Abstract
Background
Contrast‐induced nephropathy (CIN) is associated with significantly increased morbidity and mortality after percutaneous coronary intervention (PCI). Patients with acute coronary syndrome (ACS) are at higher risk for CIN. N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) is closely linked to the prognosis as a strong predictor of both short‐ and long‐term mortality in patients with ACS.
Hypothesis
We hypothesized that NT‐proBNP levels on admission can predict the development of CIN after PCI for ACS.
Methods
A total of 436 patients (age 62.27 ± 13.01 years; 64.2% male) with ACS undergoing PCI enrolled in this study. Admission NT‐proBNP levels were measured before PCI. Serum creatinine values were measured before and within 72 hours after the administration of contrast agents. Patients were divided into 2 groups: CIN group and no‐CIN group. CIN was defined as an increase in serum creatinine level of ≥0.5 mg/dL or ≥25% above baseline within 72 hours after contrast administration.
Results
CIN developed in 63 patients (14.4%). Baseline NT‐proBNP levels were significantly higher in patients who developed CIN compared to those who did not develop CIN (median 774 pg/mL, interquartile range 177.4–2184 vs median 5159 pg/mL, interquartile range 2282–9677, respectively; P < 0.001). Multivariate analysis found that NT‐proBNP (odds ratio [OR]: 3.448, 95% confidence interval [CI]: 1.394‐8.474, P = 0.007) and baseline creatinine (OR: 6.052, 95% CI: 1.860‐19.686, P = 0.003) were independent predictors of CIN.
Conclusions
Admission NT‐proBNP level is an independent predictor of the development of CIN after PCI in ACS.
Introduction
Contrast‐induced nephropathy (CIN) is a serious complication of invasive cardiovascular procedures. The incidence of CIN is 2% for the general population. However, patients undergoing percutaneous coronary intervention (PCI) are at greater risk, and patients with diabetes or previous renal impairment have a risk of almost 50%.1, 2 Development of CIN after PCI is associated with poor clinical outcomes including prolonged hospitalization, increased costs, increased rates of end‐stage renal failure, myocardial infarction, repeat revascularization, and short‐ and long‐term mortality.3, 4, 5, 6 Furthermore, patients with acute coronary syndrome (ACS) have a 3‐fold higher risk of developing CIN.7, 8 Because CIN occurs more frequently after urgent PCI in patients with ST‐segment elevation myocardial infarction (STEMI) and non‐STEMI,9 objective and rapidly available and reliable markers may be useful for identification of patients at risk of development of CIN.
N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) is synthesized and secreted from the cardiac ventricles in response to increased ventricular wall stress,10 but myocardial ischemia and infarction may also stimulate its release.11, 12 This marker is closely linked to the prognosis as a strong predictor of both short‐ and long‐term mortality in patients with ACS.13, 14, 15, 16 NT‐proBNP is associated with poor hemodynamics, neurohormonal responses, and inflammation in ACS patients, all of which play a role in the development of CIN.17, 18
In the present study, we sought to investigate whether NT‐proBNP level on admission is an independent risk factor that predicts the development of CIN in patients with ACS with ST‐segment elevation (STE‐ACS) and unstable angina/non–ST‐segment elevation (NSTE‐ACS) to undergo interventional therapy
Methods
Study Population
Between January 2013 and December 2013, a total of 530 consecutive patients (mean age, 62.27 ± 13.01 years; 64.2% male) were identified with acute STE‐ACS or NSTE‐ACS undergoing emergency PCI. After an evaluation according to inclusion and exclusion criteria, 436 patients were enrolled in our study (Figure 1). Patients with STE at the J point in 2 or more consecutive leads (with the cutoff point being >0.2 mV in leads V1, V2, or V3, and >0.1 mV in the other leads) and elevation of cardiac troponin T level greater than the upper limit of normal were defined as having STE‐ACS. Patients with ST‐segment depression, T‐wave inversion, or no electrocardiographic abnormalities and/or elevation of cardiac troponin T level greater than the upper limit of normal were defined as having NSTE‐ACS. We excluded patients receiving long‐term peritoneal or hemodialysis treatment, or those who underwent a renal transplantation or received administration of metformin, nonsteroidal anti‐inflamamatory drugs, aminoglycosides, or acetylcysteine 1 week before or after PCI. Patients were also excluded if they had intra‐aortic balloon pump support before PCI because of cardiogenic shock, cardiac surgery for coronary revascularization, severe chronic heart failure (New York Heart Association class ≥3), and contrast exposure 2 weeks before PCI or died during PCI.
Figure 1.
Diagram of the 436 patients who were enrolled in the study after inclusion and exclusion criteria evaluation. Abbreviations: ACS, acute coronary syndrome; NSTE, non–ST‐segment elevation; PCI, percutaneous coronary intervention; STE, ST‐segment elevation.
The study protocol was approved by the local ethics committee, and written informed consent was obtained from all participants.
Study Protocol and Definitions
Baseline serum creatinine and NT‐proBNP levels were measured before angiography. NT‐proBNP measurements were performed in plasma on an Elecsys 2010 analyzer, a commercially available electrochemiluminescent sandwich immunoassay (Elecsys proBNP; Roche Diagnostics, Mannheim, Germany). The lowest and highest detection limits of the assay were at 5 to 35.000 pg/mL. High‐sensitivity C‐reactive protein (hs‐CRP) levels were also measured. Immediately after intervention, all patients underwent hydration with intravenous isotonic saline (0.9%) at a rate of 1 mL/kg/h for 12 hours (or 0.5 mL/kg/h for 12 hours in cases of overt heart failure). Any nephrotoxic medications (ie, metformin, nonsteroidal anti‐inflammatory drugs) were suspended on admission. Serum creatinine was also measured at 24, 48, and 72 hours after contrast medium administration. Patients were divided into 2 groups: CIN group and no‐CIN group. CIN was defined as an increase in serum creatinine level of ≥0.5 mg/dL or ≥25% above baseline within 72 hours after contrast administration.19 High‐contrast volume was defined as the administration of a contrast volume of >140 mL.20 The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) equation.21 Left ventricular ejection fraction (LVEF) was measured using the Simpson method according to the recommendations of the American Society of Echocardiography.22
Coronary Interventions and Medications
Coronary angiography (CA) (Siemens Axiom Artis zee 2011; Siemens Healthcare, Erlangen, Germany) was performed using the femoral approach according to standard clinical practice. On admission, all patients in the emergency department received a bolus of 5,000 U of intravenous unfractionated heparin, followed by additional intraprocedural boluses to maintain an activated clotting time of 200 to 250 seconds (>300 seconds when tirofiban was not used), acetylsalicylic acid (300 mg orally), and clopidogrel loading dose of 600 mg orally. Nonionic, low‐osmolar contrast medium (iohexol, Omnipaque 350 mg/mL; GE Healthcare, Cork, Ireland) was used to visualize the coronary arteries. PCI was performed immediately after diagnostic CA when appropriate. Coronary stenting was performed using standard techniques.23 Thrombolysis in Myocardial Infarction grade 3 coronary flow in the treated vessel with a residual stenosis <50% was considered successful PCI. The use of glycoprotein IIb/IIIa inhibitor as well as bare‐metal or drug‐eluting stents was left to the discretion of the interventional cardiologist. Additional use of thrombectomy was recommended depending on thrombus in the infarct‐related artery. After CA, all patients continued to take aspirin (100 mg/d orally) indefinitely and clopidogrel (75 mg/d) for at least 12 months.
Statistical Analysis
We decided the sample size of the study by using a program named Power & Sample Size Calculator (Statistical Solutions, Cottage Grove, WI; www.statisticalsolutions.net/pss_calc.php). Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) for Windows, version 18.0 (SPSS Inc., Chicago, IL). Continuous variables were compared using the Student t test. In case of non‐normal distribution, nonparametric methods were used (Mann–Whitney U test). All values are expressed as mean ± standard deviation or median and interquartile range. Models were developed with stepwise techniques and by consideration of potential confounding factors, and of variables that are shown to be statistically significant at univariate analysis. Results of this model were presented as odds ratio (OR) and 95% confidence interval (CI). To identify independent parameters associated with CIN, multivariable logistic regression analysis was used. According to the results of univariate analysis, age, smoking, LVEF, hemoglobin, creatinine, NT‐proBNP ≥2149 pg/mL, total cholesterol, low‐density lipoprotein (LDL) cholesterol, hs‐CRP, uric acid, troponin T, SYNTAX (SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery) score, presence of multivessel disease (MVD), and presence of chronic total occlusion (CTO) were analyzed with multivariate logistic regression model. The receiver operating characteristic (ROC) analysis was performed to determine the best cutoff value of NT‐proBNP, and the sensitivity and specificity at that point were obtained for predicting the development of CIN. P values <0.05 were considered statistically significant.
Results
The study population consisted of 436 patients (mean age, 62.27 ± 13.01 years and 64.2% male) who had measurements of baseline serum NT‐proBNP and creatinine, followed by additional daily measures of creatinine up to 72 hours. A total of 63 patients (14.4%) developed CIN.
The baseline clinical characteristics of the patient population stratified by CIN are summarized in Table 1. Patients in the CIN group were significantly older than those in the no‐CIN group (72.59 ± 12.29 years vs 60.59 ± 12.79 years, respectively, P < 0.001) and had a significantly lower proportion of smoking (P < 0.001) compared with patients in the no‐CIN group. LVEF was also lower in the CIN group (P < 0.001). There were no significant differences between the groups regarding gender, hypertension, diabetes mellitus, hyperlipidemia, prior myocardial infarction, prior stroke, prior medications, in‐hospital medications, and type of ACS.
Table 1.
Baseline Characteristics of the Study Patients
| Variable | No‐CIN Group, n = 373, 85.6% | CIN Group, n = 63, 14.4% | P Value |
|---|---|---|---|
| Age, y | 60.59 ± 12.29 | 72.22 ± 12.79 | <0.001 |
| Male gender, n (%) | 241 (64.6) | 39 (61.9) | 0.679 |
| BMI, kg/m2 | 27.85 ± 4.53 | 27.13 ± 4.47 | 0.354 |
| Systolic blood pressure, mm Hg | 129 ± 25 | 133 ± 32 | 0.257 |
| Diastolic blood pressure, mm Hg | 78 ± 14 | 78 ± 16 | 0.907 |
| Hypertension, n (%) | 161 (43.2) | 35 (55.6) | 0.067 |
| Diabetes mellitus, n (%) | 119 (31.9) | 23 (36.5) | 0.471 |
| Smoking, n (%) | 176 (47.2) | 10 (15.9) | <0.001 |
| Hyperlipidemia, n (%) | 110 (29.5) | 11 (17.5) | 0.128 |
| Prior CABG, n (%) | 20 (5.4) | 3 (4.8) | 0.844 |
| Prior myocardial infarction, n (%) | 36 (9.7) | 5 (7.9) | 0.666 |
| LVEF, % | 47 ± 10 | 40 ± 10 | <0.001 |
| LVEF <40%, % | 20.7 | 38.6 | 0.003 |
| Type of ACS, n (%) | |||
| STE‐ACS | 234 (62.7) | 38 (60.3) | 0.714 |
| NSTE‐ACS | 139 (37.3) | 25 (39.7) | |
| Treatment before admission, % | |||
| ACEI or ARB | 28.1 | 31.3 | 0.794 |
| Statins | 28.3 | 27.2 | 0.814 |
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; CIN, contrast‐induced nephropathy; LVEF, left ventricular ejection fraction; NSTE, non–ST‐segment elevation; STE, ST‐segment elevation.
The baseline laboratory measurements of the study patients are shown in Table 2. NT‐proBNP levels at baseline were significantly higher in patients who developed CIN compared to those who did not (median, 774 pg/mL [interquartile range, 177.4–2184] vs median, 5159 pg/mL [interquartile range, 2282–9677], respectively; P < 0.001). NT‐proBNP levels according to risk of developing CIN are shown in Table 2 and Figure 2A.
Table 2.
Baseline Biochemical and Hematologic Measurements of Patients
| Variable | No‐CIN Group, n = 373 | CIN Group, n = 63 | P Value |
|---|---|---|---|
| Serum glucose on admission | 158 ± 87 | 158 ± 78 | 0.962 |
| HbA1c, % | 7.00 ± 1.98 | 6.97 ± 1.90 | 0.916 |
| Serum creatinine, mg/dL | 1.06 ± 0.27 | 1.37 ± 0.38 | <0.001 |
| eGFR, mL/min per 1.73 m2 | 71.9 ± 19.8 | 50.1 ± 17.6 | <0.001 |
| White blood cell count, × 103/mm3 | 10.99 ± 3.51 | 11.28 ± 3.86 | 0.557 |
| Hemoglobin, g/L | 14.11 ± 1.87 | 12.72 ± 2.09 | <0.001 |
| Platelet count, × 103/mm3 | 241 ± 66 | 247 ± 92 | 0.492 |
| Mean platelet volume, fL | 8.70 ± 1.03 | 8.89 ± 1.31 | 0.189 |
| NT‐proBNP, pg/mL | 774 (177.4–2184) | 5159 (2282–9677) | <0.001 |
| Total cholesterol, mg/dL | 191 ± 49 | 176 ± 51 | 0.030 |
| Triglyceride, mg/dL | 131 (35–815) | 112 (39–624) | 0.454 |
| Low‐density lipoprotein, mg/dL | 121 ± 42 | 106 ± 40 | 0.010 |
| High‐density lipoprotein, mg/dL | 41 ± 10 | 42 ± 11 | 0.765 |
| hs‐CRP, mg/L | 7.15 ± 3.78 | 8.48 ± 3.88 | 0.014 |
| Peak CK‐MB, ng/mL | 35.6 (0.78–425) | 50.4 (2.25–419) | 0.318 |
| Peak troponin T, ng/mL | 1060 (4.13–10000) | 1778 (43.9–10000) | 0.029 |
| Serum uric acid, mg/dL | 5.49 ± 1.53 | 6.53 ± 1.76 | <0.001 |
Abbreviations: CIN, contrast‐induced nephropathy; CK‐MB, creatine kinase‐myocardial band; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; hs‐CRP, high‐sensitivity C‐reactive protein; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Figure 2.
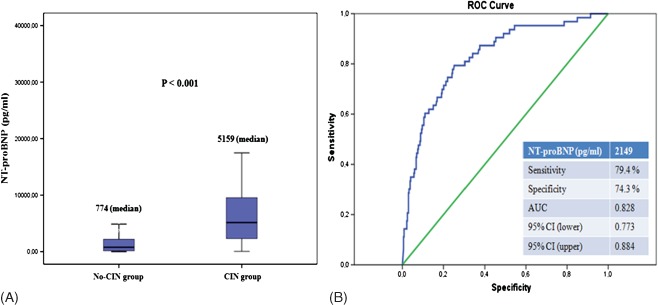
(A) Comparison of serum NT‐proBNP levels between groups. (B) The receiver operating characteristic (ROC) curve analysis for serum NT‐proBNP levels in predicting of postprocedural development of CIN. AUC = 0.828 (0.773‐0.884). Abbreviations: AUC, area under the curve; CI, confidence interval; CIN, contrast‐induced nephropathy; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
In addition to having elevated NT‐proBNP, patients who developed CIN had significantly higher baseline serum creatinine, peak troponin T, and uric acid levels, but significantly lower baseline hemoglobin, total cholesterol, LDL, and eGFR than those in whom CIN did not occur.
Patients with CIN had a higher prevalence of MVD, presence of CTO in nonculprit vessels, and higher SYNTAX score, and lower stent diameter compared with those without CIN. However, the location of the culprit lesion, the contrast volume, procedure duration, and procedure success rate did not significantly differ between patients who developed CIN and those who did not. Angiographic and procedural characteristics of the study patients are listed in Table 3.
Table 3.
Angiographic and Procedural Characteristics and Medications of Patients
| Variable | No‐CIN Group, n = 373 | CIN Group, n = 63 | P Value |
|---|---|---|---|
| Total time of procedure, min | 37.49 ± 16.58 | 40.98 ± 16.09 | 0.162 |
| Total amount of contrast, mL | 166 ± 66 | 177 ± 78 | 0.280 |
| High‐contrast volume, n (%) | 194 (61.0) | 32 (62.7) | 0.813 |
| Multivessel disease, n (%) | 207 (55.5) | 48 (76.2) | 0.002 |
| Chronic total occlusion, n (%) | 65 (17.4) | 20 (31.7) | 0.008 |
| Syntax score | 15.29 ± 8.29 | 21.49 ± 10.69 | <0.001 |
| Culprit vessel, n (%) | |||
| Left main coronary artery | 1 (0.3) | 0 (0) | 0.487 |
| Left anterior descending artery | 178 (47.7) | 31 (49.2) | |
| Left circumflex artery | 74 (19.8) | 12 (19.0) | |
| Right coronary artery | 112 (30) | 19 (30.2) | |
| Saphenous vein graft | 8 (2.1) | 0 (0) | |
| Stent implantation, n (%) | 349 (93.6) | 53 (84.1) | 0.010 |
| Total length of stent, mm | 24.00 ± 11.15 | 25.17 ± 11.36 | 0.479 |
| Stent diameter, mm | 3.17 ± 0.43 | 3.04 ± 0.35 | 0.037 |
| Procedural success, n (%) | 299 (80.2) | 44 (69.8) | 0.064 |
| In‐hospital medications, % | |||
| ACE inhibitor or ARB | 71 | 68.4 | 0.771 |
| Statin | 77.7 | 72.6 | 0.371 |
| Tirofiban | 36.5 | 26.2 | 0.120 |
| β‐blocker | 86.9 | 77.4 | 0.052 |
| Diuretic | 22.1 | 25.1 | 0.873 |
| Clopidogrel | 97.9 | 95.2 | 0.211 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CIN, contrast‐induced nephropathy.
The ROC curve analysis of NT‐proBNP for predicting CIN is shown in Figure 2B. NT‐proBNP ≥2149 pg/mL measured on admission had a 79.4% sensitivity and 74.3% specificity in predicting CIN.
The effects of multiple variables were analyzed with univariate and multivariate logistic regression analyses. According to the results of univariate analysis, age, smoking, LVEF, hemoglobin, creatinine, NT‐proBNP ≥2149 pg/mL, total cholesterol, LDL cholesterol, hs‐CRP, uric acid, troponin T, SYNTAX score, presence of MVD, and presence of CTO were analyzed with multivariate logistic regression model. At multivariate analyses, NT‐proBNP (OR: 3.448, 95% CI: 1.394‐8.474, P = 0.007) and admission creatinine (OR: 6.052, 95% CI: 1.860‐19.686, P = 0.003) were still significant independent predictors of the development of CIN after PCI in patients with ACS (Table 4).
Table 4.
Univariate and Multivariate Logistic Regression Analysis of the Association Between Contrast‐Induced Nephropathy and Multiple Parameters
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Age | 1.079 (1.053‐1.106) | <0.001 | 1.018 (0.982‐1.056) | 0.327 |
| Smoking | 0.211 (0.104‐0.427) | <0.001 | 0.473 (0.160‐1.333) | 0.157 |
| NT‐proBNP >2149 pg/mL | 11.111 (5.780‐21.276) | <0.001 | 3.448 (1.394‐8.474) | 0.007 |
| LVEF | 0.940 (0.914‐0.967) | <0.001 | 0.972 (0.934‐1.013) | 0.178 |
| Hemoglobin | 0.705 (0.614‐0.809) | <0.001 | 0.911 (0.759‐1.095) | 0.321 |
| Creatinine | 14.017 (6.071‐32.363) | <0.001 | 6.052 (1.860‐19.686) | 0.003 |
| Uric acid | 1.453 (1.238‐1.706) | <0.001 | 1.125 (0.895‐1.414) | 0.312 |
| hs‐CRP | 1.105 (1.019‐1.198) | 0.015 | 0.933 (0.832‐1.046) | 0.235 |
| Troponin T | 1.066 (1.037‐1.106) | 0.002 | 1.022(0.092‐1.036) | 0.490 |
| Total cholesterol | 0.993 (0.987‐0.999) | 0.030 | 1.017 (0.995‐1.040) | 0.130 |
| Low‐density lipoprotein | 0.990 (0.983‐0.998) | 0.010 | 0.980 (0.954‐1.006) | 0.135 |
| Syntax score | 1.077 (1.045‐1.110) | <0.001 | 1.030 (0.981‐1.081) | 0.233 |
| Multivessel disease | 2.564 (1.923‐4.793) | 0.003 | 0.665 (0.269‐1.645) | 0.377 |
| Chronic total occlusion | 2.204 (1.217‐3.992) | 0.009 | 1.251 (0.504‐3.104) | 0.629 |
Abbreviations: CI, confidence interval; hs‐CRP, high‐sensitivity C‐reactive protein; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Discussion
In the present study, we demonstrated that admission NT‐proBNP level is an independent predictor of the development of CIN in ACS patients undergoing PCI. Baseline creatinine level was the other independent predictor of CIN.
CIN after PCI is strongly associated with prolonged hospitalization, increased costs, increased rates of end‐stage renal failure, myocardial infarction, repeat revascularization, and early and late mortality.3, 4, 5 Patients who develop CIN and require dialysis after PCI have a 40% in‐hospital mortality and 80% 2‐year mortality rates.24 On the other hand, patients with ACS have a 3‐fold higher risk of CIN,3, 7, 8 and development of CIN is a sign of poor short‐ and long‐term prognosis after STE‐ACS and NSTE‐ACS despite successful early coronary revascularization.9, 25 Preexisting renal impairment, diabetes, congestive heart failure, advanced age, anemia, use of high‐contrast media volume, and reduced intravascular volume are risk factors for the development of CIN.7, 26, 27
Brain natriuretic peptides (BNPs) have a variety of effects, such as diuretic, natriuretic, and hypotensive action, along with inhibition of both the sympathetic nervous system and renin‐angiotensin‐aldosterone system (RAAS) as wells as endothelin secretion.28, 29 They also increases glomerular filtration rate by selectively dilating renal afferent arterioles and constructing renal efferent arterioles.30 Jarai et al31 demonstrated a relationship between (BNP) level on admission and development of CIN after primary PCI in STE‐ACS patients. Our study includes the whole spectrum of ACS, not only STE‐ACS, and we used NT‐proBNP, which is a more specific marker than BNP.
The N‐terminal portion of pro‐BNP appears more stable than BNP and is used as a marker instead of BNP.32 NT‐proBNP is synthesized and secreted by the left and right ventricles in response to increased left ventricular wall stretch10 and neurohormonal activation,33 and myocardial ischemia and infarction stimulate its release.11, 12, 34 Although natriuretic peptides primarily reflect central hemodynamics, their levels may also reflect a variety of processes including ischemia, inflammation, and oxidative stress.29, 35 Neurohormonal activation is known to increase oxidative stress and inflammation.36, 37 It was shown that NT‐proBNP is closely linked to the prognosis as a powerful predictor of both short‐ and long‐term mortality in patients with STEMI and ACS.13, 14, 15, 38
The pathophysiologic mechanisms of CIN is complex, multifactorial, and incompletely understood. Possible mechanisms include intrarenal vasoconstriction, reduced renal blood flow, medullary hypoxia, oxidative stress, inflammation, endothelial dysfunction, and direct tubular epithelial cell injury by contrast media.39 CIN is a specific type of cardio‐renal syndrome.40 Renal injury starts with hemodynamic effects of myocardial infarction. Abnormal hemodynamic states lead to a decrease in renal blood flow, and activation of immune and neurohormonal systems, such as sympathetic nervous system and RAAS, contribute to medullary hypoxia.41, 42 Direct cytotoxicity to renal tubular cells caused by the contrast agent then occurs. The results of the injury include renal vasoconstriction, impaired vasodilation, medullary hypoxia leading to oxidative stress, and direct tubular injury.43
Both hemodynamic impairment and neurohormonal activation involved in the development of CIN are known to stimulate NT‐proBNP.17 Therefore, NT‐proBNP is a part of neurohumoral signaling between the heart and kidney,18 which indicates the presence of pathologic processes underlying the cardio‐renal syndrome. As a diuretic, vasodilator, and negative inotrope, NT‐proBNP may also have a direct precipitating effect on the development of CIN. NT‐proBNP inhibits myocardial contractility by inhibiting sarcoplasmic reticulum Ca+2 ATPase, increasing matrix metalloproteinases, reducing the effects of catecholamines, and increasing the effect of nitric oxide44, 45 Thus, high NT‐proBNP levels in ACS may be responsible for systemic vasodilatation and renal hipoperfusion, which in turn potentiates CIN. Furthermore, plasma NT‐proBNP is found to be increased in a model of systemic inflammation in healthy men with normal heart function.46 Therefore, NT‐proBNP is accepted as an acute‐phase reactant,47 and some inflammatory cytokines, such as tumor necrosis factor, is known to stimulate NT‐proBNP.48 Thus, NT‐proBNP may be an indicator of increased immune response and inflammation in ACS, which play an important role in the development of CIN.
The hyperosmolar extracellular environment caused by radiocontrast agents induces oxidative stress via reactive oxygen species and causes renal tubular cell apoptosis.49, 50 Other factors associated with increased oxidative stress include activation of RAAS and matrix metalloproteinases, which are associated with increased NT‐proBNP levels.36 Matrix metalloproteinase‐9 activity is associated with oxidative stress in patients with ACS.51 Therefore, NT‐proBNP is an indicator of oxidative stress, which participates in the development of CIN.
In our study, MVD, presence of CTO in nonculprit vessels, high SYNTAX scores, and low stent diameters were associated with a higher incidence of CIN. In our opinion, high SYNTAX scores, MVD, and presence of CTO in nonculprit vessels are related to more serious presentations (eg, shock, arrhythmia), longer procedural duration, more contrast media use, higher incidence of no‐reflow, and lower LVEF. Thus, the renal vasoconstrictive response and the resulting cortico‐medullar hypoxia42 may be aggravated in patients with high scores. It appears (although not statistically significant) that procedural success was considerably lower in the CIN group as compared to the no‐CIN group. We think it may be due to the amount of contrast agent and total time of the procedure in the CIN group compared to the no‐CIN group (although not statistically significant).
Greater contrast volume use is associated with greater rates of CIN.52 However, it was shown that contrast media volume do not have an effect on CIN if iso‐osmolar contrast agents and adequate hydration are used.53 In our study, we did not find any effect of contrast agent volume on CIN. Two factors might play a role in this result. First, we use iso‐osmolar agents and adequate hydration for our patients. Second, amount of contrast volume used during the procedure was similar in our study population.
Study Limitations
This study has several limitations. First, we measured NT‐proBNP level only once at admission and without correction for potential variability in levels. Second, the follow‐up assessment of renal function in our study was 1 to 3 days after PCI; therefore, we might have missed a later increase in serum creatinine in some patients who did not have renal function deterioration within 72 hours of their procedure. This might have resulted in a slight underestimation of CIN. Finally, we did not measure inflammatory indices except for hs‐CRP.
Conclusion
Admission NT‐proBNP levels may predict the development of CIN after PCI in patients with ACS. Thus, NT‐proBNP can be a quick and useful marker for the early estimation of CIN.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Goldfarb S, McCullough PA, McDermott J, et al. Contrast‐induced acute kidney injury: specialty‐specific protocols for interventional radiology, diagnostic computed tomography radiology, and interventional cardiology. Mayo Clin Proc. 2009;84:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Finn WF. The clinical and renal consequences of contrast‐induced nephropathy. Nephrol Dial Transplant. 2006;21:i2–i10. [DOI] [PubMed] [Google Scholar]
- 3. McCullough PA. Contrast‐induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419–1428. [DOI] [PubMed] [Google Scholar]
- 4. Recio‐Mayoral A, Chaparro M, Prado B, et al. The reno‐protective effect of hydration with sodium bicarbonate plus N‐acetylcysteine in patients undergoing emergency percutaneous coronary intervention: the RENO Study. J Am Coll Cardiol. 2007;49:1283–1288. [DOI] [PubMed] [Google Scholar]
- 5. Lazaros G, Tsiachris D, Tousoulis D, et al. In‐hospital worsening renal function is an independent predictor of one‐year mortality in patients with acute myocardial infarction. Int J Cardiol. 2012;155:97–101. [DOI] [PubMed] [Google Scholar]
- 6. Bartholomew BA, Harjai KJ, Dukkipati S, et al. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004;93:1515–1519. [DOI] [PubMed] [Google Scholar]
- 7. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast‐induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 8. Senoo T, Motohiro M, Kamihata H, et al. Contrast‐induced nephropathy in patients undergoing emergency percutaneous coronary intervention for acute coronary syndrome. Am J Cardiol. 2010;105:624–628. [DOI] [PubMed] [Google Scholar]
- 9. McCullough PA, Stacul F, Becker CR, et al; CIN Consensus Working Panel . Contrast‐Induced Nephropathy (CIN) Consensus Working Panel: executive summary. Rev Cardiovasc Med. 2006;7:177–197. [PubMed] [Google Scholar]
- 10. de Lemos JA, McGuire DK, Drazner MH. B‐type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316–322. [DOI] [PubMed] [Google Scholar]
- 11. Weber M, Dill T, Arnold R, et al. N‐terminal B‐type natriuretic peptide predicts extent of coronary artery disease and ischemia in patients with stable angina pectoris. Am Heart J. 2004;148:612–620. [DOI] [PubMed] [Google Scholar]
- 12. Staub D, Jonas N, Zellweger MJ, et al. Use of N‐terminal pro‐B‐type natriuretic peptide to detect myocardial ischemia. Am J Med. 2005;118:1287. [DOI] [PubMed] [Google Scholar]
- 13. Omland T, de Lemos JA, Morrow DA, et al. Prognostic value of N‐terminal pro‐atrial and pro‐brain natriuretic peptide in patients with acute coronary syndromes. Am J Cardiol. 2002;89:463–465. [DOI] [PubMed] [Google Scholar]
- 14. Omland T, Persson A, Ng L, et al. N‐terminal pro‐B‐type natriuretic peptide and long‐term mortality in acute coronary syndromes. Circulation. 2002;106:2913–2918. [DOI] [PubMed] [Google Scholar]
- 15. Eren NK, Ertas F, Yuksek U, et al. Additive prognostic value of NT‐proBNP over TIMI risk score in intermediate‐risk patients with acute coronary syndrome. Turk Kardiyol Dern Ars. 2009;37:1–8. [PubMed] [Google Scholar]
- 16. Heeschen C, Hamm CW, Mitrovic V, et al; Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Investigators . N‐terminal pro‐B‐type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation. 2004;110:3206–3212. [DOI] [PubMed] [Google Scholar]
- 17. Tumlin J, Stacul F, Adam A, et al; CIN Consensus Working Panel . Pathophysiology of contrast‐induced nephropathy. Am J Cardiol. 2006;98:14K–20K. [DOI] [PubMed] [Google Scholar]
- 18. Staub D, Zeller T, Trenk D, et al. Use of B‐type natriuretic peptide to predict blood pressure improvement after percutaneous revascularisation for renal artery stenosis. Eur J Vasc Endovasc Surg. 2010;40:599–607. [DOI] [PubMed] [Google Scholar]
- 19. Thomsen HS. Guidelines for contrast media from the European Society of Urogenital Radiology. AJR Am J Roentgenol. 2003;181:1463–1471. [DOI] [PubMed] [Google Scholar]
- 20. Laskey WK, Jenkins C, Selzer F, et al; NHLBI Dynamic Registry Investigators . Volume‐to‐creatinine clearance ratio: a pharmacokinetically based risk factor for prediction of early creatinine increase after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50:584–590. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 22. Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms [review]. J Am Soc Echocardiogr. 1989;2:358–367. [DOI] [PubMed] [Google Scholar]
- 23. Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241. [DOI] [PubMed] [Google Scholar]
- 24. McCullough PA, Soman SS. Contrast‐induced nephropathy. Crit Care Clin. 2005;21:261–280. [DOI] [PubMed] [Google Scholar]
- 25. Fox CS, Muntner P, Chen AY, et al. Short‐term outcomes of acute myocardial infarction in patients with acute kidney injury: a report from the national cardiovascular data registry. Circulation. 2012;125:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCullough PA, Wolyn R, Rocher LL, et al. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–375. [DOI] [PubMed] [Google Scholar]
- 27. Wi J, Ko YG, Shin DH, et al. Prediction of contrast‐induced nephropathy with persistent renal dysfunction and adverse long‐term outcomes in patients with acute myocardial infarction using the Mehran risk score. Clin Cardiol. 2013;36:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kragelund C, Gronning B, Kober L, et al. N‐terminal pro‐B‐type natriuretic peptide and long‐term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666–675. [DOI] [PubMed] [Google Scholar]
- 29. Anker SD, Von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart. 2004;90:464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lanese DM, Yuan BH, Falk SA, Conger JD. Effects of atriopeptin III on isolated rat afferent and efferent arterioles. Am J Physiol. 1991;261(6 pt 2):F1102–F1109. [DOI] [PubMed] [Google Scholar]
- 31. Jarai R, Dangas G, Huber K, et al. B‐type natriuretic peptide and risk of contrast‐induced acute kidney injury in acute ST‐segment‐elevation myocardial infarction: a substudy from the HORIZONS‐AMI trial. Circ Cardiovasc Interv. 2012;5:813–820. [DOI] [PubMed] [Google Scholar]
- 32. Meyer T, Schwaab B, Gorge G, et al. Can serum NT‐proBNP detect changes of functional capacity in patients with chronic heart failure? Z Kardiol. 2004;93:540–545. [DOI] [PubMed] [Google Scholar]
- 33. Maisel A, Mueller C, Adams K Jr, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824–839. [DOI] [PubMed] [Google Scholar]
- 34. Sabatine MS, Morrow DA, de Lemos JA, et al. Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J Am Coll Cardiol. 2004;44:1988–1995. [DOI] [PubMed] [Google Scholar]
- 35. Kazanegra R, Cheng V, Garcia A, et al. A rapid test for B‐type natriuretic peptide correlates with falling wedge pressures in patients treated for decompensated heart failure: a pilot study. J Card Fail. 2001;7:21–29. [DOI] [PubMed] [Google Scholar]
- 36. Griendling KK, Minieri CA, Ollerenshaw JD, et al. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. [DOI] [PubMed] [Google Scholar]
- 37. Ruiz‐Ortega M, Lorenzo O, Egido J. Angiotensin III increases MCP‐1 and activates NF‐kappaB and AP‐1 in cultured mesangial and mononuclear cells. Kidney Int. 2000;57:2285–2298. [DOI] [PubMed] [Google Scholar]
- 38. Jarai R, Huber K, Bogaerts K, et al; ASSENT IV‐PCI investigators . Plasma N‐terminal fragment of the prohormone B‐type natriuretic peptide concentrations in relation to time to treatment and Thrombolysis in Myocardial Infarction (TIMI) flow: a substudy of the Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT IV‐PCI) trial. Am Heart J. 2010;159:131–140. [DOI] [PubMed] [Google Scholar]
- 39. Goldenberg I, Matetzky S. Nephropathy induced by contrast media: pathogenesis, risk factors and preventive strategies. CMAJ. 2005;172:1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ronco C, McCullough P, Anker SD, et al; Acute Dialysis Quality Initiative (ADQI) consensus group . Cardio‐renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. [DOI] [PubMed] [Google Scholar]
- 42. Heyman SN, Reichman J, Brezis M. Pathophysiology of radiocontrast nephropathy: a role for medullary hypoxia. Invest Radiol. 1999;34:685–691. [DOI] [PubMed] [Google Scholar]
- 43. McCullough PA. Acute kidney injury with iodinated contrast. Crit Care Med. 2008;36(4 suppl):S204–2S11. [DOI] [PubMed] [Google Scholar]
- 44. Tsuruda T, Boerrigter G, Huntley BK, et al. Brain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res. 2002;91:1127–1134. [DOI] [PubMed] [Google Scholar]
- 45. Kawakami R, Saito Y, Kishimoto I, et al. Overexpression of brain natriuretic peptide facilitates neutrophil infiltration and cardiac matrix metalloproteinase‐9 expression after acute myocardial infarction. Circulation. 2004;110:3306–3312. [DOI] [PubMed] [Google Scholar]
- 46. Vila G, Resl M, Stelzeneder D, et al. Plasma NT‐proBNP increases in response to LPS administration in healthy men. J Appl Physiol (1985). 2008;105:1741–1745. [DOI] [PubMed] [Google Scholar]
- 47. Madak N, Nazli Y, Mergen H, et al. Acute phase reactants in patients with coronary slow flow phenomenon. Anadolu Kardiyol Derg. 2010;10:416–420. [DOI] [PubMed] [Google Scholar]
- 48. Hopkins WE, Chen Z, Fukagawa NK, et al. Increased atrial and brain natriuretic peptides in adults with cyanotic congenital heart disease: enhanced understanding of the relationship between hypoxia and natriuretic peptide secretion. Circulation. 2004;109:2872–2877. [DOI] [PubMed] [Google Scholar]
- 49. Hizoh I, Strater J, Schick CS, et al. Radiocontrast‐induced DNA fragmentation of renal tubular cells in vitro: role of hypertonicity. Nephrol Dial Transplant. 1998;13:911–918. [DOI] [PubMed] [Google Scholar]
- 50. Qin S, Ding J, Takano T, et al. Involvement of receptor aggregation and reactive oxygen species in osmotic stress‐induced Syk activation in B cells. Biochem Biophys Res Commun. 1999;262:231–236. [DOI] [PubMed] [Google Scholar]
- 51. Bittner A, Alcaino H, Castro PF, et al. Matrix metalloproteinase‐9 activity is associated to oxidative stress in patients with acute coronary syndrome. Int J Cardiol. 2010;143:98–100. [DOI] [PubMed] [Google Scholar]
- 52. McCullough PA, Adam A, Becker CR, et al; CIN Consensus Working Panel . Risk prediction of contrast‐induced nephropathy. Am J Cardiol. 2006;98:27K–36K. [DOI] [PubMed] [Google Scholar]
- 53. Cho JY, Jeong MH, Hwan Park S, et al. Effect of contrast‐induced nephropathy on cardiac outcomes after use of nonionic isosmolar contrast media during coronary procedure. J Cardiol. 2010;56:300–306. [DOI] [PubMed] [Google Scholar]


