Abstract
Objective
Slow‐wave activity (SWA) during sleep is reduced in people with amnestic mild cognitive impairment (aMCI) and is related to sleep‐dependent memory consolidation. Acoustic stimulation of slow oscillations has proven effective in enhancing SWA and memory in younger and older adults. In this study we aimed to determine whether acoustic stimulation during sleep boosts SWA and improves memory performance in people with aMCI.
Methods
Nine adults with aMCI (72 ± 8.7 years) completed one night of acoustic stimulation (stim) and one night of sham stimulation (sham) in a blinded, randomized crossover study. Acoustic stimuli were delivered phase‐locked to the upstate of the endogenous sleep slow‐waves. Participants completed a declarative recall task with 44 word‐pairs before and after sleep.
Results
During intervals of acoustic stimulation, SWA increased by >10% over sham intervals (P < 0.01), but memory recall increased in only five of the nine patients. The increase in SWA with stimulation was associated with improved morning word recall (r = 0.78, P = 0.012).
Interpretation
Acoustic stimulation delivered during slow‐wave sleep over one night was effective for enhancing SWA in individuals with aMCI. Given established relationships between SWA and memory, a larger or more prolonged enhancement may be needed to consistently improve memory in aMCI.
Introduction
Given the increasing age of the population1 and the staggering predicted rise in Alzheimer's disease (AD) prevalence,2 understanding the pathophysiology and risk factors for AD are of paramount importance to develop preventative and therapeutic strategies. Because most clinical trials in AD have been unsuccessful,3 focus has shifted to treating earlier stages of the disease. A precursor to the dementia caused by AD is amnestic mild cognitive impairment (aMCI) – a condition of memory dysfunction without impairment in functional independence – which carries a 60–65% lifetime risk of conversion into AD.4
Compelling evidence indicates that sleep disruption is a potential risk factor for the development of aMCI and AD dementia.5, 6, 7, 8, 9, 10 Several sleep disturbances have been observed in people with aMCI; the most pronounced changes include reduced amount of time spent in the deepest stage of non‐rapid eye movement sleep (NREM), also known as slow‐wave sleep (SWS). People with aMCI have reductions in SWS,8, 9, 10, 11 sleep‐spindle count10 and density,7, 12 and slow‐wave activity (SWA),10 a quantitative measurement of SWS determined by electroencephalographic (EEG) power in the 0.5–4 Hz frequency band.8, 9, 10 Interestingly, lower SWA correlates with higher levels of amyloid‐β, a marker of AD,13 and may therefore be an important therapeutic target.5
Sleep,14, 15 — more specifically SWS and SWA,16 spindles,17 and their functional coupling,18, 19 — has been shown to contribute to memory consolidation. During SWS, thalamo‐cortical network activity contributes to the consolidation of memories, particularly declarative memories, in the cortex.16, 20 One proposed mechanism for linking sleep and age‐related memory decline is that aging leads to atrophy in the medial prefrontal cortex (mPFC), a key anatomic location for slow‐wave generation.21 Indeed, mPFC atrophy may mediate memory impairments via SWA disruption6, 22 and lead to the functional uncoupling of slow‐wave and spindle activity23 critical for memory consolidation. In aMCI the functional integrity of the thalamo‐cortical network is already impaired beyond the normal effect of aging,24, 25 and therefore the influence of sleep on memory consolidation may be even further disrupted.
Given the critical link between SWS and memory, SWA could be a therapeutic target for staving off cognitive decline if it can be improved with noninvasive methods. However, people with aMCI have reduced SWA and more sleep fragmentation,8 making targeting SWS challenging. A recent study showed that slow‐oscillatory transcranial direct current stimulation (SO‐tDCS) during naps boosted SWA in people with aMCI,26 but this methodology has practical limitations such as ease of use and long‐term safety. In contrast, acoustic stimulation has no known side effects and can be developed for use at home. We have previously shown that acoustic stimulation of slow‐waves during overnight sleep can enhance SWA and improve memory in cognitively healthy older adults.27 In this study in people with aMCI, the primary aims were to examine (1) the feasibility of acoustic stimulation during overnight sleep to enhance SWA and (2) the relationship between SWA enhancement and memory.
Methods
Participants and experimental design
Nine adults with aMCI [mean age 72 years (range 62–86), 4 men, mean education 16 years (range 12–18)] were recruited from the Northwestern University Alzheimer's Disease Center Clinical Core registry. This study was registered at ClinicalTrials.gov (NCT02608840). The Northwestern University Institutional Review Board approved this study, and all participants provided written informed consent. Participants had undergone neuropsychological and neurological research evaluations within 1 year prior to this study, according to standardized data collection procedures contained in the Uniform Data Set.28, 29 The diagnosis of aMCI was based on current guidelines: a change in cognition compared to the individual's previous level, scores of 1.5 or more standard deviations below the mean compared to age, gender, and education level in one or more cognitive domains, including declarative memory.30 Diagnosis also required preserved independence in functional abilities and no impairment in social or occupational functioning. Exclusion criteria included: (1) alcohol or substance abuse; (2) history of seizures; (3) circadian rhythm disorder; (4) current hypnotic drug use; (5) hearing loss or hearing aid use; and (6) moderate‐severe sleep apnea (apnea‐hypopnea index ≥ 15 events/h) on the home sleep apnea test. Participants wore actigraphy monitors for 1 week prior to completing the overnight visits to determine habitual sleep and wake times.
A randomized crossover sham‐controlled study design was employed. Participants completed two overnight visits at least 1 week apart. Participants received acoustic stimulation during one visit (stim) and sham‐stimulation (sham) during the other visit while blinded to experimental condition. Sham stimulation consisted of an identical procedure with EEG tracking but without sound delivery. Participants completed a word‐pair learning task and two cued‐recall tests beginning 90 min prior to their habitual sleep time (Fig. 1). Lights were turned off at habitual sleep time and participants were given at least 8 h of opportunity to sleep during which sleep was monitored with polysomnography. One hour after waking, participants completed subjective sleep quality and alertness questionnaires followed by the morning cued‐recall test.
Figure 1.
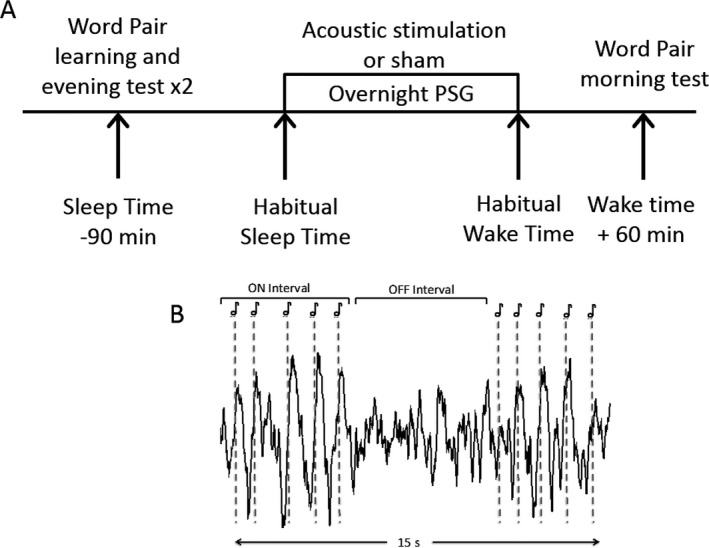
(A) Study protocol and schematic of acoustic stimulation paradigm. Lights out was set at habitual sleep time, with lights on at habitual sleep time +8 h. PSG: polysomnography. (B) Stimulation consist of five acoustic pulses separated by ~1 sec each (dotted lines, ON interval). Each ON interval was followed by ~6 sec of no sound (OFF interval). Stimulation (repeated sets of ON and OFF intervals) occurred for a mean of 122 min of the night, of which a 15 sec snippet is shown. Sham‐stimulation was identical but no sound was played.
Polysomnography recording
EEG was recorded from nine channels (Fpz, F3, F4, C3, C4, P3, P4, O1, O2) referenced to left mastoid. Impedances were lowered to <10 kΩ. Electro‐oculogram and chin electromyogram were also recorded. See Data S1 for additional EEG preprocessing details.
To examine the effect of stimulation on sleep structure, an experienced rater (RM) blinded to the experimental condition scored sleep staging and arousals offline with Polysmith (v.8.0, Nihon Kohden) reading software using the AASM scoring criteria.31 Duration (min) and percentage of time spent in each stage of sleep (N1, N2, N3, REM sleep), total sleep time, sleep efficiency, and arousal index (number/h) were calculated.
Phase‐locked acoustic stimulation
The phase‐locked loop acoustic stimulation methodology used in this study has been described extensively elsewhere32 and parameters for stimulation were in line with those used in the previous study of cognitively healthy older adults (Data S1).27 A single input from the midline frontopolar (Fpz) channel was used for online detection of sleep. Primarily during NREM stage 2 and 3, the adaptive phase‐locked algorithm delivered acoustic pulses targeted 20° before the peak of the up‐state of the endogenous slow‐oscillation (Fig. 2B). The stimulation routine consisted of 50 msec pulses of pink (1/f) noise, delivered in blocks of 5 (ON interval). Each ON interval was followed by a pause of five algorithmic oscillations (~6 sec) detected in the same way (OFF interval) (Fig. 1). Sounds were delivered through flat headphones inside a soft headband. Sound intensity was adjusted to a subjectively comfortable volume (30–50 dB) while awake but to a level that participants felt would not wake them up. Circular histograms for the mean phase of pulse delivery were calculated as previously described27 (Data S1) and used to assess slow‐wave targeting.
Figure 2.
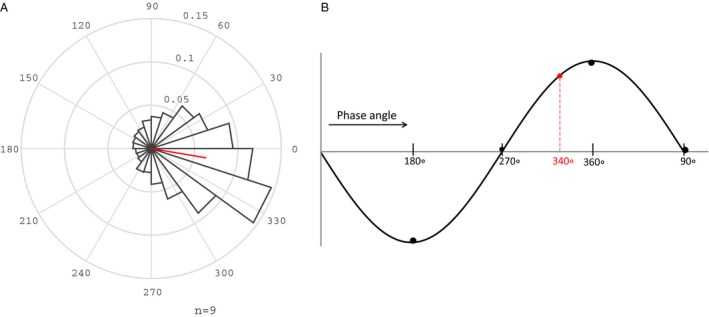
Phase targeting of acoustic pulses to the up‐state of the slow wave. (A) Distribution of phase for all acoustic pulses. Spread is depicted in 20 bins of 18°. Red line indicates mean phase vector. (B) Schematic of targeted phase angle (dotted red line) 20° prior to the peak of the up‐state (360/0°).
Cognitive assessments
To assess declarative memory, participants completed a verbal paired‐associate test including a learning phase and two cued‐recall tests before sleep and one cued‐recall test after sleep. The learning and testing paradigm (Data S1) followed previously published methods27, 33 in which participants viewed 44 moderately associated word pairs (e.g., tropics‐heat) as used in previous studies on sleep and memory.10, 33, 34, 35, 36 During each testing phase, participants completed a cued‐recall test in which they were presented with the first word of each pair and were given 10 sec to verbally recall the matching word and then given the correct word. A different learning word list was used for each overnight visit. Memory performance was measured as change in word recall from the second recall test in the evening to the morning test (morning score minus evening score).
To determine if domains other than verbal memory were influenced by stimulation, participants were also administered the NIH Toolbox Cognition Battery (NIHTB, Data S1), which includes a brief set of measures of working memory, picture memory, language and executive attention.37, 38 The NIHTB was administered in the morning in both stim and sham conditions.
Auditory event‐related potentials (ERPs)
Auditory ERPs were examined to assess the effects of stimulation on recordings from channel Fpz. ERPs were computed separately for pulse one through five, each with a 2‐sec window (500 msec before the pulse, and 1500 msec after), time‐locked to the each pulse of the ON intervals (time = 0) for stim and sham.
Sleep and cycle analysis
Spectral power analysis was conducted during acoustic stimulation to compare changes in spectral power between the ON and OFF intervals of each night and between conditions on channel Fpz. EEG data was band‐pass filtered (0.25–25 Hz). Mean power using a Fast Fourier Transform (4 sec window, 50% overlap) was calculated in the slow oscillation (SO, 0.5 to <1 Hz), “high” SWA (1–4 Hz), entire SWA band, (0.5–4 Hz), θ (>4–7 Hz), σ (>9–15 Hz) and β (>16–20 Hz) frequency bands for the ON and OFF intervals of the stim and sham nights. To account for differences between nights, average power for each frequency band was normalized to the total power in ON and OFF intervals for the entire frequency band (0.5–20 Hz). Average percent power difference between sham ON intervals and stim ON intervals was calculated. Sleep cycle analysis was used to examine the time course of SWA decline throughout the night (Data S1).
Given that spindles have been shown to play a role in memory consolidation during sleep14 and that previous studies of acoustic stimulation of slow waves have found concomitant changes in spindles,27, 36, 39 we also examined spindle changes in this study. Automated spindle detection (spindles between 9 and 16 Hz) was performed on channel Fpz for all ON and OFF intervals for stim and sham by adapting previously published methods40, 41 that have also been employed in older adults.27, 42 Additional details are described in Data S1. Spindles were examined for duration, peak amplitude, frequency, and density (number of spindles per minute of ON and OFF intervals).
Statistical analysis
Normality for all variables was tested using the Shapiro‐Wilk test. For between‐night differences in sleep characteristics (spectral power, PSG, spindles) and memory performance, paired two‐sample t‐tests were used. Circular statistics (mean, SD) for phase targeting were computed using the Matlab “circ_stat” toolbox.
Individual ERP analyses were conducted at each time point with paired sample t‐tests and corrected for multiple comparisons using a false discovery rate (FDR) of q < 0.05. To evaluate differences in ERP response from the first through the fifth pulse, a repeated measures ANOVA was used to evaluate Condition (stim/sham), Time (pulse 1–5), and Condition*Time. Mean absolute value of the ERP response following each pulse (0–1500 msec) for stim and sham were used in the ANOVA. Pearson correlations were used to test for associations between sleep characteristics and memory performance. Mean and standard error of the mean are reported as mean (SEM) unless otherwise noted.
Results
Phase targeting and polysomnography sleep characteristics
Mean SO phase for acoustic stimulation pulses was 350.1 (20.2)° (Fig. 2A). Mean duration of ON and OFF intervals of stimulation was 122.0 (20.7) min. Stimulation occurred for a mean of 75.3% (7.6) of stage N3, 37% (6.7) of stage N2, and a minimal percentage of stage N1, REM, and wake [3.6% (0.9), 1.4 (0.4)%, and 1.9% (0.3), respectively].
There were no significant differences between stim and sham in sleep‐staging characteristics, such as sleep duration, sleep efficiency, and arousal index (Table 1). There was a slight but nonsignificant reduction in time spent in stage N2 and stage N3 (P = 0.08). There were no significant differences between stim and sham in spindle characteristics or spindle density during ON intervals (Table 1).
Table 1.
Sleep macrostructure and spindle characteristics for stim and sham nights
| Sham | Stim | P | |
|---|---|---|---|
| Total sleep time (min) | 378.9 (12.7) | 364.2 (22.2) | 0.40 |
| Sleep efficiency (%) | 79.2 (2.8) | 75.8 (4.9) | 0.37 |
| Sleep latency (min) | 19.8 (4.9) | 23.8 (7.7) | 0.64 |
| Wake after sleep onset (min) | 81.6 (13.7) | 98.2 (18.1) | 0.62 |
| Stage N1 (min) | 33.1 (5.2) | 35.1 (4.2) | 0.65 |
| Stage N2 (min) | 226.3 (13.3) | 225.2 (18.4) | 0.95 |
| Stage N3 (min) | 60.3 (15.7) | 39.0 (8.2) | 0.18 |
| Stage REM (min) | 58.2 (11.9) | 64.9 (8.2) | 0.57 |
| Stage N2 + N3 (min) | 286.6 (14.8) | 264.1 (20.9) | 0.08 |
| Stage N1 (%) | 8.9 (1.5) | 9.8 (1.3) | 0.55 |
| Stage N2 (%) | 59.9 (3.5) | 61.7 (2.7) | 0.66 |
| Stage N3 (%) | 15.5 (3.6) | 10.3 (1.9) | 0.18 |
| Stage REM (%) | 15.6 (3.3) | 18.2 (2.3) | 0.31 |
| Stage N2 + N3 (%) | 32.6 (1.6) | 30.3 (2.2) | 0.10 |
| Arousal overall (number) | 81.9 (10.7) | 89.6 (13.0) | 0.54 |
| Arousal in stage N2 (number) | 34.8 (6.3) | 36.3 (5.8) | 0.80 |
| Arousal in stage N3 (number) | 1.8 (0.65) | 2.4 (0.67) | 0.46 |
| Arousal index total | 13.3 (1.9) | 15.4 (2.5) | 0.40 |
| Arousal index stage N2 (per hour) | 8.7 (1.3) | 10.0 (1.5) | 0.43 |
| Arousal index stage N3 (per hour) | 2.0 (0.72) | 3.4 (0.85) | 0.20 |
| Spindle amplitude (μV) | 19.2 (1.6) | 19.0 (1.6) | 0.86 |
| Spindle duration (msec) | 702.5 (37.2) | 694.0 (37.5) | 0.54 |
| Spindle frequency (Hz) | 11.3 (0.15) | 11.4 (0.11) | 0.31 |
| Spindle density (number/min) | 3.8 (0.53) | 3.9 (0.43) | 0.50 |
Spindle characteristics are described for ON intervals for sham and stim.
Event related potentials
The average ERP, aligned to the first pulse of the block, revealed a significant increase in both negative and positive potentials at about 500 and 1200 msec for each pulse, except for pulse five which was significant only at 500 msec (Fig. 3). An ANOVA revealed a significant Condition effect (P = 0.007) indicating an increase in ERP amplitude in stim compared to sham, which was observed across all five pulses (Time*Condition: P = 0.52).
Figure 3.
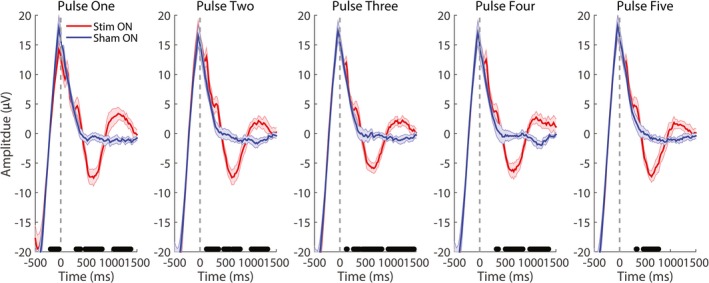
Event‐related potentials (ERPs) in response to acoustic stimuli. Grand average ERPs for stim and sham for each pulse of the series (one through five) in the ON interval aligned to the respective pulse. Black bars indicate P < 0.05 between stim and sham, FDR corrected for multiple comparisons.
Sleep spectral analysis
As depicted in Figure 4A, SO activity and SWA increased by 15.1% (2.8) and 11.4% (2.9), respectively, during stim ON compared to sham ON intervals (P < 0.01). “High” SWA was increased by 8.6% (5.1) compared to sham ON (data not shown, P < 0.01). Sigma activity was also increased by 16.2% (8.1), but this effect did not reach significance (P = 0.09). Theta and β activity remained unchanged by stimulation (Fig. 4A). SO and SWA decreased by 8.1% (3.3) and 6.6% (2.9) during stim OFF intervals compared to sham OFF intervals (P = 0.040 and P = 0.046, respectively, Figure 4B). No other effects were observed in θ, σ, or β activity (P > 0.8).
Figure 4.
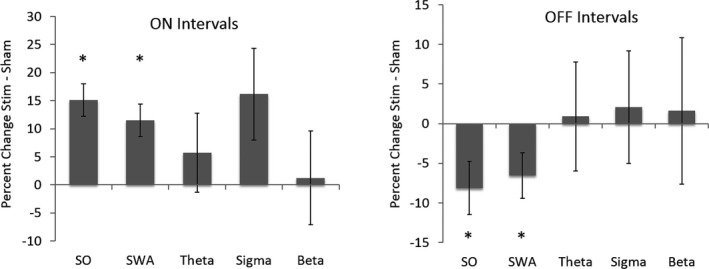
Percentage change in spectral power for SO (>0.5–1 Hz), SWA (>0.5–4 Hz), θ (>4–8 Hz), σ (>9–15 Hz), and β (>16–25 Hz) for stim and sham ON and OFF intervals. (A) SO and SWA power was significantly increased in stim ON compared to sham ON conditions, with a trend for σ power (SO: P = 0.001, SWA: P = 0.003, σ: P = 0.09). (B) SO and SWA power were significantly reduced in stim OFF compared to sham OFF condition (SO: P = 0.03, SWA: P = 0.04).
To measure within‐night effects of stimulation, we examined changes in ON compared to OFF intervals. Acoustic stimulation led to an SO increase in 22.2% (5.1, P = 0.003), a SWA increase in 17.9% (4.12, P = 0.004), and a θ increase in 5.0% (1.3, P = 0.02). Such differences were not present in the sham condition. No differences were observed for the σ and β bands in the stim condition (P > 0.15).
There was no difference in mean NREM SWA between stim and sham conditions [stim: 110.1 (10.1) μV2/Hz, sham: 119.3 (14.0) μV2/Hz, P = 0.08]. There was no difference between stim and sham in normalized SWA power (P = 0.30) nor for any other power bands during NREM sleep.
Examining SWA during ON intervals across the first four sleep cycles, there was a trend for increased SWA in stim ON compared to sham ON intervals that did not reach significance (Fig. S1A, condition: P = 0.05, cycle: P < 0.001, condition*cycle: P = 0.70) and no difference between stim OFF and sham OFF intervals (Fig. S1B).
Cognitive measures
In the two conditions, participants recalled a similar number of words both during the second recall test prior to sleep [stim: 12.6 (11.4), sham: 14.7 (11.2), P = 0.43] and after sleep [stim: 14.0 (13.0), sham: 14.8 (14.0), P = 0.39]. When examined as an overnight change (morning minus evening) performance was better in the stim condition, though this effect did not reach significance [Fig. 5A; stim: 1.4 (0.4), sham: 0.11 (1.3), P = 0.56]. Notably, most participants (5/9) recalled more words in the stim condition over sham (Fig. 5A). Morning NIHTB scores were the same between conditions (Table S1). Participants did not differ in self‐reported sleep quality in the morning after stim or sham intervention (Data S1).
Figure 5.
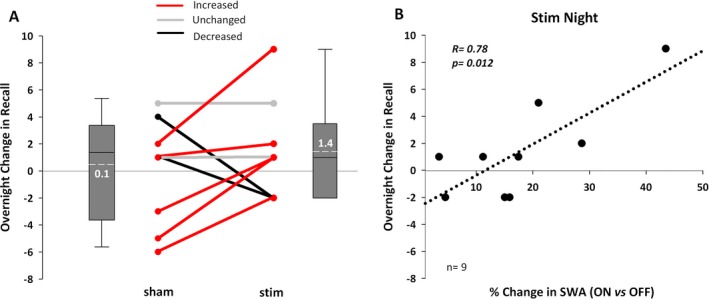
Word recall performance and associations with SWA. (A) Overnight change in number of words recalled (morning minus evening) for stim and sham. Red indicates five participants with a larger overnight improvement in recall in the stim compared to the sham condition. Gray represents two participants who performed the same, while black indicates two who performed worse in stim compared to sham. Box plots represent the increase in recall from evening to morning; the mean change was +1.4 words for stim and +0.1 words for sham (P = 0.39). (B) A positive association is shown between the increase in SWA in ON versus OFF intervals and the number of word pairs recalled in stim.
Despite the similarity in memory performance between conditions, there were significant associations between SWA enhancement and memory. The degree to which the stimulation enhanced SWA in ON versus OFF intervals positively correlated with overnight word recall (Fig. 5B, r = 0.78, P = 0.012). SO activity (r = 0.72, P = 0.029) and σ power (r = 0.72, P = 0.028) in ON versus OFF intervals were also associated with overnight change in recall, while θ and β power were not. The amount of stimulation based SWA increase in ON versus OFF intervals also correlated with total cognitive scores on the NIHTB in the stim condition (r = 0.75, P = 0.031), but not in the sham condition (r = −0.31, P = 0.46). No power bands in sham ON versus OFF intervals were associated with overnight word recall (all P's>0.20). There were no associations between word recall and spindle characteristics (e.g., density, amplitude) or sleep macrostructure (e.g., SWS, sleep efficiency) in stim or sham.
Discussion
In this study we provide initial evidence that acoustic stimulation is a viable technique for enhancing SWA during overnight sleep in people with aMCI. Furthermore, the degree of SWA enhancement was associated with overnight declarative memory improvement, indicating the potential of slow‐wave stimulation for mitigating memory difficulties.
Given that slow‐wave amplitudes are reduced in individuals with aMCI,10 whether the stimulation procedure would be viable and effective was questionable. Here, using the same parameters as in cognitively healthy older adults,27 acoustic stimuli successfully targeted the up‐state of slow‐waves. Our results indicate that slow‐waves in aMCI were (1) suitable for tracking by the algorithm for delivery of stimuli and (2) amenable to being enhanced.
Acoustic stimulation significantly increased SO by ~15% and SWA by ~11% in stim ON compared to sham ON intervals, similar to the enhancement seen in cognitively healthy older adults.27 Additionally, there was a trend for an increase in SWA during ON intervals throughout the first four cycles of sleep, particularly in cycle 2 and 3 of sleep showing a similar trend observed in young adults.45 Similarly, the lack of a difference in SWA in cycle 1 may might be due to variability or to ceiling effects.
We observed a re‐organization of power in which stimulation resulted in SWA enhancement during ON intervals followed by a reduction during OFF intervals, with no net effect on NREM SWA. This re‐organization of SWA has also been observed in older adults27 and younger adults45 using the same methodology. The increase in ON intervals was counteracted by a reduction in OFF intervals resulting in an absence of overall change in sleep macrostructure (e.g., SWS duration) and NREM SWA power across the night found in this study and elsewhere.27, 41 The lack of overall changes to NREM SWA may also be due to the method of stimulation (5 pulses ON, 5 OFF), which differs from other studies that use 1–2 pulses at fixed intervals36, 41, 46, 47 or due to a refractoriness in large‐scale neural synchronization. Other studies have found that longer trains of noninvasive stimulation using acoustic stimuli35 and SO‐tDCS48 can induce a reduction in power in slower oscillatory frequencies, though no adverse consequences on cognitive performance were observed.
As predicted from a previous study,27 the degree of SWA enhancement in ON versus OFF intervals was associated with memory performance on the verbal paired‐associates task and total score on the NIHTB. A greater enhancement in SO, SWA, and σ activity was associated with a greater increase in the number of correctly recalled words from evening to morning, consistent with previous findings in cognitively healthy older adults.27 This relationship suggests that the transient state or re‐organization of power induced by stimulation, rather than overall sleep changes, is critical for sleep‐dependent memory consolidation. The transient enhancement of SWA in ON versus OFF intervals is supported by a recent study in young adults using the same acoustic stimulation paradigm. Changes in ON versus OFF intervals were significantly associated with autonomic nervous system function,45 suggesting that the phenomena of SWA reorganization has widespread implications for physiology. Indeed there is evidence that the brain follows a cyclical cortical excitability between 0.02 and 0.2 Hz49, 50 and it may be that acoustic stimulation in 5 sec intervals capitalizes on this existing cyclical modulation.
In light of these findings, it appears that individuals with aMCI continue to maintain enough brain plasticity to allow for modulation during the ON versus OFF intervals of acoustic stimulation, adding credence to the feasibility of use as an interventional tool. Furthermore, it may be that a transient reorganization of SWA is enough for neuronal synchronization within the thalamo‐cortical network to potentiate memory consolidation.51, 52 Indeed, induced refractoriness from continuous acoustic stimuli ultimately did not negatively impact memory consolidation.36 Beyond a larger scale reorganization, our method of stimulation may also transiently induce coupling between SWA and spindles that supports memory consolidation, even though the strength of such coupling has been shown to deteriorate in older age.23 Nonetheless, the reorganization of SWA with acoustic stimulation is a relatively new observation,27, 45 warranting additional studies to further elucidate what mechanisms contribute to the observed phenomenon.
The finding of similar memory performance in the stim and sham conditions does not fit with the association between memory and the SWA ON versus OFF ratio. Five participants recalled fewer than 10 words prior to sleep, indicating difficulty with memory encoding and potentially limiting the ability of acoustic stimulation to affect sleep‐dependent consolidation. Furthermore, the degree of SWA enhancement was highly variable, as some participants responded while others did not (range: 1.4–29.8%). Further adjustments to the stimulation technique, such as an increase in the number or duration of stimuli or even multiple nights of stimulation, may be needed to achieve a consistently larger effect on slow‐wave enhancement or memory performance.
Despite an increase in σ power in stim ON intervals, we did not find significant differences in direct spindle characteristics, such as density or amplitude. In cognitively healthy older adults, acoustic stimulation has been shown to increase both σ power and spindle density during overnight sleep.27 Though there are no acoustic stimulation studies in aMCI for comparison, one study has demonstrated that SO‐tDCS enhances SWA and spindle activity during naps.26 Our results may differ, in part, due to differences in sleep physiology between daytime naps and overnight sleep as well as methodological differences between acoustic versus electrical stimulation. Alternatively, acoustic stimulation may not be as effective as SO‐tDCS in eliciting spindles in individuals with aMCI due to atrophy in the prefrontal cortex,51 hippocampus,52, or thalamus.24, 25
This study has several limitations. First, this study had a small sample size. However, the effect on SWA was rather robust, demonstrating the feasibility of acoustic stimulation as a tool for manipulating SWA. Second, the association between the SWA increase in ON/OFF intervals and word recall is impacted by a participant who had a large response to stimulation and a large overnight memory improvement. However, the response observed is not an outlier and is in line with the range of responsiveness observed in data reported previously in healthy controls, where at least five participants had a >35% increase in SWA.27 This study primarily aims to test feasibility of increasing SWA in aMCI, and therefore future studies must include larger sample sizes to further elucidate what contributes to responsiveness to acoustic stimulation during sleep and what impact this may have on memory. Future studies on repeated stimulation over multiple nights and wider cognitive assessments will be important to further determine the utility of acoustic stimulation as a potential therapeutic tool and intervention for sleep and memory decline in individuals with aMCI.
In conclusion, acoustic stimulation during sleep can enhance SWA in aMCI. This finding supports the hypothesis that deep sleep can be improved in individuals with aMCI. Although memory improved with stimulation in five of nine participants, it was not significantly better with stimulation. Yet, the degree of SO, SWA, and σ activity enhancement was positively associated with overnight memory consolidation, supporting the hypothesis that SWA remains important for memory consolidation in patients with cognitive decline. Furthermore, acoustic stimulation may prove useful in elucidating the relationship between sleep in memory in disease and as a potential early‐stage sleep intervention for aMCI.
Author Contributions
NAP, SW, SG, KAP, PCZ, RGM designed the study. NAP, TC, DG, RGM acquired and analyzed the data. NAP, SW, DG, KAP, PCZ, RGM prepared the manuscript.
Conflict of Interest
Dr. Santostasi currently serves as the scientific officer at DeepWave Technologies. Dr. Santostasi and Dr. Zee are inventors on a patent application for the phase‐locked loop technique of acoustic stimulation (described in manuscript) which has been filed by Northwestern University with the United States Patent and Trademark Office (US Patent Application Number 15/517,458). The remaining authors have no relevant conflicts of interest to disclose.
Supporting information
Figure S1. Slow wave activity (SWA) by cycle of sleep for ON and OFF intervals of stim and sham. The amount of SWA (0.5–4 Hz, channel Fpz) was calculated for ON (A) and OFF (B) intervals for each cycle of sleep normalized to SWA in all ON and OFF intervals for the entire night. Stim showed a trend for more SWA in ON intervals compared to sham, but the result did not reach statistical significance. There was no difference in stim OFF and sham OFF intervals.
Table S1. NIH Cognition Toolbox scores for each cognitive subtest and the composite scores for stim and sham Data S1. Supplemental methods and results.
Acknowledgments
This work was supported by grants from the Alzheimer's Association (NIRG‐15‐364483), the National Institute on Aging (P30AG013854, PI: Mesulam; P01AG11412, PI: Zee), the Illinois Department of Public Health (63282002D), the Northwestern University Center for Circadian and Sleep Medicine, a National Institutes of Health T32NS047987 and National Science Foundation Graduate Research Fellowship Program DGE‐1324585. We gratefully acknowledge the cooperation of the Northwestern Alzheimer's Disease Center Clinical Core and its participants and the staff of the Mesulam Center for Cognitive Neurology and Alzheimers Disease.
Funding information
This work was funded by Alzheimer's Association (NIRG 15‐364483), Illinois Department of Public Health (63282002D), National Institute on Aging (P01AG11412 (PI: Zee) and P30AG013854 (PI: Mesulam)), National Institutes of Health (T32NS047987), National Science Foundation Graduate Research Fellowship Program (DGE‐1324585) and Northwestern University Center for Circadian and Sleep Medicine.
Funding Statement
This work was funded by Alzheimer's Association grant NIRG 15-364483; Illinois Department of Public Health grant 63282002D; National Institute on Aging grants P01AG11412 (PI: Zee) and P30AG013854 (PI: Mesulam); National Institutes of Health grant T32NS047987; National Science Foundation Graduate Research Fellowship Program grant DGE‐1324585; Northwestern University Center for Circadian and Sleep Medicine grant .
References
- 1. Colby SL, Ortma JM. Projections of the size and composition of the U.S. Population: 2014 to 2060, current population reports. United States Census Bur. 2015;25–1143.
- 2. Association A. 2018 Alzheimer's disease facts and figures includes a special report on the financial and personal benefits of early diagnosis. Alzheimers Dement 2018;14:367–429. [Google Scholar]
- 3. Cummings JL, Morstorf T, Zhong K. AD drug development pipeline few candidates frequent failures. Alzheimers Res Ther 2014;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Busse A, Angermeyer MC, Riedel‐Heller SG. Progression of mild cognitive impairment to dementia: a challenge to current thinking. Br J Psychiatry 2006;189:399–404. [DOI] [PubMed] [Google Scholar]
- 5. Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer's disease? Trends Neurosci 2016;39:552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron 2017;94:19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Naismith SL, Mowszowski L. Sleep disturbance in mild cognitive impairment: a systematic review of recent findings. Curr Opin Psychiatry 2018;31:153–159. [DOI] [PubMed] [Google Scholar]
- 8. Hita‐Yañez E, Atienza M, Cantero JL. Polysomnographic and subjective sleep markers of mild cognitive impairment. Sleep 2013;36:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hita‐Yañez E, Atienza M, Gil‐Neciga E, Cantero JL. Disturbed sleep patterns in elders with mild cognitive impairment: the role of memory decline and ApoE ε4 genotype. Curr Alzheimer Res 2012;9:290–297. [DOI] [PubMed] [Google Scholar]
- 10. Westerberg CE, Mander BA, Florczak SM, et al. concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J Int Neuropsychol Soc 2012;18:490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raskind M, Peskind E, Gerber C, et al. Sleep, EEG and mental function changes in senile dementia of the Alzheimer's type. Neurobiol Aging 1982;3:361–370. [DOI] [PubMed] [Google Scholar]
- 12. Gorgoni M, Lauri G, Truglia I, et al. Parietal fast sleep spindle density decrease in Alzheimer's disease and amnesic mild cognitive impairment. Neural Plast 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Varga AW, Wohlleber ME, Giménez S, et al. Reduced slow‐wave sleep is associated with high cerebrospinal fluid Aβ42 levels in cognitively normal elderly. Sleep 2016;39:2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rasch B, Born J. About sleep's role in memory. Physiol Rev 2013;93:681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gais S, Born J. Declarative memory consolidation: mechanisms acting during human sleep. Learn Mem 2004;11:679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep‐dependent memory consolidation. Sleep Med Rev 2009;13:309–321. [DOI] [PubMed] [Google Scholar]
- 17. Schabus M, Gruber G, Parapatics S, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep 2004;27:1479–1485. [DOI] [PubMed] [Google Scholar]
- 18. Cox R, van Driel J, de Boer M, et al. Slow oscillations during sleep coordinate interregional communication in cortical networks. J Neurosci 2014;34:16890–16901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cox R, Hofman WF, Talamini LM. Involvement of spindles in memory consolidation is slow wave sleep‐specific. Learn Mem 2012;19:264–267. [DOI] [PubMed] [Google Scholar]
- 20. Diekelmann S. Sleep for cognitive enhancement. Front Syst Neurosci 2014;8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murphy M, Riedner BA, Huber R, et al. Source modeling sleep slow waves. Proc Natl Acad Sci USA 2009;106:1608–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mander BA, Rao V, Lu B, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal‐dependent memory in aging. Nat Neurosci 2013;16:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Helfrich RF, Mander BA, Jagust WJ, et al. Old brains come uncoupled in sleep: slow wave‐spindle synchrony, brain atrophy, and forgetting. Neuron 2017;97:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cantero JL, Atienza M, Gomez‐Herrero G, et al. Functional integrity of thalamocortical circuits differentiates normal aging from mild cognitive impairment. Hum Brain Mapp 2009;30:3944–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alderson T, Kehoe E, Maguire L, et al. Disrupted thalamus white matter anatomy and posterior default mode network effective connectivity in amnestic mild cognitive impairment. Front Aging Neurosci 2017;9:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ladenbauer J, Ladenbauer J, Külzow N, et al. Promoting sleep oscillations and their functional coupling by transcranial stimulation enhances memory consolidation in mild cognitive impairment. J Neurosci 2017;37:7111–7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Papalambros NA, Santostasi G, Malkani RG, et al. Acoustic enhancement of sleep slow oscillations and concomitant memory improvement in older adults. Front Hum Neurosci 2017;11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 2006;20:210–216. [DOI] [PubMed] [Google Scholar]
- 29. Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord 2010;23:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 31. Iber C, Ancoli‐Israel S, Chesson AL Jr, Quan SF. The AASM manual for the scoring of sleep and associated events: rules terminology and technical specifications, 1st ed Westchester, Illinois: American Academy of Sleep Medicine, 2007. [Google Scholar]
- 32. Santostasi G, Malkani RG, Riedner BA, et al. Phase‐locked loop for precisely timed acoustic stimulation during sleep. J Neurosci Methods 2016;259:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Westerberg CE, Florczak SM, Weintraub S, et al. Memory improvement via slow‐oscillatory stimulation during sleep in older adults. Neurobiol Aging 2015;36:2577–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marshall L, Mölle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci 2004;24:9985–9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ngo HV, Miedema A, Faude I, et al. Driving sleep slow oscillations by auditory closed‐loop stimulation–a self‐limiting process. J Neurosci 2015;35:6630–6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ngo HV, Martinetz T, Born J, Mölle M. Auditory closed‐loop stimulation of the sleep slow oscillation enhances memory. Neuron 2013;78:545–553. [DOI] [PubMed] [Google Scholar]
- 37. Weintraub S, Dikmen SS, Heaton RK. Cognition assessment using the NIH Toolbox. Neurology 2013;80: S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weintraub S, Dikmen SS, Heaton RK, et al. The cognition battery of the NIH Toolbox for assessment of neurological and behavioral function: validation in an adult sample. J Int Neuropsychol Soc 2014;20:567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ngo HV, Claussen JC, Born J, Mölle M. Induction of slow oscillations by rhythmic acoustic stimulation. J Sleep Res 2013;22:22–31. [DOI] [PubMed] [Google Scholar]
- 40. Ferrarelli F, Huber R, Peterson MJ, et al. Reduced sleep spindle activity in Schizophrenia patients. Am J Psychiatry 2007;164:483. [DOI] [PubMed] [Google Scholar]
- 41. Warby SC, Wendt SL, Welinder P, et al. Sleep‐spindle detection: crowdsourcing and evaluating performance of experts, non‐experts and automated methods. Nat Methods 2014;11:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosinvil T, Lafortune M, Sekerovic Z, et al. Age‐related changes in sleep spindles characteristics during daytime recovery following a 25‐hour sleep deprivation. Front Hum Neurosci 2015;9:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grimaldi D, Papalambros NA, Reid KJ, et al. Strengthening sleep‐autonomic interaction via acoustic enhancement of slow oscillations. Sleep 2019;42;zsz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leminen MM, Virkkala J, Saure E, et al. Enhanced memory consolidation via automatic sound stimulation during non‐REM sleep. Sleep 2017;40:zsx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Debellemaniere E, Chambon S, Pinaud C, et al. Performance of an ambulatory dry‐EEG device for auditory closed‐loop stimulation of sleep slow oscillations in the home environment. Front Hum Neurosci 2018;12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ketz N, Jones A, Bryant N, et al. Closed‐loop slow‐wave tACS improves sleep dependent long‐term memory generalization by modulating endogenous oscillations. J Neurosci 2018;38:7314–7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vanhatalo S, Palva JM, Holmes MD, et al. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc Natl Acad Sci USA 2004;101:5053–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lecci S, Fernandez LMJ, Weber FD, et al. Coordinated infraslow neural and cardiac oscillations mark fragility and offline periods in mammalian sleep. Sci Adv 2017;3:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rasch JBB, Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist 2006;12:410–424. [DOI] [PubMed] [Google Scholar]
- 50. Born J, Wilhelm I. System consolidation of memory during sleep. Psychol Res 2012;76:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mander BA, Marks SM, Vogel JW, et al. β‐amyloid disrupts human NREM slow waves and related hippocampus‐dependent memory consolidation. Nat Neurosci 2015;18:1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fogel S, Vien C, Karni A, et al. Sleep spindles: a physiological marker of age‐related changes in gray matter in brain regions supporting motor skill memory consolidation. Neurobiol Aging 2017;49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Slow wave activity (SWA) by cycle of sleep for ON and OFF intervals of stim and sham. The amount of SWA (0.5–4 Hz, channel Fpz) was calculated for ON (A) and OFF (B) intervals for each cycle of sleep normalized to SWA in all ON and OFF intervals for the entire night. Stim showed a trend for more SWA in ON intervals compared to sham, but the result did not reach statistical significance. There was no difference in stim OFF and sham OFF intervals.
Table S1. NIH Cognition Toolbox scores for each cognitive subtest and the composite scores for stim and sham Data S1. Supplemental methods and results.


