ABSTRACT
Background
The purpose of this study was to determine the long‐term prognostic implications of incidental pleural effusion (PE) detected during echocardiographic examination and its relationship with concomitant diseases.
Hypothesis
The study hypothesis is to test whether incidental pleural PE detected during echocardiographic examination be used as a prognostic marker.
Methods
The study was performed by evaluating patient records (N = 251) in whom PE was incidentally detected during echocardiographic examination in a tertiary hospital between 1999 and 2012. The patients were classified into 4 major groups according to the concomitant primary disease: malignancy, and cardiovascular, renal, and pulmonary diseases. The total survival time was obtained from hospital records for patients who died during the hospital stay and social security institution records for patients with out‐of‐hospital death.
Results
One‐year and 5‐year life expectancies of PE cases concomitant with different disorders were as follows; heart failure (n = 151), 81% and 70%; malignancies (n = 45), 53% and 44%; pulmonary diseases (n = 37), 89% and 78%; renal diseases (n = 18), 100% and 83%; respectively. PE associated with heart failure, renal disease, and pulmonary disease had similar (P > 0.05 for all) and favorable outcomes compared to PE associated with malignancies (P < 0.001).
Conclusions
The prognosis of incidental PE was the worst in patients with concomitant malignancies; however, PE associated with nonmalignant diseases including heart failure, pulmonary disease, and renal disease have similar and favorable outcomes.
Introduction
Diagnosis of pleural effusion (PE) is generally based on chest x‐ray findings. Ultrasound is more sensitive in the detection of PE.1 PE is also frequently detected during echocardiographic examination. The prognostic significance of incidentally detected PE during echocardiographic examination remains unclear.
Pleural space has important roles in respiratory functions. This space lies between visceral pleura, which stems from mesothelial cells and covers the lungs and parietal pleura that is attached to the chest wall.2 Pleural space normally contains 5 to 10 mL of fluid.3 There is continuous fluid excretion from parietal pleura and lymphatic drainage from visceral pleura.4 PE is diagnosed when the amount of fluid exceeds 15 to 20 mL. Abnormalities of hydrostatic pressure, oncotic pressure, pleural membrane permeability, and lymphatic drainage are probable etiologic factors for PE.5 Heart failure, pneumonia, malignant diseases, pulmonary embolism, and viral diseases are the leading causes of PE.5 The purpose of this study was to determine the long‐term prognostic implications of PE that is incidentally detected during echocardiographic examination and its relationship with concomitant diseases.
Methods
The study was performed by evaluating patient records in whom PE was incidentally detected during echocardiographic examination in a tertiary teaching hospital between 1999 and 2012. If there was more than 1 record for any patient, only the first record was included in the study. The diagnoses of patients were accepted according to International Classification of Diseases (ICD) codes recorded in their respective clinics. The patients were classified into 4 major groups: cardiovascular diseases, malignancy, renal dysfunction, and pulmonary diseases. Patients in whom PE was attributed to very rare causes such as rheumatologic (n = 3), gastrointestinal (n = 4), and unknown etiology were excluded. Finally, a total of 251 patients with adequate echocardiographic and follow‐up data were included in the study. The total survival time was obtained from hospital records for patients who died in the hospital and social security institution records for patients experiencing out‐of‐hospital death.
Echocardiographic Examination
Echocardiographic measurements were performed with VINGMED CFM‐800 (GE Healthcare, Little Chalfont, Buckinghamshire, UK) equipment until 2002, afterward all patients were evaluated by Vivid 7, GE VINGMED Ultrasound AS (GE Healthcare) equipment.
PE is diagnosed as an echo‐free space posterior to the heart in a patient in supine or left lateral position. The following criteria have been applied for differentiating PE from pericardial fluid in our echocardiographic laboratory: fluid appearing exclusively behind the left atrium is more likely to represent pleural than pericardial effusion. One of the more reliable distinguishing features between a pericardial effusion and PE is the location of the fluid‐filled space with respect to the descending thoracic aorta. The pericardial reflection is typically anterior to the descending aorta, and therefore fluid appearing posterior to the descending thoracic aorta is more likely to be pleural, whereas fluid appearing anterior to the aorta is more likely to be pericardial (Figure 1).6
Figure 1.
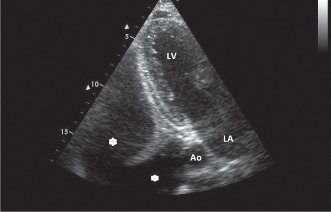
Echocardiographic image shows massive pleural effusion. Star‐shaped symbols indicate pleural effusion. Abbreviations: Ao, descending aorta; LA, left atrium; LV, left ventricle.
Statistical Analyses
The data were processed with Microsoft Excel 2007 (Microsoft Corp., Redmond, WA) and analyzed with the Statistical Package for the Social Sciences version 15.0 (IBM/SPSS, Armonk, NY). When available, cause of death was indicated using the medical record. Survival was calculated using a life tables analysis. The log‐rank test was used to determine whether there were any statistical differences between the distributions of the curves. We used the Student t test to compare the mean values obtained for PE. The level of statistical significance was set at P < 0.05.
Results
The prognostic data of PE according to concomitant illnesses are described below (Table 1).
Table 1.
Patients' Demographic Values and Data Regarding Pleural Effusion According to Concomitant Illness
| Concomitant Illness | Cardiovascular Diseases | Malignant Disorders | Pulmonary Diseases | Renal Diseases | ||||
|---|---|---|---|---|---|---|---|---|
| Alive (n = 100) | Dead (n = 51) | Alive (n = 20) | Dead (n = 25) | Alive (n = 28) | Dead (n = 9) | Alive (n = 15) | Dead (n = 3) | |
| Age, y | 61.3 ± 17.0 | 59.1 ± 15.7 | 59.8 ± 17.6 | 54.9 ± 16.2 | 55.5 ± 19.7 | 56.7 ± 16.9 | 51.2 ± 17.3 | 44.6 ± 17.9 |
| Gender, M/F, n | 62/38 | 35/16 | 14/6 | 12/13 | 13/15 | 6/3 | 11/4 | 1/2 |
| EF, % | 47.3 ± 13.3 | 47.0 ± 14.3 | 59.3 ± 8.1 | 60.9 ± 3.8 | 62.3 ± 3.9 | 60.3 ± 7.2 | 54.0 ± 14.0 | 50.6 ± 10.0 |
| Survey, mo | 95.1 ± 32.1 | 25.4 ± 30.2 | 80.9 ± 16.7 | 6.0 ± 8.5 | 82.6 ± 33.1 | 24.1 ± 33.7 | 90 ± 23.2 | 28.5 ± 17.9 |
Abbreviations: EF, ejection fraction; F, female, M, male.
PE Concomitant With Heart Failure
PE was due to heart failure in 151 patients. The mean age of these patients was 60 ± 16 years, with 97 male (64.2%) and 54 female (35.8%). The mean duration of follow‐up was 71.5 ± 45.6 months. Fifty‐one patients (33.8%) died during this follow‐up period. The mean duration of life after the index echocardiographic examination was 71.5 ± 45.6 months. There was no significant difference regarding age and sex between survivors and those died during follow‐up. Life expectancies of these patients were 81% for 1 year and 70% for 5 years.
PE Concomitant With Malignancies
There were a total of 45 patients, 26 male (57.8%) and 19 female (42.2%) in whom PE was due to a malignant disease. In this group, the mean age was 57 ± 16 years. There were 9 patients with lung cancer, 7 patients with hematological malignancies (lymphoma, leukemia, multiple myeloma), 6 patients with breast cancer, 5 patients with mesothelioma, 5 patients with gastrointestinal malignancies, 3 patients with soft tissue sarcomas, 2 patients with pancreatic cancer, 1 patient with carcinoid tumor, and 5 patients with malignancies of unknown origin. The mean duration of follow‐up was 39 ± 3.9 months. During this time period, 25 patients (55.6%) expired. The mean survival time after echocardiographic examination for this group was 6.0 ± 8.5 months. One‐year and 5‐year life expectancies of these patients was 53% and 44%, respectively.
PE Concomitant With Pulmonary Diseases
Pulmonary disease was responsible for PE in 37 patients including 19 male (51.4%) and 18 female (48.6%). The mean age was 55 ± 18 years. The leading diseases were chronic obstructive pulmonary disease, pulmonary embolism, empyema, and pulmonary tuberculosis. The mean duration of follow‐up 68.4 ± 41.5 months. Nine patients (24.3%) expired in this period. The mean survival time after the index evaluation was 24.1 ± 33.7 months. For these patients, life expectancy for 1 year was 89% and for 5 years 78%.
PE Concomitant With Renal Diseases
There were a total of 18 patients, 12 male (66.7%) and 6 female (33.3%), with PE due to renal dysfunction. The mean age was 50 ± 17 years. Fifteen patients had chronic kidney disease, 2 had nephritic syndrome, and 1 patient had acute renal failure. The mean duration of follow‐up was 79.8 ± 32.2 months. There were 3 deaths during follow‐up; 2 of them were female. The mean survival time after the index echocardiographic examination was 28.5 ± 17.9 months. One‐year and 5‐year life expectancies of these patients were 100% and 83%, respectively.
PE concomitant with heart failure, renal disease, and pulmonary disease have similar (P > 0.05 for all) and favorable outcomes compared to PE concomitant with malignancies (P < 0.001).
Discussion
In our study, we evaluated the prognostic significance of incidental PE diagnosed by echocardiography. According to our findings: (1) the worst prognosis of incidental PE is the setting of PE concomitant with malignancies, and (2) except PE concomitant with malignancies, other effusions with cardiovascular, pulmonary, and renal diseases were associated with similar prognostic significance. In the comprehensive workup of patients with PE, etiology should always be clearly determined.
PE Concomitant With Heart Failure
In accordance with other studies in this field, we found that PE is most commonly associated with heart failure (60.1%). PE, as an indicator of volume overload, is also an important finding of cardiac decompensation.7 Chest x‐rays, especially in intensive care units, are of low sensitivity in detecting PE. Ultrasonography is superior in the detection of PE in most aspects (eg, lack of radiation, ease of access and low cost).8 Kataoka and Takada revealed that ultrasonographic detection of PE is an important finding of decompensation.9 In addition, they revealed that PE, as detected by ultrasonography, is helpful in determining prognosis and correlated with clinical findings in patients with heart failure.9
One‐year mortality in heart failure is 20%, and half of the patients die within the first 5 years of diagnosis.10, 11 Poor prognostic factors for heart failure are principally increased age, hypotension, renal dysfunction, reduced left ventricular ejection fraction, and hyponatremia. The last few decades witnessed the improvement in prognosis for heart failure with reduced ejection fraction, but the prognosis is still considerably worse compared to patients with preserved ejection fraction.12 Pocock et al. evaluated 7599 patients with heart failure and reported a 2% frequency for PE in a 38‐month follow‐up period.13 However, the prognostic significance of PE is still not clear. In a study conducted in patients with acute heart failure, PE detected by conventional radiographic methods was not associated with prognosis. However, Devroey and Van Casteren reported that PE is associated an with increased rate of hospitalizations.7, 10 One‐year survival for the patients in our study (83%) was more favorable compared to Devroey and Van Casteren's findings. Long‐term survival expectancy was 74%. It is obvious that 1‐year and 5‐year life expectancies were more favorable in our study compared to other studies. This could be attributed to relatively younger age and higher left ventricular ejection fractions of patients in our study.
PE Concomitant With Malignancy
Malignancy is the second leading cause of PE after cardiovascular pathologies in the Western world.14 PE is seen in the half of the patients with a malignancy who develop metastasis.15 Lung and breast cancers and leukemias constitute 75% of the total cases of PE.16 Life expectancy in patients with malignant PE is lower than 1 year.16 The presence of malignant cells in PE specimens renders curative therapy almost impossible. and many of these patients receive symptomatic/palliative treatment. In patients with breast cancer, the mean time interval from diagnosis to the development of PE is 20 to 63.6 months, whereas the time from the onset of PE to death is 6 to 15.7 months.17 Life expectancy in these patients is as short as 4 months. In our study, life expectancy in patients with PE associated with malignancy was 53% for 1 year and 44% for 5 years. In 1 study, PE associated with malignancy could be commonly due to causes not directly associated with the malignity itself (eg, infection, lymphatic obstruction). We had no pleural cytological examination of our patients. The longer life expectancies observed in our study might be attributed to the absence of metastasis in many of the patients.
PE Concomitant With Pulmonary Disease
In our study, pulmonary disease was the third most common cause of PE. In the United States, there are nearly 1 million hospitalizations due to pneumonia, and half of these patients develop parapneumonic effusions.18 Increased consolidations seen in conventional radiographic imaging modalities render the diagnosis of PE more unlikely, especially if effusion is mild to moderate; ultrasonographic evaluation is more accurate in these patients. The frequency of PE is 32% for intensive care patients with chronic obstructive pulmonary disease.19 The presence of PE is associated with an increased mortality rate in these patients.18 PE is also associated with an increased duration of hospitalizations.19 In a study by Falguera et al. radiologic findings of PE were present in 19% of patients with community‐acquired pneumonias, and age (<60 years), tachycardia, pleuritic chest pain, alcoholism, and leukocytosis were independent predictors of PE.20, 21 Life expectancies for 1 year was 89% and for 5‐years was 78%.
PE Concomitant With Renal Diseases
End‐stage renal dysfunction was the principal cause of renal dysfunction‐associated PE in our study. Dialysis is associated with improved quality of life and prognosis in patients with end‐stage renal dysfunction.22 Hypervolemia was a serious problem observed in these patients. In patients receiving chronic hemodialysis, the incidence of PE was 20% in hospitalized patients.23, 24 Murtagh et al compared conservative treatment with dialysis in patients with end‐stage renal dysfunction ages 75 years and older.25 Life expectancy for 1 year and 2 years in the dialysis arm was 84% and 76%, respectively, whereas rates for the conservative treatment arm was considerably lower at 68% and 47%, respectively. Kang et al showed that the most important factor for determining prognosis in patients receiving peritoneal dialysis was age. In these stable end‐stage patients, cumulative life expectancy was 91.6% for 3 years and 78.7% for 5 years.26 In our study, renal dysfunction not only in dialysis patients was the fourth leading cause of PE. Life expectancy in patients with PE and renal dysfunction was 100% for 1 year and 83% for 5 years.
Study Limitation
The study findings should be applied only to the incidental PE diagnosed during echocardiography. Because only large collections can be visualized on echocardiography, nonmassive PE cases are not included in the study. Additionally, discrimination between bilateral, left‐, or right‐sided PEs was not made, therefore we cannot report on the influence of localization on the prognosis in this study. The second limitation is that the retrospective nature of the study precludes detailed analyses of effusion properties. Therefore, we cannot determine specific etiologic causes of PE; however, we can determine PE with concomitant primary illness. Another limitation is that we excluded rheumatologic (n = 3) and gastrointestinal (n = 4) etiologies from the study because of the too small patient numbers, which might lead misinterpretation. Thus, our study is applicable to all PEs except for rheumatologic and gastrointestinal etiologies.
Conclusion
The prognosis of incidental diagnosis of PE by echocardiography concomitant with malignancies is worse than other associated disorders. PE associated with nonmalignant diseases, heart failure, renal disease, and pulmonary diseases have similar and favorable outcomes.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Rumende CM. The role of ultrasonography in the management of lung and pleural diseases. Acta Med Indones. 2012;44:175–183. [PubMed] [Google Scholar]
- 2. Noppen M, De Waele M, Li R. Volume and cellular content of normal pleural fluid in humans examined by pleural lavage. Am J Respir Crit Care Med. 2000;162:1023–1026. [DOI] [PubMed] [Google Scholar]
- 3. Diaz‐Guzman E, Budev MM. Accuracy of the physical examination in evaluating pleural effusion. Cleve Clin J Med. 2008;75:297–303. [DOI] [PubMed] [Google Scholar]
- 4. McGrath EE, Anderson PB. Diagnosis of pleural effusion: a systematic approach. Am J Crit Care. 2011;20:119–127. [DOI] [PubMed] [Google Scholar]
- 5. Thomas R, Lee YC. Causes and management of common benign pleural effusions. Thorac Surg Clin. 2013;23:25–42. [DOI] [PubMed] [Google Scholar]
- 6. Feigenbaum H: The echocardiographic examination In Feigenbaum's Echocardiography. 6 edition Edited by: Feigenbaum H, Armstrong WF, Ryan T. Philadelphia: Lippincott Williams; 2005:251–255. [Google Scholar]
- 7. Davutoglu V, Yildirim C, Kucukaslan H, et al. Prognostic value of pleural effusion, CA‐125 and NT‐proBNP in patients with acute decompensated heart failure. Kardiol Pol. 2010;68:771–778. [PubMed] [Google Scholar]
- 8. Kataoka H. Ultrasound pleural effusion sign as a useful marker for identifying heart failure worsening in established heart failure patients during follow‐up. Congest Heart Fail. 2012;18:272–277. [DOI] [PubMed] [Google Scholar]
- 9. Kataoka H, Takada S. The role of thoracic ultrasonography for evaluation of patients with decompensated chronic heart failure. J Am Coll Cardiol. 2000;35:1638–1646. [DOI] [PubMed] [Google Scholar]
- 10. Devroey D, Van Casteren V. Symptoms and clinical signs associated with hospital admission and mortality for heart failure. Cent Eur J Public Health. 2010;18:209–214. [DOI] [PubMed] [Google Scholar]
- 11. Pantilat SZ, Steimle AE. Palliative care for patients with heart failure. JAMA. 2004;291:2476–2482. [DOI] [PubMed] [Google Scholar]
- 12. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 13. Pocock SJ, Wang D, Pfeffer MA, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. [DOI] [PubMed] [Google Scholar]
- 14. Light RW. Clinical practice. Pleural effusion N Engl J Med. 2002;346:1971–1977. [DOI] [PubMed] [Google Scholar]
- 15. Kastelik JA. Management of malignant pleural effusion. Lung. 2013;191:165–175. [DOI] [PubMed] [Google Scholar]
- 16. Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii32–ii40. [DOI] [PubMed] [Google Scholar]
- 17. dos Santos GT, Prolla JC, Camillo ND, et al. Clinical and pathological factors influencing the survival of breast cancer patients with malignant pleural effusion. J Bras Pneumol. 2012;38:487–493. [DOI] [PubMed] [Google Scholar]
- 18. Thomas R, Lee YC. Causes and management of common benign pleural effusions. Thorac Surg Clin. 2013;23:25–42. [DOI] [PubMed] [Google Scholar]
- 19. Meveychuck A, Osadchy A, Chen B, et al. Pleural effusion in chronic obstructive pulmonary medicine (COPD) patients in a medical intensive care unit: characteristics and clinical implications [in Hebrew]. Harefuah. 2012;151:198–201, 255. [PubMed]
- 20. Falguera M, Carratala J, Bielsa S, et al. Predictive factors, microbiology and outcome of patients with parapneumonic effusion. Eur Respir J. 2011;38:1173–1179. [DOI] [PubMed] [Google Scholar]
- 21. Chalmers JD, Singanayagam A, Murray MP, et al. Risk factors for complicated parapneumonic effusion and empyema on presentation to hospital with community‐acquired pneumonia. Thorax. 2009;64:592–597. [DOI] [PubMed] [Google Scholar]
- 22. Stuart S, Booth TC, Cash CJ, et al. Complications of continuous ambulatory peritoneal dialysis. Radiographics. 2009;29:441–460. [DOI] [PubMed] [Google Scholar]
- 23. Jarratt MJ, Sahn S. Pleural effusions in hospitalized patients receiving long‐term hemodialysis. Chest. 1995;108:470–474. [DOI] [PubMed] [Google Scholar]
- 24. Bakirci T, Sasak G, Ozturk S, et al. Pleural effusion in long‐term hemodialysis patients. Transplant Proc. 2007;39:889–891. [DOI] [PubMed] [Google Scholar]
- 25. Murtagh FE, Marsh JE, Donohoe P, et al. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant. 2007;22:1955–1962. [DOI] [PubMed] [Google Scholar]
- 26. Kang SH, Cho KH, Park JW, et al. Risk factors for mortality in stable peritoneal dialysis patients. Ren Fail. 2012;34:149–154. [DOI] [PubMed] [Google Scholar]


