ABSTRACT
Background
Chronic obstructive pulmonary disease (COPD) has been shown to be associated with lower levels of vitamin D, and the latter has been associated with total and cardiovascular disease (CVD) mortality.
Hypothesis
We hypothesized that lower vitamin D levels will further enhance the association of COPD and its severity with total and CVD mortality.
Methods
We studied 7746 US adults age ≥40 years without known CVD (mean age, 59.8 years) and followed up through 2006 (8.2 ± 2.5 years) for total and CVD mortality. Serum 25‐hydroxyvitamin D levels were categorized as tertiles: first tertile, <50.9 nm/L; second tertile, 50.9 to 73.6 nm/L; and third tertile, >73.6 nm/L. Severity of COPD was classified on the basis of forced expiratory volume per second (FEV1): mild COPD (FEV1 ≥80%) and moderate or severe COPD (FEV1 <80%), and requires a FEV1/forced vital capacity ratio <70%. With Cox regression, we examined the hazard ratio (HR) and 95% confidence interval (CI) for total and CVD mortality according to COPD/vitamin D category, using those with no COPD in the first tertile of vitamin D as the reference group.
Results
From Cox regression, unadjusted HRs increased successively with increasing COPD severity and decreasing vitamin D group to 4.5 (95% CI: 3.3‐6.1) for total and 3.4 (95% CI: 2.2‐5.3) for CVD mortality among those with moderate/severe COPD who were in the first tertile of vitamin D. After adjustment for age, sex, ethnicity, and other risk factors, these associations were attenuated but remained increased.
Conclusions
Lower levels of vitamin D may be associated with further increases in total and CVD mortality associated with COPD; however, age and cardiovascular risk factors appear to explain much of this association.
Introduction
Chronic obstructive pulmonary disease (COPD) is a complex inflammatory disease associated with comorbidities1, 2 and the third leading cause of death in the United States.3, 4, 5, 6, 7 Furthermore, the financial burden of COPD was estimated in 2005 by the National Heart, Lung, and Blood Institute (NHLBI) as approximately $38.8 billion annually.8 However, apart from smoking cessation, no treatment has proven to modify COPD disease progression, although several large randomized controlled trials demonstrated some beneficial effects on exacerbation rates using inhalers.9, 10
Traditionally, vitamin D has been associated with bone health, and the role of vitamin D in calcium homeostasis has been well established.11, 12, 13 However, large epidemiological studies have also linked low 25‐hydroxyvitamin D levels with autoimmune diseases, cancer, cardiovascular disease (CVD), and infection, including respiratory infections and tuberculosis.14, 15, 16, 17, 18 Vitamin D seems to have a pleiotropic effect on the lungs, which includes modulation, infection protection, asthma, and tuberculosis in addition to bone metabolism. Relationships have been demonstrated between serum levels of 25‐hydroxyvitamin D and pulmonary function, including COPD.19, 20 In the general population, decreased mortality has been shown with enhanced vitamin D levels.21, 22
Few studies have examined the impact of vitamin D in COPD. The progression of COPD within patients who have moderate or severe cases may not have an affiliation with vitamin D,23 and thus the role of vitamin D in the progression or prognosis of COPD is unclear. Our study examined in a large population of US adults whether CVD and all‐cause mortality associated with COPD varies according to levels of serum vitamin D.
Methods
Study Sample
We included 7746 US adults age ≥40 years who participated in the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994) and were free of prevalent CVD. The clinical and demographic data of these participants were then linked with the National Death Index‐linked mortality data through 2006. All of the data used for this study were publicly available and thus exempt from review by the institutional review board and did not involve any patient interaction.
Definition of Cardiovascular Disease and Measurement of Risk Factors and Serum 25‐Hydroxyvitamin D Levels
We defined prevalent CVD as a self‐reported history of heart failure, coronary heart disease, angina, myocardial infarction, or stroke. Blood pressure was measured using a mercury sphygmomanometer and averaging 4 readings. Levels of systolic blood pressure (SBP) and diastolic blood pressure (DBP) were defined as the average of up to 6 measurements (3 each at the end of the home interview and at the physical examination).24 High‐density lipoprotein cholesterol (HDL‐C) was measured after the precipitation of the other lipoprotein/divalent cation mixture, with low HDL‐C defined as <40 mg/dL for men and <50 mg/dL for women.25 Total cholesterol and triglycerides were measured enzymatically after hydrolyzation to glycerol on a Hitachi 704 analyzer (Roche Diagnostics, Indianapolis, IN). We defined serum 25‐hydroxyvitamin D levels using tertiles: <50.9 nmol/L (20.4 ng/mL) was the first tertile, 50.9 nmol/L (20.4 ng/mL) to 73.6 nmol/L (29.5 ng/mL) was the second tertile, and >73.6 nmol/L (29.5 ng/mL) was the third and highest tertile. Hypertension was defined as a mean SBP ≥140 mm Hg or mean DBP ≥90 mm Hg, or individuals who were told by their physician that they had hypertension. Diabetes mellitus (DM) was defined as a fasting glucose level ≥126 mg/dL, a glucose level ≥200 mg/dL when not fasting, or patient self‐report of DM diagnosis by a doctor or if they are on hyperglycemic therapy.
Spirometry and Definition of Chronic Obstructive Pulmonary Disease
Each subject in this study was asked to perform ≥5 forced vital capacity (FVC) maneuvers, with an additional goal of meeting the American Thoracic Society (ATS) acceptability and reproducibility criteria.3 One‐second forced expiratory volumes (FEV1) were measured using a dry rolling‐seal spirometer. The spirometer used a digital shaft encoder to measure volume with a volume resolution of 2.6 mL and a sampling interval of 10 ms. All of the digital volume‐time curves were saved on digital tape (as much as 20 seconds of exhalation), allowing recalculation of all parameters and test performance with regard to ATS acceptability and reproducibility criteria. The spirometry system has been independently tested and found to exceed the ATS spirometry equipment recommendations.26 The spirometric system measures of the 1979 and updated 1987 National Institute for Occupational Safety and Health (NIOSH) and the ATS were used, which follow the recommendations of the American Heart Association. Expected FVC values were determined using equations develop by Hankinson et al.26, 27, 28 From prebronchodilator spirometric data, we utilized FEV1 and FEV1/FVC ratios for categorizing those with COPD.18 Of the individuals with a FEV1/FVC ratio <70%, severities were categorized as mild (FEV1 ≥ 80%) and moderate/severe (FEV1 ≤ 80%; Global Initiative for Obstructive Lung Disease [GOLD] criteria). Measures of weight (in kg) and height (in cm) were also available.
Statistical Analysis
We initially used the χ2 test of proportions to compare the proportions and means of the relevant risk factors throughout the various severities of COPD. Then, Cox proportional hazards regression examined combined categories of COPD and vitamin D in relation to CVD and total mortality, as well as the hazard ratio (HR) and the 95% confidence interval (CI) for total and CVD mortality. For the analyses, those in the third vitamin D tertile with no COPD were used as the reference group. Finally, we also calculated the total and CVD mortality rates per 1000 person‐years throughout the severity groups, and stratified by the vitamin D tertile groups. This statistical analysis used SAS version 9.0.3 (SAS Institute, Cary NC) and SUDAAN (Survey Data Analysis) version 9.0.1 (Research Triangle Institute, Research Triangle Park, NC).
Results
Patients with COPD had a higher mean age, SBP, and history of smoking. These individuals were also more likely to be Caucasian males who have hypertension and to die from pulmonary and CVD causes of mortality. Increased severity of COPD was significantly associated with decreased FEV1, FEV1/FVC ratio, body mass index, and triglyceride levels, and the proportion of Hispanics was also lower among higher‐severity groups (Table 1). Follow‐up time for both CVD and total mortality from the time of survey averaged 8.2 ± 2.5 years.
Table 1.
Means and Proportions of Demographics and Risk Factors According to Different Severities of COPD
| No COPD, n = 5877 (75.9) | Mild COPD, n = 932 (12.0) | Mod/Sev COPD, n = 937 (12.1) | P Value Across All Categories | |
|---|---|---|---|---|
| Age, y | 57.5 ± 13.1 | 67.8 ± 12.5a | 66.0 ± 11.9a | <0.001 |
| FEV1, L/sec | 2.8 ± 0.8 | 2.5 ± 0.7a | 1.8 ± 0.6a | <0.001 |
| FEV1/FVC ratio | 0.8 ± 0.1 | 0.7 ± 0.0a | 0.6 ± 0.1a | <0.001 |
| TC, mg/dL | 218.2 ± 43.5 | 218.3 ± 44.2 | 214.2 ± 43.5b | 0.0356 |
| HDL‐C, mg/dL | 50.7 ± 15.9 | 50.0 ± 15.2 | 51.3 ± 16.6 | 0.2229 |
| SBP, mm Hg | 131.8 ± 19.7 | 137.5 ± 19.4a | 137.2 ± 19.7a | <0.001 |
| BMI, kg/m2 | 28.1 ± 5.6 | 26.1 ± 4.5a | 26.2 ± 5.6a | <0.001 |
| TG, mg/dL | 165.9 ± 137.4 | 158.3 ± 102.0 | 154.2 ± 108.0a | 0.020 |
| Male sex | 2634 (44.8) | 576 (61.8)a | 555 (59.2)a | <0.001 |
| DM (%) | 722 (12.3) | 90 (9.7)c | 104 (11.1) | 0.292 |
| First vit D tertile | 1813 (32.0) | 403 (44.9)a | 286 (32.3) | <0.001 |
| Second vit D tertile | 1901 (33.6) | 288 (32.1) | 275 (31.1) | 0.271 |
| Third vit D tertile | 1944 (34.4) | 206 (23.0)a | 323 (36.5) | <0.001 |
| CRP >3 mg/L | 1929 (34.4) | 270 (29.7)c | 409 (46.2)a | <0.001 |
| Hypertension (%) | 2881 (49.0) | 520 (55.8)a | 560 (59.8)a | <0.001 |
| Ethnicity | ||||
| Caucasian | 2824 (50.0) | 641 (68.8)a | 609 (65.0)a | <0.001 |
| African | 1391 (24.6) | 76 (8.2)a | 281 (30.0)a | <0.001 |
| Hispanic | 1434 (25.4) | 215 (23.1) | 47 (5.0)a | <0.001 |
| Current smokers | 1263 (21.5) | 234 (25.1)c | 406 (43.3)a | <0.001 |
| CVD‐related mortality (%) | 466 (7.9) | 130 (14.0)a | 139 (14.8)a | <0.001 |
| Total mortality (%) | 931 (15.6) | 275 (29.5)a | 386 (41.2)a | <0.001 |
Abbreviations: BMI, body mass index; CRP, C‐reactive protein; CVD, cardiovascular events; DM, diabetes mellitus; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; Mod/Sev, moderate or severe; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol; TG, triglycerides; vit D, vitamin D.
Data are presented as mean ± SD or n (%).
P < 0.001,
P < 0.01,
P < 0.05, in reference to No COPD group.
Analyses of CVD and total mortality per 1000 person‐years show the lowest vitamin D tertile (first tertile vitamin D) has the highest CVD and total mortality risk compared with the higher tertiles, and with increasing COPD severity, the risk of CVD mortality also increased (Figure 1). For total mortality, this trend is more noticeable (Figure 2).
Figure 1.
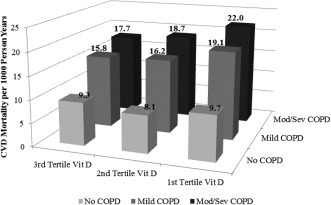
Cardiovascular disease (CVD) mortality per 1000 person‐years by vitamin D tertile group–COPD group (P < 0.001). Abbreviations: COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; mod/sev, moderate/severe; Vit D, vitamin D.
Figure 2.
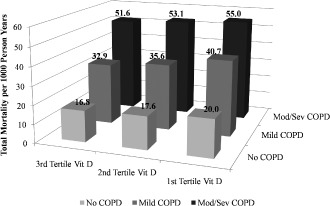
Total mortality per 1000 person‐years by vitamin D tertile group–COPD group (P < 0.001). Abbreviations: COPD, chronic obstructive pulmonary disease; mod/sev, moderate/severe; Vit D, vitamin D.
From Cox proportional hazards regression, increasing severity of COPD was also associated with increases in risk for CVD and total mortality. When comparing a patient with moderate or severe COPD in the first vitamin D tertile to someone in the reference group, the unadjusted HR and 95% CI for that category are 3.4 (2.2‐5.3) for CVD and 4.5 (3.3‐6.1) for total mortality. These findings were attenuated, but remained significant, after adjusting for baseline age, sex, ethnicity, body mass index, total cholesterol and HDL‐C, triglycerides, SBP, DM, smoking, and month of blood sampling, with HRs of 1.4 (95% CI: 0.9‐2.2) and 2.1 (95% CI: 1.5‐2.9), respectively (Table 2). We also performed these regressions stratified by sex and noted that females generally had a slightly higher HR than men; for example, in the highest risk group (moderate or severe COPD, first tertile vitamin D), the HR for total mortality in females was 2.4 (95% CI: 1.4‐4.0, P < 0.01), as opposed to 1.9 (95% CI: 1.3‐2.8, P < 0.001) for males, after adjustment.
Table 2.
Cox Regression of Combined Categories of COPD and Vitamin D in Relation to CVD and Total Mortality
| Total Mortality | CVD Death | |
|---|---|---|
| Unadjusted HR (95% CI) | ||
| No COPD, third tertile vit D | 1.0 | 1.0 |
| No COPD, second tertile vit D | 1.2 (1.0‐1.6) | 1.0 (0.7‐1.4) |
| No COPD, first tertile vit D | 1.7 (1.3‐2.1)a | 1.3 (0.9‐1.8) |
| Mild COPD, third tertile vit D | 2.1 (1.5‐3.0)a | 1.6 (1.1‐2.5)b |
| Mild COPD, second tertile vit D | 2.8 (2.0‐4.0)a | 1.9 (1.2‐3.2)b |
| Mild COPD, first tertile vit D | 3.2 (2.1‐4.9)a | 3.2 (1.8‐5.8)a |
| Mod/sev COPD, third tertile vit D | 3.5 (2.6‐4.8)a | 1.9 (1.2‐3.2)c |
| Mod/sev COPD, second tertile vit D | 4.5 (3.3‐6.1)a | 2.2 (1.3‐3.6)c |
| Mod/sev COPD, first tertile vit D | 4.5 (3.3‐6.1)a | 3.4 (2.2‐5.3)a |
| Adjusted HR (95% CI) | ||
| No COPD, third tertile vit D | 1.0 | 1.0 |
| No COPD, second tertile vit D | 1.1 (0.9‐1.4) | 0.9 (0.6‐1.2) |
| No COPD, first tertile vit D | 1.5 (1.1‐1.9)c | 1.2 (0.8‐1.6) |
| Mild COPD, third tertile vit D | 1.1 (0.8‐1.5) | 0.7 (0.5‐1.1) |
| Mild COPD, second tertile vit D | 1.0 (0.7‐1.4) | 0.6 (0.3‐1.0)b |
| Mild COPD, first tertile vit D | 1.2 (0.8‐1.8) | 1.1 (0.6‐1.9) |
| Mod/sev COPD, third tertile vit D | 1.8 (1.3‐2.4)a | 0.9 (0.5‐1.4) |
| Mod/sev COPD, second tertile vit D | 1.8 (1.3‐2.5)a | 0.7 (0.4‐1.2) |
| Mod/sev COPD, first tertile vit D | 2.1 (1.5‐2.9)a | 1.4 (0.9‐2.2) |
Abbreviations: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; HR, hazard ratio; Mod/sev, moderate or severe; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; vit D, vitamin D.
P < 0.001,
P < 0.05,
P < 0.01, adjusted for baseline age, sex, ethnicity, BMI, TC, TG, HDL‐C, SBP, DM, smoking, and month of blood sampling.
Discussion
Our study suggests an association between 25‐hydroxyvitamin D and CVD and total mortality among people with COPD, with decreasing tertiles of vitamin D levels being associated with increases in total and CVD mortality among those without and with mild or moderate/severe COPD. Our study is the first population‐based prospective study to demonstrate the combined associations of vitamin D levels and COPD with mortality but is consistent with other cross‐sectional studies examining relations of COPD with vitamin D or with mortality separately.19, 20, 21, 22, 29 Black et al examined spirometric data from NHANES III, a cross‐sectional survey of 14 091 US adults age ≥21 years, and after adjustment for potential confounders, a strong relationship was found between serum levels of 25‐hydroxyvitamin D and pulmonary function assessed using FEV1 and FVC.19 Janssens et al also reported that vitamin D deficiency occurs frequently in COPD and correlates with COPD severity.20 Melamed et al showed that the lowest quartile of 25‐hydroxyvitamin D levels (<17.8 ng/mL) is independently associated with all‐cause mortality in the general population.21 In 2011, Ford et al also demonstrated that concentrations of vitamin D were weakly and inversely related to all‐cause mortality in US adults (<20 ng/mL; HR: 1.65).22
Chronic obstructive pulmonary disease has become a major medical burden to society, and, despite the need for urgent treatment and prevention, treatment options remain very limited. When focusing on the calcemic effects, vitamin D insufficiency is best described as 25‐hydroxyvitamin D <20 ng/mL. A sensitive parameter to determine vitamin D deficiency is using the serum levels of parathyroid hormone. Based on observational studies, several experts have suggested that, for noncalcemic effects, serum levels of ≥30 ng/mL are required. Patients with COPD are often at higher risk of becoming vitamin deficient for a variety of associated factors: low food intake, reduced capacity in vitamin synthesis of the skin due to aging and smoking, the absence of outdoor activity and sun exposure, impaired renal function, and a lower storage capacity in muscles and fat.30 As 25‐hydroxyvitamin D is an inactive metabolite involved in vitamin D metabolism, this serves as a useful measure to vitamin D studies because of the long half‐life of 2 to 3 weeks, as opposed to the bioactive 1,25‐hydroxyvitamin D, which has a half‐life of 4 to 6 hours.
To our knowledge, this is the first study that describes all‐cause and CVD mortality rates according to extent of COPD and vitamin D deficiency. Because CVD is known as a comorbidity of COPD, vitamin D deficiency may have a direct impact on mortality.31 Also, although substantial studies have demonstrated the association of vitamin D and CVD, the mechanism remains unclear. A recent study by Pilz et al shows that vitamin D sufficiency can have a protective effect against all‐cause mortality, but it did not draw conclusions on its specific effects on cardiovascular mortality.32
However, a recent study by Holmgaard et al of 462 patients with moderate to severe COPD through a 10‐year follow‐up concluded that 25‐hydroxyvitamin D levels do not seem to be associated with mortality rate, suggesting little or no role of 25‐hydroxyvitamin D in disease progression in patients with moderate or severe COPD.23 This is possibly due to the power issue from low sample size and the high degree of ethnic homogeneity in the patient group consisting of those with severe levels of COPD (median FEV1 38.5% predicted). Similarly, another year‐long study concluded that among 973 patients diagnosed with severe COPD (mean FEV1 40% predicted), baseline levels of 25‐hydroxyvitamin D were not predictive of acute exacerbations of COPD.30 Furthermore, in a randomized trial among 182 patients who had moderate to severe levels of COPD and recent exacerbations, high doses of vitamin D did not reduce the incidence of acute exacerbations of COPD, except among those with severe vitamin D deficiencies (<10 ng/mL) compared with baseline.14 Possibly, the studies that have demonstrated an association may have overestimated mortality due to cardiovascular‐associated risk factors. After adjustment for confounders in our study, the mortality and CVD death trends in our study were diminished, suggesting that at least part of our observed associations may be explained by age and cardiovascular risk factors.
Previous studies have targeted moderate to severe cases of COPD, which may not be viable stages for treatment due to advancement of the disease.14, 23, 33 For example, the protective effects of vitamin D may be attenuated in patients with severe COPD or those with prolonged smoking. However, milder COPD cases are often neglected because symptoms are not as prevalent; yet these may be the ideal targets for early intervention. Future studies should consider the potential benefits of targeting the earlier stages of COPD severity in assessing the impact of vitamin D. With the conflicting findings, the precise role of vitamin D in mitigating mortality remains ambiguous, and future prospective, large, population‐based clinical trials need to be conducted to produce more definitive answers.
The strength of our study was the benefit of a US‐population‐representative sample with a standardized collection of both CVD risk‐factor and pulmonary‐function data to classify COPD. However, due to the cross‐sectional nature of our study, direct causation could not be established. In addition, despite adjustment for common confounders, not every potential variable could be controlled for, and it is possible that other factors we did not adjust for would explain additional variance in the relation between vitamin D and mortality. Finally, the vitamin D data used in the study did not precisely account for the seasonal variation in vitamin D concentration, which may have confounded the results.34 However, the timing of the blood collection in NHANES III participants minimizes the effects of seasonal variation from sunlight exposure on vitamin D levels because the data are collected primarily during warm‐weather seasons. We also attempted to partially control for this by including the month of blood sampling as a covariate in our multivariate models, which did not change the HRs of the analyses upon addition. Additionally, the GOLD criteria were used to determine the COPD classification and may have overestimated the severities in the largely older population in this sample, who would generally have lower FEV1 levels. Finally, though we initially attempted to separate the moderate and severe classification, due to the relatively low number of subjects fitting these classifications causing a power issue, we combined the 2 categories as an aggregate.
Conclusion
Our study suggests that although lower levels of vitamin D can predict further increases in total and CVD mortality associated with COPD, much of this association may be explained by age and cardiovascular risk factors rather than vitamin D level. Clinical trials are needed to show if vitamin D supplementation can reduce risk in those with COPD before routine vitamin D measurement can be recommended.
Presented in part at the American College of Chest Physicians Conference, October 20–25, 2012, Atlanta, Georgia.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Gan WQ, Man SF, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta‐analysis. Thorax. 2004;59:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sin DD, Anthonisen NR, Soriano JB, et al. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28:1245–1257. [DOI] [PubMed] [Google Scholar]
- 3. American Thoracic Society . Standardization of spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. [DOI] [PubMed] [Google Scholar]
- 4. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. [DOI] [PubMed] [Google Scholar]
- 5. Berger JS, Sanborn TA, Sherman W, et al. Effect of chronic obstructive pulmonary disease on survival of patients with coronary heart disease having percutaneous coronary intervention. Am J Cardiol. 2004;94:649–651. [DOI] [PubMed] [Google Scholar]
- 6. Celli BR, Cote CG, Marin JM, et al. The body‐mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. [DOI] [PubMed] [Google Scholar]
- 7. Curkendall SM, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16:63–70. [DOI] [PubMed] [Google Scholar]
- 8. Foster TS, Miller JD, Marton JP, et al. Assessment of the economic burden of COPD in the U.S.: a review and synthesis of the literature. COPD. 2006;3:211–218. [DOI] [PubMed] [Google Scholar]
- 9. Group TTS. The TORCH (Towards a Revolution in COPD Health) survival study protocol. Eur Respir J. 2004;24:206–210. [DOI] [PubMed] [Google Scholar]
- 10. Tashkin DP, Celli B, Senn S, et al. A 4‐year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. [DOI] [PubMed] [Google Scholar]
- 11. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. [DOI] [PubMed] [Google Scholar]
- 12. Bischoff‐Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25‐hydroxyvitamin D for multiple health outcomes [published corrections appear in Am J Clin Nutr. 2006;84:1253 and Am J Clin Nutr. 2007;86:809]. Am J Clin Nutr. 2006;84:18–28. [DOI] [PubMed] [Google Scholar]
- 13. Thomas MK, Lloyd‐Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–783. [DOI] [PubMed] [Google Scholar]
- 14. Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156:105–114. [DOI] [PubMed] [Google Scholar]
- 15. Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. [DOI] [PubMed] [Google Scholar]
- 16. Dawson‐Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151–1154. [DOI] [PubMed] [Google Scholar]
- 17. Decramer M, Cooper CB. Treatment of COPD: the sooner the better? Thorax. 2010;65:837–841. [DOI] [PubMed] [Google Scholar]
- 18. Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease [published correction appears in Thorax. 2008;63:753]. Thorax. 2002;57:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Black PN, Scragg R. Relationship between serum 25‐hydroxyvitamin D and pulmonary function in the Third National Health and Nutrition Examination Survey. Chest. 2005;128:3792–3798. [DOI] [PubMed] [Google Scholar]
- 20. Janssens W, Bouillon R, Claes B, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D‐binding gene. Thorax. 2010;65:215–220. [DOI] [PubMed] [Google Scholar]
- 21. Melamed ML, Michos ED, Post W, et al. 25‐hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ford ES, Zhao G, Tsai J, et al. Vitamin D and all‐cause mortality among adults in USA: findings from the National Health and Nutrition Examination Survey Linked Mortality Study. Int J Epidemiol. 2011;40:998–1005. [DOI] [PubMed] [Google Scholar]
- 23. Holmgaard DB, Mygind LH, Titlestad IL, et al. Serum vitamin D in patients with chronic obstructive lung disease does not correlate with mortality—results from a 10‐year prospective cohort study. PLoS One. 2013;8:e53670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. US Department of Health and Human Services . Plan and operation of the third National Health and Nutrition Examination Survey, 1988–1994. Vital and Health Statistics, 1994; Series 1, No. 32. 1994. http://www.cdc.gov/nchs/nhanes/nh3data.htm. Accessed January 17, 2012.
- 25. Menke A, Muntner P, Wildman R, et al. Measures of adiposity and cardiovascular disease risk factors. Obesity (Silver Spring). 2007;15:785–795. [DOI] [PubMed] [Google Scholar]
- 26. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
- 27. US Centers for Disease Control and Prevention , Third National Health and Nutrition Examination Survey III. Spirometry Procedure Manual. http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/spiro.pdf. Published August 1988. Accessed January 17, 2012. [Google Scholar]
- 28. Hankinson JL, Crapo RO, Jensen RL. Spirometric reference values for the 6‐s FVC maneuver. Chest. 2003;124:1805–1811. [DOI] [PubMed] [Google Scholar]
- 29. Landbo C, Prescott E, Lange P, et al. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1856–1861. [DOI] [PubMed] [Google Scholar]
- 30. Janssens W, Lehouck A, Decramer M, et al. Vitamin D and chronic obstructive pulmonary disease In: Litonjua AA, ed. Vitamin D and the Lung: Mechanisms and Disease Associations. New York, NY: Springer‐Humana Press; 2012:239–260. [Google Scholar]
- 31. Lee HM, Lee J, Lee K, et al. Relation between COPD severity and global cardiovascular risk in US adults. Chest. 2012;142:1118–1125. [DOI] [PubMed] [Google Scholar]
- 32. Pilz S, Tomaschitz A, März W, et al. Vitamin D, cardiovascular disease and mortality. Clin Endocrinol (Oxf). 2011;75:575–584. [DOI] [PubMed] [Google Scholar]
- 33. Kunisaki KM, Niewoehner DE, Connett JE. Vitamin D levels and risk of acute exacerbations of chronic obstructive pulmonary disease: a prospective cohort study. Am J Respir Crit Care Med. 2012;185:286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Boer IH, Levin G, Robinson‐Cohen C, et al. Serum 25‐hydroxyvitamin D concentration and risk for major clinical disease events in a community‐based population of older adults: a cohort study. Ann Intern Med. 2012;156:627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]


