3) peak cTnI <2 ng/dL were highly unlikely to have an ACS consistent with type 1 AMI, with 98% negative predictive value, ROC AUC of 0.86, and no ACS‐related deaths during follow‐up. This model can provide reassurance to clinicians when confronted with a hospitalized patient with elevated cTnI, and additional invasive testing could be prevented among low‐risk patients meeting these criteria.
This combination of factors provides a superior risk‐prediction model than each of the criteria could separately. For example, relying solely on elevated cTnI for diagnosis of ACS is inadequate without additional clinical information.1 When used alone to predict ACS in hospitalized patients, prior data found that a cTnI level of 21.7 ng/dL had a sensitivity of 78% and specificity of 44% for the detection of ACS, with an ROC AUC of 0.63.14 Our study also found that cTnI alone was only predictive of ACS at very high levels (Figure 2), which is generally not clinically useful. Therefore, in this study, peak cTnI was dichotomized at a level of 2 ng/dL to provide a clinically meaningful cutoff.
Preexisting CAD is a well‐known risk factor for future ischemic events and has been reported to have an OR of 3.2 (95% CI: 2.17‐4.71, P < 0.001) for ACS in patients presenting with chest pain.15 Echocardiographic WMA was shown to be an independent predictor of AMI, death, or need for revascularization, and was superior to age, sex, or the combination of history, ECG, and cTnI level.16 Studies of echocardiography for diagnosis or risk prediction typically occur in the acute setting among patients presenting with chest pain.8, 9, 10, 11, 12 To our knowledge, the utility and accuracy of echocardiography among a broader population of hospitalized patients with elevated cTnI has not been previously studied.
Existing ACS risk‐prediction tools also focus on diagnosis and risk stratification of patients presenting to emergency departments. For example, the Time Insensitive Predictive Instrument (TIPI), published in the early 1990s, attempted to diagnose ACS in patients presenting with chest pain. Seven pieces of data including age, sex, and various features of chest pain and ECG changes predicted ACS with an ROC of 0.88.17 Risk‐prediction tools such as Thrombolysis In Myocardial Infarction (TIMI),18 Goldman,19 and Sanchis20 are useful for prognostication among patients with ACS, but these were not designed to differentiate among causes of cTnI elevation. In a study that applied these tools for prediction of a diagnosis of ACS, low risk scores had negative predictive values of 91% (TIMI), 92% (Goldman), and 92% (Sanchis).21 Our prediction model uses only 3 pieces of simple data, applies to a broader hospitalized population, and has a negative predictive value of 98%.
As novel measures of high‐sensitivity cTn become more widely available, more patients can be expected to have detectable cTn. The ability to accurately distinguish those suffering from ACS from patients with other causes of cTn elevation is critical.22 Recent algorithms using high‐sensitivity cTn show promise for distinguishing AMI from noncoronary causes.23, 24, 25 Our study demonstrates that diagnostic algorithms should also be developed for hospitalized patients, who frequently lack the typical signs and symptoms of coronary ischemia.
Elevated cTn, regardless of etiology, has been associated with worse outcomes.26, 27, 28 The short‐ and long‐term management of patients with non‐ACS is not well‐defined, and future research is needed to determine the optimal management of patients with elevated cTn unrelated to ACS.
Study Limitations
This was a retrospective study and therefore subject to bias and confounding that may have influenced our results. However, each chart was carefully reviewed with detailed data extraction, and consensus was reached on the characterization of the clinical events during hospitalization. The cTnI measurements were analyzed in a dichotomous fashion; although this allowed for greater simplicity as a bedside prediction tool, the model may have been more refined by including cTnI as a continuous variable. The ΔTn also may be an important tool for distinguishing ACS from non‐ACS causes, but, due to the retrospective nature of this study, there was insufficient standardization in timing of Tn measurements to include this in our prediction model. Multivariate modeling was performed, but residual unmeasured confounders may exist. The retrospective nature of this review reflected typical practice. Of note, patients underwent echocardiography at the discretion of the treating clinicians; thus, if patients clearly had a non‐ACS cause of Tn elevation, an echocardiogram may not have been performed and was not included in our study. The results of our study should not in any way replace good clinical judgment. Few patients underwent additional stress testing and cardiac catheterization. Additional testing may have provided a gold standard for comparison, but many patients in this study had relative contraindications for invasive catheterization. Furthermore, the presence of obstructive coronary disease does not necessarily lead to a diagnosis of type 1 AMI.1
Our study represents a first step in ruling out ACS (type 1 plaque rupture) among hospitalized patients, but additional tools for distinguishing demand‐type ischemia vs mechanical Tn release (such as cardiopulmonary resuscitation, implantable cardioverter‐defibrillator placement, and radiofrequency ablation procedures) would also be useful areas of future research in a larger study.
Conclusion
To our knowledge, this is the first model to predict the presence or absence of ACS among hospitalized patients with elevated cTnI. Patients who have (1) no history of CAD, (2) a peak cTnI <2 ng/dL, and (3) no new echocardiographic WMA appear to have a very low likelihood of ACS. Prospective validation of this model in an independent sample of larger size is warranted.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Newby LK, Jesse RL, Babb JD, et al. ACCF 2012 expert consensus document on practical clinical considerations in the interpretation of troponin elevations: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2012;60:2427–2463. [DOI] [PubMed] [Google Scholar]
- 2. Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. [DOI] [PubMed] [Google Scholar]
- 3. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. [DOI] [PubMed] [Google Scholar]
- 4. Thygesen K, Mair J, Katus H, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J. 2010;31:2197–2204. [DOI] [PubMed] [Google Scholar]
- 5. Korff S, Katus HA, Giannitsis E. Differential diagnosis of elevated troponins. Heart. 2006;92:987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roongsritong C, Warraich I, Bradley C. Common causes of troponin elevations in the absence of acute myocardial infarction: incidence and clinical significance. Chest. 2004;125:1877–1884. [DOI] [PubMed] [Google Scholar]
- 7. Di Pasquale P, Cannizzaro S, Scalzo S, et al. Sensitivity, specificity and predictive value of the echocardiography and troponin‐T test combination in patients with non–ST‐elevation acute coronary syndromes. Int J Cardiovasc Imaging. 2004;20:37–46. [DOI] [PubMed] [Google Scholar]
- 8. Kontos MC, Arrowood JA, Paulsen WH, et al. Early echocardiography can predict cardiac events in emergency department patients with chest pain. Ann Emerg Med. 1998;31:550–557. [DOI] [PubMed] [Google Scholar]
- 9. Sabia P, Afrookteh A, Touchstone DA, et al. Value of regional wall motion abnormality in the emergency room diagnosis of acute myocardial infarction: a prospective study using two‐dimensional echocardiography. Circulation. 1991;84(3 suppl):I85–I92. [PubMed] [Google Scholar]
- 10. Sasaki H, Charuzi Y, Beeder C, et al. Utility of echocardiography for the early assessment of patients with nondiagnostic chest pain. Am Heart J. 1986;112:494–497. [DOI] [PubMed] [Google Scholar]
- 11. Lewis WR. Echocardiography in the evaluation of patients in chest pain units. Cardiol Clin. 2005;23:531–539, vii. [DOI] [PubMed] [Google Scholar]
- 12. Mohler ER, Ryan T, Segar DS, et al. Clinical utility of troponin T levels and echocardiography in the emergency department. Am Heart J. 1998;135:253–260. [DOI] [PubMed] [Google Scholar]
- 13. Harrell F. Regression Modeling Strategies With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001. [Google Scholar]
- 14. Blich M, Sebbag A, Attias J, et al. Cardiac troponin I elevation in hospitalized patients without acute coronary syndromes. Am J Cardiol. 2008;101:1384–1388. [DOI] [PubMed] [Google Scholar]
- 15. Gorelik O, Almoznino‐Sarafian D, Yarovoi I, et al. Patient‐related variables predicting acute coronary syndrome following admission for chest pain of possible coronary origin. Coron Artery Dis. 2006;17:15–21. [DOI] [PubMed] [Google Scholar]
- 16. Muscholl MW, Oswald M, Mayer C, et al. Prognostic value of 2D echocardiography in patients presenting with acute chest pain and non‐diagnostic ECG for ST‐elevation myocardial infarction. Int J Cardiol. 2002;84:217–225. [DOI] [PubMed] [Google Scholar]
- 17. Selker HP, Griffith JL, D'Agostino RB. A tool for judging coronary care unit admission appropriateness, valid for both real‐time and retrospective use. A time‐insensitive predictive instrument (TIPI) for acute cardiac ischemia: a multicenter study [published correctiona appears in Med Care. 1992;30:188]. Med Care. 1991;29:610–627. [DOI] [PubMed] [Google Scholar]
- 18. Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non‐ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. [DOI] [PubMed] [Google Scholar]
- 19. Goldman L, Cook EF, Johnson PA, et al. Prediction of the need for intensive care in patients who come to the emergency departments with acute chest pain. N Engl J Med. 1996;334:1498–1504. [DOI] [PubMed] [Google Scholar]
- 20. Sanchis J, Bodí V, Núñez J, et al. New risk score for patients with acute chest pain, non‐ST‐segment deviation, and normal troponin concentrations: a comparison with the TIMI risk score. J Am Coll Cardiol. 2005;46:443–449. [DOI] [PubMed] [Google Scholar]
- 21. Manini AF, Dannemann N, Brown DF, et al. Limitations of risk score models in patients with acute chest pain. Am J Emerg Med. 2009;27:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Twerenbold R, Jaffe A, Reichlin T, et al. High‐sensitive troponin T measurements: what do we gain and what are the challenges? Eur Heart J. 2012;33:579–586. [DOI] [PubMed] [Google Scholar]
- 23. Haaf P, Drexler B, Reichlin T, et al. High‐sensitivity cardiac troponin in the distinction of acute myocardial infarction from acute cardiac noncoronary artery disease. Circulation. 2012;126:31–40. [DOI] [PubMed] [Google Scholar]
- 24. Reichlin T, Schindler C, Drexler B, et al. One‐hour rule‐out and rule‐in of acute myocardial infarction using high‐sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211–1218. [DOI] [PubMed] [Google Scholar]
- 25. Gassenmaier T, Buchner S, Birner C, et al. High‐sensitive troponin I in acute cardiac conditions: implications of baseline and sequential measurements for diagnosis of myocardial infarction. Atherosclerosis. 2012;222:116–122. [DOI] [PubMed] [Google Scholar]
- 26. Mehta NJ, Khan IA, Gupta V, et al. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol. 2004;95:13–17. [DOI] [PubMed] [Google Scholar]
- 27. Lim W, Qushmaq I, Devereaux PJ, et al. Elevated cardiac troponin measurements in critically ill patients. Arch Intern Med. 2006;166:2446–2454. [DOI] [PubMed] [Google Scholar]
- 28. Peacock WF, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358:2117–2126. [DOI] [PubMed] [Google Scholar]


