Abstract
Background
Recently, mild therapeutic hypothermia (MTH) has been integrated into the European resuscitation guidelines to improve outcomes after out‐of‐hospital cardiac arrest (OHCA). Data on long‐term results are limited, especially in patients with acute ST‐elevation myocardial infarction (STEMI).
Hypothesis
Invasive MTH influences long‐term prognosis after OHCA due to STEMI.
Methods
We analyzed 48 patients who underwent emergency coronary angiography for STEMI after witnessed OHCA. In 24 consecutive patients, MTH was performed via intravascular cooling (CoolGard System, 34°C maintained for 24 hours) after initialization by rapid infusion of cold saline. Clinical, procedural, and mortality data were compared to 24 historical controls. Neurological recovery was assessed using the Cerebral Performance Category score (CPC) at 30‐day and 1‐year follow‐up.
Results
Median time delay until arrival of emergency medical service was 6 minutes (MTH group) vs 6.5 minutes (controls) (P = 0.16). Initial rhythm was ventricular fibrillation in 75% vs 66.7% (P = 0.75). There were no differences regarding baseline characteristics, angiographic findings, and success of cardiac catheterization procedures. MTH was not associated with a higher frequency of bleeding complications or of pneumonia. Thirty‐day mortality was 33.3% in both groups. One‐year mortality was 37.5% (MTH group) vs 50% (controls) (P = 0.56). At 1 year, favorable neurological outcome (CPC ≤2) was significantly more frequent in the MTH group (58.3% vs 20.8%, P = 0.017). Multivariate analysis identified MTH as independent predictor of favorable neurological outcome (P < 0.02, odds ratio: 12.73).
Conclusions
MTH via intravascular cooling improves neurological long‐term prognosis after OHCA due to STEMI and is safe in clinical practice.
Introduction
Recently, mild therapeutic hypothermia (MTH) has been integrated into international resuscitation guidelines1, 2, 3 to improve survival in comatose survivors of out‐of‐hospital cardiac arrest (OHCA). But survivors of OHCA are a very inhomogeneous patient population with a wide variety of risk and outcomes. This concerns the circumstances of the index event, such as witnessed versus unwitnessed cardiac arrest, but also the underlying cause.4, 5, 6, 7, 8 For example, patients with ST‐elevation myocardial infarction (STEMI) have a better prognosis than patients with other causes of cardiac arrest.9 Data on the specific influence of MTH on patients who were resuscitated in the context of STEMI are rare,6, 7, 10, 11 especially data on long‐term prognosis. In fact, STEMI patients were excluded from some prospective studies.12
However, STEMI is a frequent cause for OHCA, which requires specific data on the value of MTH in these patients. Therefore, we analyzed the effect of MTH on long‐term prognosis of a homogeneous, clinically relevant group of patients with witnessed OHCA due to STEMI and successful resuscitation with return of spontaneous circulation (ROSC) before admission, and who underwent immediate invasive coronary angiography. In contrast to existing data,10, 11 we performed MTH by an intravascular cooling strategy.
Methods
We retrospectively analyzed all consecutive patients admitted to our intensive care unit (ICU) between April 2002 and December 2009 after witnessed OHCA and ROSC following successful resuscitation by the emergency medical service (EMS) who underwent emergency coronary angiography for acute STEMI (our standard procedure in all STEMI cases). Unwitnessed OHCA was the only exclusion criterion. Our institution's referral area encompasses the city of Erlangen (population 100 000) and surroundings. Advanced cardiovascular life support was performed by a team of trained paramedics and an emergency physician according to the current guidelines of the European Resuscitation Council.
MTH was integrated into the institution's standard care in December 2005, allowing the comparison to consecutive patients before December 2005 who served as historical controls. MTH was performed using an intravascular cooling system consisting of an external heat exchanger (CoolGard System 3000; Zoll Medical Corp., Chelmsford, MA) and a closed loop heat exchange catheter (Alsius Icy Catheter; Zoll), which was placed in the inferior vena cava via the femoral vein. Cooling was initiated immediately upon admission by rapid infusion of cold saline at 4°C. The amount of cold saline was not adjusted to body weight,1, 12 but 1000 mL were rapidly infused upon admission followed by another 1000 mL during heart catheterization procedures (if no clinical/radiological signs of lung edema).
Intravascular MTH was started immediately after the emergency coronary procedure (the cooling catheter had been placed just before cardiac catheterization laboratory (cath lab) procedures in most cases). Target temperature was 34°C, maintained for 24 hours, followed by controlled rewarming of 0.2°C/h and a period of controlled normothermia (37°C) for another 12 hours. Patient's core temperature was measured continuously by a sensor probe integrated into the urinary catheter. An infrared tympanic thermometer was used for all measurements in the historical controls (3–5 times within the first 24 hours) and to estimate the initial temperature on admission in all patients.
In the MTH group, analgosedation was induced by continuous infusion of fentanyl (0.05–0.08 mg/h) and midazolam (6–8 mg/h), adapted to obtain a Ramsay‐Sedation Score of 2 to 3.13 Relaxation to prevent shivering was performed by continuous infusion of cisatracurium, which was terminated as soon as core the temperature reached 36°C during rewarming. In the historical controls, analgosedation was performed in the same manner, but no relaxation was applied.
The MTH group received prophylactic antibiotic treatment with piperacillin/combactam/ciprofloxacin (adapted to renal function) from admission day, which was continued depending on the clinical course. The controls received calculated antibiotic treatment if indicated by clinical and laboratory findings.
Data were obtained from records of EMS, cath lab, and ICU. Thirty‐day and 1‐year follow‐up were carried out by contacting all patients and/or direct family members and the patients general practitioners by telephone or questionnaire. Informed consent was obtained from each patient. No patients were lost to follow‐up, and the retrospective analysis of data was approved by the institutional review board.
Definitions
Definitions of the data analyzed are as follows:
Assessment of neurological recovery by Cerebral Performance Category (CPC) scale:14 CPC1 = conscious, no neurological disability; CPC2 = conscious, moderate neurological disability, can work; CPC3 = conscious, severe neurological disability, dependent; CPC4 = permanent vegetative status.
Acute STEMI: persisting ST‐elevations >0.1 mV in at least 2 standard leads, or >0.2mV in at least 2 contiguous precordial leads, and creatine kinase elevation >170 U/L, and/or troponin I >0.5 ng/mL within the first 6 hours after admission.
Door‐to‐start invasive cooling time: interval between hospital admission and return to ICU after cath lab procedures, estimated as start point of intravascular cooling.
Successful percutaneous coronary intervention (PCI): residual stenosis <30% and TIMI flowgrade 3 by visual assessment.
Multivessel disease: at least 1 additional ≥70% stenosis in a major coronary vessel besides the culprit lesion.
Left ventricular ejection fraction: visually estimated from echocardiography performed within 4 hours after admission.
Moderate bleeding is bleeding necessitating blood transfusion, according to the GUSTO III (Global Use of Strategies to Open Occluded Coronary Arteries) criteria15; severe bleeding is bleeding leading to hemodynamic instability.
Primary Coronary Intervention/Adjunctive Therapy
All patients received 500 mg aspirin and 5000 IU unfractionated heparin intravenously before or upon admission and another 5000 IU during PCI. Patients undergoing stent placement received 300 to 600 mg clopidogrel immediately before or after PCI. Clopidogrel 75 mg was maintained for 4 to 6 weeks after bare‐metal stent placement and 6 months after drug‐eluting stent. After European guidelines changed in 2008,16 clopidogrel was generally recommended for 12 months.
Statistical Analysis
Numerical data were compared by Mann‐Whitney U rank sum test, presented as median values and interquartile range (IQR) (IQR between 25th and 75th percentile). Categorical variables were compared by Fisher exact test. Univariate and multivariate analyses (logistic regression) of predictors of favorable neurological outcome (CPC ≤2) were performed. Because of the small study population, only age, gender, and the variables that had been identified as possible predictors by univariate analysis as well as factors commonly assumed to be correlated with neurological prognosis could have been included in the multivariate analysis (MTH, initial rhythm shockable, EMS arrival time, bystander cardiopulmonary resuscitation, and serum lactate). Results were presented as odds ratio (OR) with 95% confidence interval (CI). P values <0.05 were considered significant. All P values are the results of 2‐tailed tests. All calculations were carried out using SPSS version 19.02 (IBM SPSS, Armonk, NY).
Results
Forty‐eight consecutive patients were included. The cardiac arrests occurred at home (n = 26), in public places (n = 12), at work (n = 6), or during physical exercises (n = 4).
Differences Between MTH and Non‐MTH Groups
The number of patients was coincidentally equal in both groups: 24/48 patients underwent MTH. Their data were compared to 24 consecutive historical controls without MTH (Table 1). All patients were admitted intubated/mechanically ventilated by EMS after ROSC. There were no significant differences regarding baseline characteristics or angiographic and procedural data (Table 1). The rate of PCI was 95.8% in the MTH group and 87.5% for the controls (P = 0.60), resulting in successful reperfusion after PCI in 82.6% vs 85.7% (P = 1.00).
Table 1.
Baseline, Procedural, and Outcome Data of 48 Patients With OHCA and Emergency Coronary Angiography for STEMI
| STEMI Complicated by Witnessed OHCA (n = 48) | P | ||
|---|---|---|---|
| Therapeutic Hypothermia (n = 24) | No Hypothermia (n = 24) | ||
| Baseline data | |||
| Age, median (IQR), y | 62 (51–74) | 64 (50–75) | 0.90 |
| Female | 16.7% (4) | 29.2% (7) | 0.49 |
| Diabetes | 25% (6) | 29.2% (7) | 1.00 |
| Hypertension | 62.5% (15) | 66.7% (16) | 1.00 |
| Smoking | 50% (12) | 58.3% (14) | 0.77 |
| Prior myocardial infarction | 8.3% (2) | 4.2% (1) | 1.00 |
| Prior stroke | 8.3% (2) | 12.5% (3) | 1.00 |
| Bystander CPR | 45.8% (11) | 41.7% (10) | 1.00 |
| Initial rhythm shockable | 75.0% (18) | 66.7% (16) | 0.75 |
| pH on admission, median (IQR) | 7.2 (7.07–7.26) | 7.2 (7.0–7.28) | 0.64 |
| Lactate on admission, median (IQR), mg/dL | 68 (60–90) | 95 (60–123) | 0.34 |
| Creatinine on admission, median (IQR), mg/dL | 1.43 (1.18–1.63) | 1.40 (1.19–1.59) | 0.85 |
| Creatine kinase on admission, median (IQR), U/L | 213 (159–321) | 179 (113–320) | 0.29 |
| Left ventricular ejection fraction <30% | 20.8% (5) | 33.3% (8) | 0.52 |
| Vasopressors on admission | 66.7% (16) | 79.2% (19) | 0.52 |
| Mechanical ventilation | 100% (24) | 100% (24) | 1.00 |
| Temperature on admission, median (IQR), mg/dL , °C | 36.4 (35.9–36.9) | 36.8 (36.3–37.7) | 0.17 |
| Infarction‐related coronary vessel | |||
| Left main coronary artery | 0 | 8.3% (2) | 0.49 |
| Left anterior descendent artery | 50% (12) | 58.3% (14) | 0.77 |
| Circumflex artery | 12.5% (3) | 8.3% (2) | 1.00 |
| Right coronary artery | 25.0% (6) | 20.8% (5) | 1.00 |
| Coronary artery bypass graft | 4.2% (1) | 0 | 1.00 |
| Unclear | 8.3% (2) | 4.2% (1) | 1.00 |
| Presence of multivessel disease | 50% (12) | 58.3% (14) | 0.77 |
| Procedural data | |||
| PCI performed | 95.8% (23) | 87.5% (21) | 0.61 |
| Stent implantation performed | 78.3% (18/23) | 85.7% (18/21) | 0.70 |
| Implantation of drug eluting stent | 16.7% (3/18) | 11.1% (2/18) | 1.00 |
| PCI in >1 vessel | 17.4% (4/23) | 9.5% (2/21) | 0.67 |
| Successful reperfusion (TIMI 3) | 82.6% (19/23) | 85.7% (18/21) | 1.00 |
| Intra‐aortic balloon pump | 12.5% (2) | 29.2% (7) | 0.14 |
| Use of GP IIb/IIIa antagonists | 29.2% (7) | 41.7% (10) | 0.55 |
| Time intervals, median (IQR), min | |||
| Emergency call to EMS arrival | 6 (5–8) | 6.5 (6–9) | 0.16 |
| Emergency call to door | 58 (40–72) | 59 (49–78) | 0.39 |
| Emergency call to balloon | 126 (115–169) | 138 (107–164) | 0.92 |
| Emergency call to start invasive cooling | 155 (126–182) | — | — |
| Arrival EMS to balloon time | 120 (110–163) | 128 (101–156) | 0.82 |
| Door to balloon | 85 (70–100) | 77 (65–93) | 0.43 |
| Door to start invasive cooling | 104 (77–121) | — | — |
| Duration catheter lab procedures | 40 (34–62) | 41 (28–67) | 0.73 |
| Intensive care unit data | |||
| Temperature 24 hours after admission (°C) | 34.0 (34.0–34.1) | 37.9 (37.0–38.3) | <0.001 |
| Duration of stay in the ICU, d | 8 (5–17) | 11 (3–22) | 0.50 |
| Maximum creatine kinase, U/L | 4000 (2666–5150) | 2726 (878–4801) | 0.17 |
| Severe bleeding (hemodynamic instability) | 0 | 0 | |
| Moderate bleeding (blood transfusion) | 29.2% (7) | 25.0% (6) | 1.00 |
| Mortality data | |||
| 3‐day mortality | 20.8% (5) | 20.8% (5) | 1.00 |
| 30‐day mortality | 33.3% (8) | 33.3% (8) | 1.00 |
| 1‐year mortality | 37.5% (9) | 50% (12) | 0.56 |
Abbreviations: CPR, cardiopulmonary resuscitation; EMS, emergency medical service; GP, glycoprotein; IQR, interquartile‐range (25th–75th percentile); MTH, mild therapeutic hypothermia; OHCA, out‐of‐hospital cardiac arrest; PCI, percutaneous coronary intervention; STEMI, ST‐elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction.
Study included patients who underwent MTH (n = 24) vs historical controls (n = 24).
There were no significant differences regarding the intervals emergency call to door, arrival EMS to balloon, and door to balloon, respectively. Mean door to start invasive cooling time (start of CoolGard System after cath lab procedures) was 104 minutes (IQR, 77–121) leading to an estimated emergency call to start invasive cooling time of 155 minutes (IQR, 126–182). After the start of the CoolGard System, time to target temperature in the MTH group was not documented in all cases, but was maximally 2.5 hours.
Three‐day and 30‐day mortality were the same in both groups. One‐year mortality was numerically lower in the MTH group without reaching statistical significance (37.5% vs 50.0%, P = 0.56). Bleeding complications requiring blood transfusion occurred with almost the same frequency in both groups but were moderate in severity (Table 1).
All patients of the MTH group received prophylactic antibiotic treatment as described above. In comparison, all but 2 of the controls received a calculated antibiotic treatment that had been started after 2 to 4 days because of markedly increasing C‐reactive protein (CRP) levels. Duration of the antibiotic treatment was similar in both groups (median, 9 days; IQR 7–12 days [MTH‐group] and 7–17days [controls]; P = 0.53). Maximum CRP levels were higher in the MTH group (median, 205 mg/L [IQR, 173–232 mg/L] vs 129 mg/L [IQR, 90–158 mg/L]; P = 0.037]. The frequency of clinically/radiologically diagnosed pneumonia during the ICU period was analyzed in survivors of the first 3 days and was 36.8% (7/19) in the MTH group vs 52.6% (10/19) in controls (P = 0.52).
Differences Between Survivors and Nonsurvivors
Among all 48 patients, 30‐day nonsurvivors (n = 16) were older (P < 0.005) (median, 71 years [IQR, 64–77 years] vs 55 years [IQR, 47–72 years). Compared to 30‐days survivors (n = 32) they also presented higher creatinine levels (median, 1.6 mg/dL [IQR, 1.4–1.8 mg/dL] vs 1.3 mg/dL [1.1–1.5 mg/dL]; P = 0.011), higher lactate levels (median, 122 mg/dL [IQR, 90–140 mg/dL] vs 67 mg/dL [IQR, 57–90 mg/dL]; P < 0.005), and more frequent vasopressor therapy on admission (100% vs 59.4%, P = 0.002).
There were no differences regarding frequency of pneumonia (50% in 30‐day nonsurvivors vs 43.8% in survivors, P = 1.00), and maximum CRP levels (median, 144 mg/L [IQR, 120–209 mg/L] vs 173 mg/L [IQR, 100–233 mg/L]; P = 0.69).
The mean delay emergency call to EMS arrival tended to be longer in 30‐day nonsurvivors (median, 7.5 minutes [IQR, 6–9.5 minutes] vs 6 minutes [IQR, 5–7.5 minutes], P = 0.056), and these patients also had significantly longer emergency call to balloon times (median, 169 minutes [IQR, 141–198 minutes] vs 117 minutes [IQR, 108–157 minutes], P = 0.012), whereas door to balloon times did not differ significantly (median, 82 minutes [IQR, 76–95 minutes] vs 80 minutes [IQR, 57–101 minutes], P = 0.517). Finally, in 30‐day nonsurvivors, successful reperfusion tended to be less frequent (62.5% vs 84.4%, P = 0.144).
Importantly, the proportion of patients who had undergone MTH was 50% in both 30‐day nonsurvivors and survivors.
Most of the 30‐day nonsurvivors (8/24 of MTH group, 8/24 of controls) died from cardiovascular causes (7/8 of MTH group, 7/8 of controls) and the rest as result of neurological injury. Thirty‐day survivors who died within 1 year after the OHCA (1/16 of MTH group, 4/16 of controls), had all been in CPC status >2 on hospital discharge and died as consequence of the neurological injury in most cases (3/4 of the controls), the rest as a result of myocardial reinfarction.
Neurological Prognosis of Survivors
At 30 days, favorable neurological outcome (CPC <2) was observed in 50% (12/24) of the MTH group and 16.7% (4/24) of the controls (P = 0.031) and at 1 year in 58.3% (14/24) vs 20.8% (5/24), respectively (P = 0.017). Accordingly, regarding survivors, 75% of the 30‐day survivors of the MTH group (12/16) but only 25% of the 30‐day survivors of the controls (4/16) survived with CPC status ≤2 (P = 0.012). Similarly, after 1 year a favorable neurological outcome was significantly more frequent in survivors of the MTH group (93.3% [14/15] vs 41.7% [5/12], P = 0.009) (Table 2). Figure 1 demonstrates CPC scores.
Table 2.
Frequency of Favorable Neurological Outcome (CPC ≤2) in Survivors of OHCA and STEMI
| Frequency of CPC Score ≤2 in Survivors | P | ||
|---|---|---|---|
| Survivors of Hypothermia Group |
Survivors of No Hypothermia Group |
||
| CPC <2 after 30 days | 75.0% (12/16) | 25.0% (4/16) | 0.012 |
| CPC <2 after 1 year | 93.3% (14/15) | 41.7% (5/12) | 0.009 |
Abbreviations: CPC, Cerebral Performance Category; MTH, mild therapeutic hypothermia; OHCA, out‐of‐hospital cardiac arrest; STEMI: ST‐elevation myocardial infarction.
Patients who underwent MTH vs historical controls.
Figure 1.
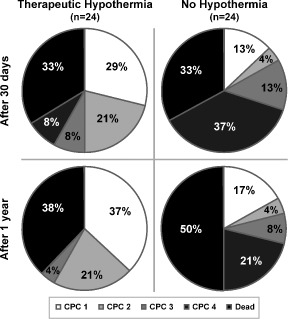
Outcome after 30 days and 1 year of the groups OHCA + STEMI, MTH (n = 24) and OHCA + STEMI, No‐MTH (n = 24). Neurological recovery was assessed by CPC scale: CPC1 = conscious, no neurological disability; CPC2 = conscious, moderate neurological disability, can work; CPC3 = conscious, severe neurological disability, dependent; and CPC4 = permanent vegetative status. Abbreviations: CPC, Cerebral Performance Category; MTH, mild therapeutic hypothermia; OHCA, out‐of‐hospital cardiac arrest; STEMI, ST‐elevation myocardial infarction.
Differences between the 30‐day survivors of both groups with favorable neurological outcome (n = 16) and the remaining 30‐day survivors of both groups with severe neurological damage (CPC >2, n = 16) were analyzed by univariate analysis (Table 3). In 30‐day survivors with favorable neurological outcome, MTH had been performed significantly more frequent (75% vs 25%, P = 0.012). Multivariate analysis confirmed MTH (OR: 12.7; 95% CI: 1.59‐102) and initial rhythm shockable (OR: 16.1; 95% CI: 1.22‐212) as independent predictors of a favorable neurological prognosis (Figure 2).
Table 3.
Univariate Analysis of Predictors of Neurological Prognosis in 30‐Day Survivors After OHCA and STEMI
| 30‐Day Survivors | P | OR | 95% CI | ||
|---|---|---|---|---|---|
| CPC ≤2, n = 16 | CPC >2, n = 16 | ||||
| Therapeutic hypothermia | 75.0% (12) | 25.0% (4) | 0.012 | 9.00 | 1.87–44.61 |
| Successful reperfusion (TIMI 3) | 100% (16) | 75.0% (12) | 0.10 | 11.88 | 0.58–241.9 |
| EF ≤30 | 12.5% (2) | 25.0% (4) | 0.65 | 0.43 | 0.07–2.766 |
| Vasopressors on admission | 43.8% (7) | 75.0% (12) | 0.15 | 0.26 | 0.06–1.165 |
| Lactate on admission ≤80 | 68.8% (11) | 62.5% (10) | 1.00 | 1.32 | 0.30–5.706 |
| Emergency call, EMS arrival <6 minutes | 68.8% (11) | 50.0% (8) | 0.47 | 2.20 | 0.52–9.303 |
| Creatinine on admission <1.40 mg/dL | 75.0% (12) | 56.3% (9) | 0.46 | 2.33 | 0.52–10.48 |
| pH on admission >7.2 | 68.8% (11) | 37.5% (6) | 0.16 | 3.67 | 0.85–15.85 |
| Initial rhythm shockable | 93.8% (15) | 56.3% (9) | 0.037 | 11.67 | 1.23–111.0 |
| Bystander CPR | 50.0% (8) | 56.3% (9) | 1.00 | 0.78 | 0.19–3.128 |
| Age <64 years | 62.5% (10) | 62.5% (10) | 1.00 | 1.00 | 0.24–4.186 |
| Bleeding necessitating transfusion | 37.5% (6) | 25.0% (4) | 0.70 | 1.80 | 0.40–8.218 |
| Use of GP IIb/IIIa antagonists | 37.5% (6) | 50.0% (8) | 0.72 | 0.60 | 0.15–2.456 |
| Presence of multivessel disease | 50.0% (8) | 62.5% (10) | 0.72 | 1.67 | 0.41–6.821 |
| Female | 12.5% (2) | 25.0% (4) | 0.65 | 0.43 | 0.07–2.766 |
Abbreviations: CI, confidence interval; CPC, Cerebral Performance Category score, CPR, cardiopulmonary resuscitation; EF, ejection fraction; EMS, emergency medical service; GP, glycoprotein; OHCA, out‐of‐hospital cardiac arrest; OR, odds ratio; STEMI, ST‐elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction.
Figure 2.
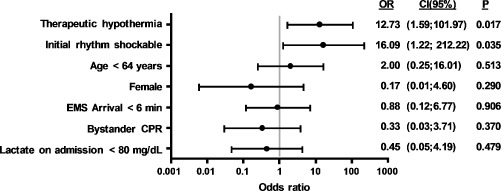
Multivariate analysis (log regression): predictors of favorable neurological outcome (CPC ≤2) in 30‐day survivors after out‐of‐hospital cardiac arrest and ST‐elevation myocardial infarction. Abbreviations: CI, confidence interval; CPR, cardiopulmonary resuscitation; EMS, emergency medical services; OR, odds ratio.
Discussion
In contrast to most MTH studies,4, 5, 6, 17, 18 we analyzed a very homogeneous group of patients. Furthermore, compared to the few existing studies dealing with MTH after OHCA due to STEMI,10, 11 we used an intravascular cooling system, providing both controlled hypothermia and rewarming.19, 20
Besides the only 2 randomized trials that observed the beneficial effect of MTH on survival as well as on neurological outcome after cardiac arrest of any cardiac causes,4, 5 data about a positive effect of MTH on survival, especially in STEMI patients, are rare.10, 11
In our study, MTH did not seem to influence 30‐days survival, an observation that is concordant with the results of other authors,6, 10, 17 who included not only STEMI6, 17 or also analyzed unwittnessed cardiac arrest and noncardiac causes of cardiac arrest.17 Our study population is small, but conceivably short‐term mortality after OHCA depends on several critical factors, which MTH possibly might not influence, such as multiorgan failure. Correspondingly, initial lactate levels were higher in 30‐day nonsurvivors, indicating a longer delay until ROSC, which portends a grave prognosis in general. Furthermore, these patients more often presented well‐known predictors of mortality in STEMI such as older age, vasopressor therapy, renal insufficiency, and longer prehospital delays.
At 1‐year follow‐up there was an absolute 12.5% difference regarding mortality in the 2 groups favoring MTH without reaching statistical significance. However, in our study, 30‐day survivors after MTH significantly more often presented a favorable neurological status, which probably has a beneficial effect on long‐term survival per se, as most patients after OHCA die due to neurological injury.21, 22 Correspondingly, although almost all of our patients who died within the first 30 days died from cardiovascular causes, patients who died after the first 30 days died as a consequence of neurological injury in most cases.
Our main finding was the clear difference in neurological prognosis after MTH. At 30 days, favorable neurological outcome (CPC ≤2) was observed in 50% of the MTH group vs 16.7% of the controls (P = 0.031) and at 1 year in 58.3% vs 20.8%, respectively (P = 0.017). These results correspond to those of Knafelj et al.,11 who used external ice packs in 40 STEMI patients after OHCA and described in‐hospital survival with CPC <2 in 55% (MTH) vs 16% (controls). Wolfrum et al.10 also analyzed STEMI patients with MTH after OHCA using external cooling devices; compared to 17 historical controls, their patients treated with MTH (n = 16) tended to have a lower mortality and improved neurological outcome after 6 months.
Nevertheless, data of the 2 large randomized trials establishing hypothermia after resuscitation from cardiac arrest due to ventricular fibrillation showed a neurological recovery in 55% vs 39%4 and 49% vs 26%,5 respectively.
Our multivariate analysis model confirmed MTH as independent predictor of favorable neurological outcome. The second factor identified was initial rhythm shockable. Correspondingly, most existing studies demonstrated improved neurological outcome after therapeutic hypothermia for survivors of ventricular fibrillation,4, 5, 11, 23 and MTH seems to be more effective in patients with shockable rhythm compared to those with nonshockable rhythm.24
Rapid infusion of cold saline decreases the core temperature significantly,1, 11, 12 and we used this technique to initiate our cooling procedure until intravascular MTH via the CoolGard System was applied. As the incidence of complications such as arrhythmias, infections, and coagulopathy seems to increase with core temperatures below 32°C2 we defined our target temperature at 34°C. For the same reason, we chose a slow rewarming of 0.2°C/h, whereas current recommendations cite 0.25 to 0.5°C/h.1
Existing data describe MTH as a safe procedure even in combination with reperfusion strategies in acute myocardial infarction.10, 11, 18, 25, 26 Nevertheless, Wolfrum et al.10 described a significantly higher need of blood transfusion in STEMI patients who underwent PCI and MTH after OHCA than in historical controls (38% vs 6%, P = 0.04).
In our analysis, in spite of the use of an intravascular cooling system, bleeding complications necessitating blood transfusion (29.2% vs 25.0%, P = 1.00) were only moderate in both groups, even if there was a tendency toward a more frequent use of glycoprotein IIb/IIIa antagonists in the historical controls.
Because other authors reported a higher occurrence of infections associated with MTH,2, 10, 11 we administered standardized prophylactic antibiotic treatment to all patients in our MTH group from admission, whereas most of the historical controls received a calculated antibiotic treatment once indicated. We found a tendency toward a lower rate of pneumonia in the MTH group (36.8% vs 52.6%, P = 0.52).
Limitations
Our retrospective data reflect current treatment strategies of a single referral center, and therefore our study population is small. We used an historical control group of the precooling era, but we believe that in‐hospital logistic procedures and management of STEMI did not significantly change within both time intervals. Regarding clinical aspects, we analyzed a very homogeneous group of patients, but significant confounders may still exist. Documented short time delays until arrival of EMS (range, 5–9 minutes) were consistent with the local EMS statutes, which require a maximum of 12 minutes. These conditions might be incomparable to other geographic regions and may have influenced the results of this study. Relatively long door‐to‐balloon times are due to the logistic constraints of performing coronary angiography in patients on life support systems.
Conclusion
Invasive MTH improves neurological long‐term prognosis of STEMI patients after OHCA. A combination of initial infusion of cold saline followed by an intravascular cooling system performing controlled MTH and rewarming is safe in these selected patients.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Deakin CD, Nolan JP, Soarc J, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 4. Adult advanced life support. Resuscitation. 2010;81:1305–1352. [DOI] [PubMed] [Google Scholar]
- 2. Nolan JP, Morley PT, Vanden Hoek TL, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;108:118–121. [DOI] [PubMed] [Google Scholar]
- 3. Neumar RW, Nolan JP, Adrie C, et al. Post‐cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication a consensus statement from the International Liaison Committee on Resuscitation. Circulation. 2008;118:2452–2483. [DOI] [PubMed] [Google Scholar]
- 4. The Hypothermia After Cardiac Arrest (HACA) Study Group . Mild therapeutic hypothermia to improve the neurological outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. [DOI] [PubMed] [Google Scholar]
- 5. Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. [DOI] [PubMed] [Google Scholar]
- 6. Bro‐Jeppesen J, Kjaergaard J, Horsted TI, et al. The impact of therapeutic hypothermia on neurological function and quality of life after cardiac arrest. Resuscitation. 2009;80:171–176. [DOI] [PubMed] [Google Scholar]
- 7. Nielsen N, Hovdenes J, Nilsson F, et al; Hypothermia Network. Outcome, timing and adverse events in therapeutic hypothermia after out‐of‐hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53:926–934. [DOI] [PubMed] [Google Scholar]
- 8. Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol. 2009;133:223–228. [DOI] [PubMed] [Google Scholar]
- 9. Pleskot M, Hazukova R, Stritecka H, et al. Long‐term prognosis after out‐of‐hospital cardiac arrest with/without ST elevation myocardial infarction. Resuscitation. 2009;80:795–804. [DOI] [PubMed] [Google Scholar]
- 10. Wolfrum S, Pierau C, Radke PW, et al. Mild therapeutic hypothermia in patients after out‐of‐hospital cardiac arrest due to acute ST‐segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med. 2008;36:1780–1786. [DOI] [PubMed] [Google Scholar]
- 11. Knafelj R, Radsel P, Ploj T, et al. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST‐elevation acute myocardial infarction. Resuscitation. 2007;74:227–234. [DOI] [PubMed] [Google Scholar]
- 12. Kim F, Olsufka M, Carlbom D, et al. Pilot study of rapid infusion of 2 L of 4°C normal saline for Induction of mild hypothermia in hospitalized, comatose survivors of out‐of‐hospital cardiac arrest. Circulation. 2005;112:715–719. [DOI] [PubMed] [Google Scholar]
- 13. Ramsay MA, Savege TM, Simpson BR, et al. Controlled sedation with alphaxalone‐alphadolone. Br Med J. 1974;2:656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. [DOI] [PubMed] [Google Scholar]
- 15. The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO III) Investigators. A comparison of reteplase with alteplase for acute myocardial infarction. N Engl J Med. 1997;337:1124–1130. [DOI] [PubMed] [Google Scholar]
- 16. The Task Force on the Management of ST‐Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology, ESC Guidelines; Van de Werf F, Bax J, Betriu A, et al. Management of acute myocardial infarction in patients presenting with persistent ST‐segment elevation. Eur Heart J. 2008;29:2909–2945. [DOI] [PubMed] [Google Scholar]
- 17. Pfeifer R, Jung C, Purle S, et al. Survival does not improve when therapeutic hypothermia is added to post‐cardiac arrest care. Resuscitation. 2011;82:1168–1173. [DOI] [PubMed] [Google Scholar]
- 18. Dumas F, White L, Stubbs BA, et al. Long‐term prognosis following resuscitation from out of hospital cardiac arrest, role of percutaneous coronary intervention and therapeutic hypothermia. J Am Coll Cardiol. 2012;60:21–27. [DOI] [PubMed] [Google Scholar]
- 19. Hoedemakers CW, Ezzahti M, Gerritsen A, et al. Comparison of cooling methods to induce and maintain normo‐ and hypothermia in intensive care unit patients: a prospective intervention study. Crit Care. 2007;11:R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al‐Senani FM, Graffagnino C, Grotta JC, et al. A prospective, multicenter pilot study to evaluate the feasibility and safety of using the CoolGard System and Icy catheter following cardiac arrest. Resuscitation. 2004;62:143–150. [DOI] [PubMed] [Google Scholar]
- 21. Laver S, Farrow C, Turner D, et al. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–2128. [DOI] [PubMed] [Google Scholar]
- 22. Edgren E, Hedstrand U, Kelsey S, et al.: Assessment of neurological prognosis in comatose survivors of cardiac arrest. BRCT I Study Group. Lancet. 1994;343:1055–1059. [DOI] [PubMed] [Google Scholar]
- 23. Belliard G, Catez E, Charron C, et al. Efficacy of therapeutic hypothermia after out‐of‐hospital cardiac arrest due to ventricular fibrillation. Resuscitation. 2007;75:252–259. [DOI] [PubMed] [Google Scholar]
- 24. Laish‐Farkash A, Matetzky S, Oieru D, et al. Usefulness of mild therapeutic hypothermia for hospitalized comatose patients having out‐of‐hospital cardiac arrest. Am J Cardiol. 2011;108:173–178. [DOI] [PubMed] [Google Scholar]
- 25. Schefold JC, Storm C, Joerres A, et al. Mild therapeutic hypothermia after cardiac arrest and the risk of bleeding in patients with acute myocardial infarction. Int J Cardiol. 2009;132:387–391. [DOI] [PubMed] [Google Scholar]
- 26. Batista LM, Lima FO, Januzzi FL Jr, et al. Feasibility and safety of combined percutaneous coronary intervention and therapeutic hypothermia following cardiac arrest. Resuscitation. 2010;81:398–403. [DOI] [PubMed] [Google Scholar]


