ABSTRACT
Primary malignancies of the heart and pericardium are rare. All the available data come from autopsy studies, case reports, and, in recent years, from large, specialized, single‐center studies. Nevertheless, if primary malignancy is present, it may have a devastating implication for patients. Malignancies may affect heart function, also causing left‐sided or right‐sided heart failure. In addition, they can be responsible for embolic events or arrhythmias. Today, with the widespread use of noninvasive imaging modalities, heart tumors become evident, even as an incidental finding. A multimodality imaging approach is usually required to establish the final diagnosis. Despite the increased awareness and improved diagnostic techniques, clinical manifestations of primary malignancy of the heart and pericardium are so variable that their occurrence may still come as a surprise during surgery or autopsy. No randomized clinical trials have been carried out to determine the optimal therapy for these primary malignancies. Surgery is performed for small tumors. Chemotherapy and radiation therapy can be of help. Partial resection of large neoplasms is performed to relieve mechanical effects, such as cardiac compression or hemodynamic obstruction. Most patients present with marginally resectable or technically nonresectable disease at the time of diagnosis. It seems that orthotopic cardiac transplantation with subsequent immunosuppressive therapy may represent an option for very carefully selected patients. Early diagnosis and radical exeresis are of great importance for long‐term survival of a primary cardiac malignancy. This can rarely be accomplished, and overall results are very disappointing.
Introduction
Primary malignancies of the heart and its sac are very uncommon and challenge clinicians' diagnostic ability and surgical skills.1 The overall incidence of both malignant and benign primary tumors is 0.02% in the United States, based upon data from autopsy series. The reported prevalence is between 0.0017% and 0.028%.2 Approximately one‐quarter to one‐third of all primary heart and pericardium tumors are malignant. But available data from single‐center studies vary, and the reported prevalence is between 3% and 28.7%, as shown in Table 1.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 With the exception of mesothelioma, or primary malignancy of the pericardium, primary malignancy of the heart consists of different sarcomas and lymphomas.1
Table 1.
| Authors | Year of Publication | Period of Data Collection | Total No. of Patients | Primary Tumors of the Heart and Pericardium, N (%) | Histopathological Entity, N (%) |
|---|---|---|---|---|---|
| Barreiro et al3 | 2013 | 1979–2012 | 73 | 11 (15.1) | Undifferentiated sarcoma, 4 (36.4); rhabdomyosarcoma, 2 (18.2); angiosarcoma, 2 (18.2); pericardial mesothelioma, 2 (18.2); osteosarcoma, 1 (9) |
| Thiene et al4 | 2013 | 1970–2010 | 239 | 26 (10.5) | Leiomyosarcoma, 5 (19); angiosarcoma, 5 (19); undifferentiated sarcoma, 4 (15.5); histiocytoma, 3 (11.5); mesothelioma, 3 (11.5); lymphoma, 3 (11.5); fibrosarcoma, 2 (8); malignant schwannoma, 1 (4) |
| Agaimy et al5 | 2012 | 1999–2011 | 74 | 5 (6.7) | Undifferentiated sarcoma, 4 (80); angiosarcoma, 1 (20) |
| Carrel et al6 | 2011 | 1996–2010 | 155 | 11 (7) | Angiosarcoma, 6 (54.5); leiomyosarcoma, 3 (27.5); unclassified sarcoma, 2 (18) |
| Kumar et al7 | 2011 | 1995–2010 | 188 | 14 (7.6) | Undifferentiated sarcoma, 6 (42.8); leiomyosarcoma, 1 (7.2); rhabdomyosarcoma, 2 (14.2); angiosarcoma,1 (7.2); non‐Hodgkin lymphoma, 2 (14.2); synovial sarcoma, 1 (7.2); PNET, 1 (7.2) |
| Patel et al 8 | 2010 | 1999–2008 | 94 | 27 (28.7) | Unclassified sarcoma, 11 (40.7); leiomyosarcoma, 5 (18.5); lymphoma, 4 (14.8); angiosarcoma, 3 (11.1); mesothelioma, 2 (7.4); synovial sarcoma, 1 (3.7); liposarcoma, 1 (3.7) |
| Matebele et al9 | 2010 | 2001–2008 | 19 | 1 (5.2) | Angiosarcoma, 1 (100) |
| Elbardissi et al10 | 2008 | 1957–2006 | 323 | 19 (6) | Leiomyosarcoma, 8 (42); angiosarcoma, 6 (31.5); fibrohistiosarcoma, 3 (16); rhabdomyosarcoma, 2 (10.5) |
| Yu et al11 | 2007 | 1996–2005 | 242 | 18 (7.4) | Angiosarcoma, 7 (38.9); mesenchymoma, 6 (33.3); leiomyosarcoma, 3 (16.6); mesothelioma, 1 (5.6); fibromyxosarcoma, 1 (5.6) |
| Bossert et al12 | 2005 | 1994–2003 | 77 | 4 (5.1) | Sarcoma, 1 (25); angiosarcoma, 1 (25); leiomyosarcoma, 2 (50) |
| Agarwal et al13 | 2003 | 1989–2001 | 34 | 2 (5.8) | Leiomyosarcoma, 2 (100) |
| Grande et al14 | 1993 | 1980–1992 | 31 | 5 (16.1) | Fibrosarcoma, 4 (80); lymphoma, 1 (20) |
Abbreviations: PNET, primitive neuroectodermal tumor.
Common Clinical Features
Despite increased awareness and improved diagnostic techniques, clinical manifestations of primary malignancies of the heart and pericardium are so variable that their discovery may still be incidental during surgery or autopsy. A wide range of unique clinical presentations may simulate other clinical entities. In some instances, the clinical presentation may mimic ischemic heart disease. Chest pain as a result of pericardial, pleural, and/or coronary involvement is typical of the primary malignancy of the heart.
The clinical features depend on tumor size, invasiveness, friability, rate of growth, and location.15
Signs and Symptoms
The majority of primary cardiac tumors are asymptomatic until they become large enough to cause signs and symptoms. Barreiro et al3 reported >90% symptomatic patients at the time of diagnosis, typically with heart failure (70%).
Symptoms may vary from specific cardiac symptoms to nonspecific symptoms of a neoplastic disease, which occurs in one‐third of patients.1, 2, 15 Most often, dyspnea, chest pain, syncope, and hemoptysis can be observed. A large variety of symptoms occurs, through several possible mechanisms (Table 2).2 Sometimes, sudden cardiac death can be the first and only presentation. It can be explained by occurrence of arrhythmias, coronary obstruction or embolization, tamponade caused by bleeding or effusion, and valvular obstruction.
Table 2.
Main Characteristics of Primary Malignancies of the Heart
| Dominant Chamber Location | Specific Signs and Symptoms According to Location | Mechanisms That Lead to Symptom Occurrence (Common to All Malignancies) |
|---|---|---|
| Right side of the heart (predominantly RA) | The tricuspid and pulmonary valve obstruction cause tricuspid regurgitation, thereby causing right‐sided HF and symptoms of obstructed right heart: | Mechanical obstruction and HF (right‐sided or left‐sided) |
| Angiosarcoma | Ascites | Impaired valvular function |
| Lymphoma | Hepatomegaly | Embolism: pulmonary, cerebrovascular, peripheral |
| Peripheral edema | Pericardial effusion and tamponade | |
| SVCS (with cyanosis, distended neck veins, and edema of the face and upper extremities) or obstruction of the IVC (with signs of right‐sided HF) | ||
| Left side of the heart | Tumors in the LA can obstruct the mitral or aortic valve. If the tumor obstructs the pulmonary venous return to the heart left side, it may lead to pulmonary congestion/edema. Symptoms such as dyspnea and orthopnea are common in these settings. | |
| Sarcomas of various lines of differentiation: undifferentiated sarcomas, osteosarcoma, leiomyosarcoma, fibrosarcoma, liposarcoma, synovial sarcoma, malignant nerve‐sheath tumors | They can interfere with the mitral valve, causing mitral regurgitation. Systemic embolization is associated with left‐sided tumors; consequently, strokes or TIAs are not unusual first symptoms. | |
| Any chamber | ||
| Rhabdomyosarcoma | ||
| Usefulness and the goals of imaging (echo, MRI, MDCT) in primary cardiac malignancies | ||
| Step 1: After abnormal clinical examination, initial technique is echo to ascertain if the mass and/or pericardial effusion are present and define the mass location, extent, and relationship. | ||
| Step 2a: MRI and/or MDCT to distinguish if the mass is a thrombus or a tumor and distinguish between benign and malignant lesions. | ||
| Step 2b: MDCT is superior to MRI for mesothelioma, or primary malignancy of the pericardium. | ||
Abbreviations: HF, heart failure; IVC, inferior vena cava; LA, left atrium; MDCT, multidetector computed tomography; MRI, magnetic resonance imaging; RA, right atrium; SVCS, superior vena cava syndrome; TIA, transient ischemic attack.
General symptoms of neoplastic disease include fever, malaise, and weight loss and can mimic an infectious disease. Chills and night sweats can be observed. Arthralgia and anemia due to tumors from neoplastic tissues can also be present.2 In addition, if metastases occur to distant organs (lungs, mediastinum, vertebral column, brain, thyroid gland, bones, and other organs), the spectrum of signs and symptoms can be even wider.
Electrocardiographic and Laboratory Analyses
Intramyocardial and intracavitary tumors both may affect cardiac rhythm through direct infiltration of the conduction tissue or through the irritation of the myocardium itself.1 Common electrocardiographic abnormalities include atrial fibrillation and ventricular tachycardia. Elbardissi et al10 reported that atrial fibrillation accompanied 16% of malignant cardiac tumors and ventricular tachycardia was present in 7%. Complete atrioventricular block has been described.16, 17 Another common finding is electrical alternans characterized by beat‐to‐beat alternation of the QRS complex due to swinging heart within a large pericardial effusion.
Most often, laboratory abnormalities include leukocytosis, elevated erythrocyte levels and sedimentation rate, hemolytic anemia, thrombocytopenia, and elevated C‐reactive protein levels, interleukin‐6, and other neuroendocrine factors.2 Release of systemic inflammatory biomarkers can cause difficulties and cause delay in diagnosis. Usually these laboratory abnormalities are observed in other clinical entities such as endocarditis, connective‐tissue disorders, and other malignancies.
Radiography: Chest X‐Ray
As an initial modality in symptomatic patients, a chest x‐ray is routinely performed. It can reveal abnormal findings including cardiomegaly, signs of heart failure, pleural effusion, pericardial effusion, pulmonary consolidation, and abnormalities in cardiac contour. Various cardiac intracavitary tumors enlarge a specific chamber.18
All presented abnormalities are common for other cardiac diseases and cannot be of help in distinguishing the final diagnosis of malignancy.5, 18
Imaging of Primary Heart and Pericardial Malignancies
Today, with the increased use of noninvasive imaging modalities, heart tumors become evident, even as an incidental finding. A multimodality imaging approach is usually required when investigating a suspected cardiac mass,19 as shown in Table 2. The choice of the imaging technique is guided by patient‐related factors, local availability, and provider experience.20 Imaging is valuable for the diagnosis, characterization, evaluation, and follow‐up of the tumor lesion.
Transthoracic echocardiography is the mainstay imaging technique for cardiac tumor detection and is performed after abnormal clinical examination. It is usually adequate for providing diagnostic information, such as a specific cardiac chamber and the size of a lesion. The technique has a limited acoustic window in some patients, for example in patients with large body habitus or emphysema.21 Transesophageal echocardiography affords improved spatial resolution. It is excellent for imaging of the left atrium, small cardiac lesions (<1 cm), and extracavital involvement. However, it is invasive, and imaging of anterior structures, the aortic arch, and left pulmonary artery is more difficult. It provides limited tissue characterization, making it often impossible to distinguish between thrombi and benign and malignant tumors. Contrast echocardiography allows better visualization between the tumor and an intramural thrombus.
Nowadays, 3‐dimensional transthoracic and transesophageal echocardiography offer the cardiologist and cardiovascular surgeon accurate preoperative and intraoperative assessment of cardiac masses and correlate with the pathological findings.19
Besides echocardiography, cardiac magnetic resonance imaging (MRI) has become the imaging modality of choice. Magnetic resonance imaging can significantly contribute in differentiating between benign and malignant cardiac tumors. Imaging findings suggestive of a malignant cardiac tumor include right‐atrial location, size >5 cm, hemorrhagic pericardial effusion, and delayed enhancement.20 Malignant tumors usually have a wide point of attachment, are large, and occupy almost the entire cardiac chamber. They can involve >1 cardiac chamber or great vessels. Further, they tend to be lobular, with not well‐defined margins and with a large base. Pericardial and extracardiac extension is often present. The wide field of view, together with its ability to acquire images of multiple plans, make this method effective in demonstrating intracardiac and extracardiac tumor extension. Tumor necrosis can also be seen, as well as multiple foci of calcification. These are the reasons why primary malignant heart tumors have distinctive features on MRI that can be used to differentiate them from primary benign heart tumors.22, 23, 24 Magnetic resonance imaging is the best‐facilitated surgical planning and post‐treatment follow‐up because of its ability to locate and delimit these tumors and also because of its 3‐dimensional assessment facilities.
Recent technological advances in multidetector computed tomography (MDCT), including conjunction with electrocardiographic gating, have made MDCT very important for evaluating a cardiac mass. In some respects it is superior to MRI because of spatial resolution, such as in primary pericardial malignancy. Multidetector CT is superior to MRI in mesothelioma, or primary malignancy of the pericardium, because of the lung parenchyma and pleura that can be simultaneously evaluated. Signs of asbestos‐related diseases, such as calcified pleural plaques, diffuse pleural thickening, and interstitial fibrosis, can be observed. Rahbar et al25 recently suggested that newly developed techniques, such as 18F‐fluorodeoxyglucose (FDG) positron emission tomography/CT, may be helpful in detecting metastasis from malignant cardiac tumors preoperatively.
Cardiac Sarcomas
Sarcoma accounts for the majority of primary malignancies of the heart. Patients may present with congestive heart failure refractory to medical therapy, arrhythmias, and myocardial ischemia due to intramyocardial invasion or hemopericardium. Mayer et al26 reported that in half the patients with cardiac sarcomas, many present with high‐grade tumors and distant metastases, especially in the lungs (35.7%), lymph nodes (14.2%), and liver (7.14%). Tumor spread from primary cardiac sarcoma to the bone is very rare and has a poor prognosis; only 6 cases have been reported by Strina et al.27
Histologically, sarcomas are classified into subgroups: angiosarcoma, sarcomas of various lines of differentiation, and rhabdomyosarcoma (Table 2).15
Angiosarcoma
Angiosarcomas represent approximately 30% to 45% of the malignant sarcomas.28 This is an aggressive primary malignancy that usually occurs over a wide age range, from 36 months to 80 years, with peak incidence at middle age. More frequently it appears in males, with a male‐to‐female ratio of 2:1.29 These are malignant tumors originating from vascular endothelium and are more often found in the right side of the heart (80% of cases in right atrium). They often replace the atrial wall and fill the entire atrium. Angiosarcomas may rapidly invade adjacent structures, such as the tricuspid valve, right‐ventricle free wall, ventricular septum, the great veins, and, in some cases, even the right coronary artery. In rare instances, angiosarcomas may develop within the wall of the pulmonary artery or the vena cava close to the heart. They may also arise from the epicardial surface of the heart and penetrate into the pericardial space. The majority have evidence of right‐sided heart failure and/or pericardial disease because of pericardial effusion, which can lead to tamponade. By the time of diagnosis, most patients have metastases (47% to 89%), most commonly to the lung, liver, brain, and bone.
Histologically, angiosarcomas consist of endothelial cells lining not well‐defined anastomotic vascular spaces. There may be large avascular spaces with areas of spindle cells. Immunohistochemical staining can be positive for factor VIII, von Willebrand factor, and CD31.
On echocardiography they typically appear as an echogenic nodular or lobulated mass in the right atrium with pericardial effusion or direct pericardial extension.15 If performed, MRI sequences tend to be sensitive to hemorrhage (T1‐weighted images). Areas of hemorrhage can be diffuse or nodular. After intravenous contrast with gadopentetate dimeglumine (Gd‐DTPA), enhancement along vascular lakes may be seen. Their appearance is described as “sunray.”15 They can be associated with sheetlike pericardial thickening.20
Prognosis is poor; without resection, 90% of patients die within 9 to 12 months of diagnosis. Treatment includes surgical resection with or without chemotherapy and radiation therapy. Complete excision is rarely possible. In some cases, surgery is performed when the diagnosis is not clear; other surgical exploration allows for tumor debulking. Chemotherapy or radiation therapy might be needed to prolong life expectancy.
Sarcomas of Various Lines of Differentiation
This subgroup of cardiac sarcomas contains heterologous elements. It is further subclassified as undifferentiated sarcoma, leiomyosarcoma, fibrosarcoma, liposarcoma, osteosarcoma, and cardiac Ewing sarcoma (Figure 1). These usually originate along the posterior wall and tend to exhibit slow, infiltrative growth.
Figure 1.
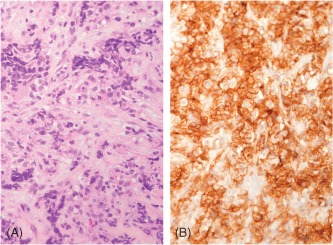
Primary cardiac Ewing sarcoma/PNET. (A) The tumor is composed of cells with indistinct cytoplasm and round to oval nuclei showing lobular growth pattern with intervening hyaline stroma (HE, 400× magnification). (B) The tumor cells show typical diffuse membranous staining for CD99 (CD99 IHC, 400× magnification). The diagnosis was confirmed by positive FISH study using the EWSR1 probe. Abbreviations: FISH, fluorescence in situ hybridization; HE, hematoxylin and eosin stain; IHC, immunohistochemical stain; PNET, primitive neuroectodermal tumor.
Undifferentiated Sarcoma
In recent reports, undifferentiated sarcoma has been found to account for 24% of primary heart malignancies.19 Most are found in the left atrium and have poor prognosis. On imaging studies, they appear as a discrete mass or irregular and infiltrative with necrosis and hemorrhage. Histological pictures and findings are shown in Figure 2.
Figure 2.
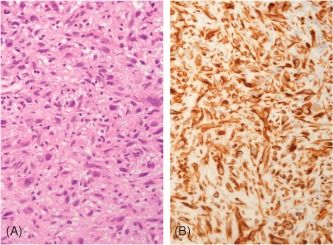
Primary cardiac undifferentiated sarcoma. (A) The tumor shows a solid growth pattern of pleomorphic cells with prominent nuclear atypia (HE, 400× magnification). (B) Only vimentin positively stains the tumor cells, whereas muscle markers (smooth muscle actin, desmin, and myogenin) and vascular markers (CD31, CD34, and factor VIII) were all negative (vimentin IHC, 400× magnification). Abbreviations: HE, hematoxylin and eosin stain; IHC, immunohistochemical stain.
Osteosarcoma
This primary malignancy of the heart has an incidence of 3% to 9% and is approximately twice as common in men.18 Predominantly it is attached to the wall of the left atrium, causing respiratory symptoms and left‐sided heart failure.2
Leiomyosarcoma
Leiomyosarcomas are very rare (8% of cardiac sarcomas) but highly aggressive invasive tumors, most often diagnosed in adults at about age 35 years.2, 18, 30, 31 They are often located in the posterior wall of the left atrium and tend to invade the pulmonary veins or the mitral valve. Rarely, a right‐atrial location has been reported.32
Patients present with signs and symptoms of right‐sided heart failure. Rhythm alternation, hemopericardium, and sudden death have been described. The optimum treatment of leiomyosarcoma is not known. Of the several reports in literature, patients subjected to multimodality treatment, including heart transplantation, have a longer survival time. The most common cause of death is local recurrence of the tumors, in 50% of the cases.32, 33
Other Cardiac Sarcomas
Fibrosarcomas and pleomorphic undifferentiated sarcoma (histiocytomas) constitute about 5% of primary malignant cardiac tumors in surgical series.19 Primary liposarcomas are extremely rare, accounting for <1% of cardiac sarcomas,19 and may occur in any cardiac chamber.
Rhabdomyosarcoma
Rhabdomyosarcomas are malignant tumors that present striated muscle differentiation and account for about 5% of all adult cardiac tumors. The patients range in age from 3 months to 80 years. It appears that rhabdomyosarcomas can arise in any of the cardiac chambers without specific predilection. Pericardium is usually involved by direct extension from the myocardium. These bulky (>10 cm in diameter) tumors can also extend to valve leaflets.2
Similar to other primary malignancies of the heart, the majority of patients with rhabdomyosarcoma present with nonspecific symptoms. The tumors are often nodular, soft with central necrosis, as confirmed by MDCT. Diagnosis is made by finding a typical rhabdomyoblast and supported by appropriate immunohistochemical stains (eg, myogenin, α‐sarcomeric actin).
Lymphoma
Lymphoma accounts for 1% to 2% of primary malignant cardiac tumors. The patients range in age from 18 to 77 years, with no specific differences in incidence among males and females. Incidence of lymphomas is increasing, as they are frequently observed in patients with immunodeficiency syndromes. Up to 20% of patients with non‐Hodgkin lymphoma will have evidence of cardiac lymphoma at autopsy.15 There has been an increased connection to acquired immune deficiency syndrome (AIDS) and transplant patients (eg, cardiac transplantation). The typical lymphoma is of non‐Hodgkin type and involves the heart and pericardium, without extracardiac involvement. The right atrium and right ventricle are the most frequently involved areas. The majority (80%) are diffuse large B‐cells (Figure 3), followed by T‐cell lymphoma, small‐cell lymphoma, and Burkitt lymphoma.
Figure 3.

Primary cardiac diffuse large B‐cell lymphoma. (A) The tumor shows sheets of cells with large atypical nuclei and prominent nucleoli (HE, 400× magnification). (B) The tumor cells show diffuse and strong staining for CD20 (CD20 IHC, 400× magnification) and positive staining for the proliferation marker Ki‐67 in 75% of the cells (not shown). Abbreviations: HE, hematoxylin and eosin stain; IHC, immunohistochemical stain.
Presenting symptoms are tamponade, atrial fibrillation, symptoms of right heart failure, and superior vena cava syndrome.
Echocardiography demonstrates hypoechoic myocardial masses in the right atrium or ventricle with associated pericardial effusion.18 Cardiac MRI has the highest sensitivity for detecting cardiac lymphoma because of its superior soft‐tissue contrast; MRI demonstrates poorly marginated and heterogeneous lesions that are isointense to slightly hypointense relative to cardiac muscle on T1‐weighted MR images and isointense on proton‐density‐weighted and T2‐weighted images. Diagnosis is confirmed by cytology of the serous fluid from pericardial or pleural effusion or biopsy of the pericardial mass.34, 35, 36
Treatment with anthracycline‐based chemotherapy and anti‐CD20 treatment, with or without radiation therapy, is considered the treatment of choice because of the sensitivity of lymphoma to chemotherapy. Surgical excision is not recommended. Palliative cardiac surgery is performed for tumor debulking. Rituximab in combination with standard chemotherapy has shown to improve survival. Multimodality treatment may include autologous stem‐cell transplantation.
Prognosis is poor; 60% of patients die within 2 months of diagnosis, regardless of treatment given. If it is caught in the early stage, durable remission has been reported in literature. In some cases it has been prolonged to 5 years with palliative treatment.
Primary Malignancy of the Pericardium
Malignant mesothelioma is neoplasm that can arise from the pericardial mesothelial‐cell layer and accounts for 50% of primary pericardial tumors. Patients range in age from 2 to 78 years with a mean age of 46 years and male‐to‐female ratio of 2:1.18
Most of the pericardial mesotheliomas are diffuse, cover visceral and parietal surfaces, and grow by direct extension into surrounding surfaces. Epicardial myocardium may be focally involved, but the tumor does not extend to the endocardial surface. Distant metastases are extremely unusual. Prognosis is poor, with few patients surviving beyond 12 months from the time of diagnosis.37 An association with asbestos exposure is assumed but not yet confirmed because of the rarity of this tumor. Because of its potential connection to asbestos‐related disease, MDCT is superior to MRI, as described previously. Pericardial mesotheliomas appear as multiple enhancing and coalescing pericardial masses that envelop the pericardial space. A long delay time is recommended for the initial set of images because of the poor vascularization of the tumor.38 Surgery combined with radiation therapy may be palliative.
Pericardial synovial sarcomas are very aggressive tumors. They are composed of spindle and epithelioid cells with imaging characteristics that at some point overlap with angiosarcoma. Thus, MDCT is suggested to distinguish the differential diagnosis. A heterogeneously enhanced, multilobulated mass with extensive pericardial infiltration and deep invasion is expected to be observed.20
Treatment and Prognosis: New Perspectives
Early diagnosis and radical exeresis are of great importance for long‐term survival of primary cardiac malignancy. This is rarely accomplished, and overall results are very disappointing. Unfortunately, most patients will die due to tumor‐related progressive heart failure. A minority will die due to distant metastasis.5 This is why it is not surprising that the optimal treatment of primary malignancy of the heart and pericardium still remains to be discovered.6 Because presenting symptoms appear late, these delayed manifestations generally reflect a wide local extension with severe cardiac damage. Orthotopic cardiac transplantation may represent an option in the setting of unresectable but locally aggressive tumors involving only the heart in the absence of metastases. Agarwal et al13 published the results of 8 patients who underwent orthotopic cardiac transplantation for primary cardiac neoplasms; 4 of them had malignant tumors. In the case of tumor‐free surgical margins, orthotopic heart transplantation provided long‐term survival without recurrence, despite immunosuppression.39 Removal of all cardiopulmonary structures involved by tumor in the patient population has been proposed to improve long‐term survival for malignancy of the heart. Talbot et al presented the data after heart and lung transplantation for highly selected patients with cardiac sarcomas. The median survival was 31 months, with a high incidence of metastatic disease limiting its utility.40
Conclusion
Our understanding of primary malignancies of the heart and pericardium has arisen during the last several decades. With the introduction of echocardiography, MRI, and CT in clinical practice, these malignancies have become evident and a part of clinical work. Yet, it seems that the treatment algorithm for patients with malignant cardiac tumors will remain poorly defined, because no randomized clinical trials have been carried out to determine the optimal therapy. Future work will lead to a better prognosis and survival rate of these rare but aggressive diseases.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Shapiro L. Cardiac tumors: diagnosis and management. Heart. 2001;85:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Castello J, Silvay G. Characterization and management of cardiac tumors. Sem Cardiothorac Vasc Anesth. 2010;14:6–20. [DOI] [PubMed] [Google Scholar]
- 3. Barreiro M, Renilla A, Jimenez JM, et al. Primary cardiac tumors: 32 years of experience from a Spanish tertiary surgical center. Cardiovasc Pathol. 2013;22:424–427. [DOI] [PubMed] [Google Scholar]
- 4. Thiene G, Basso C, Rizzo S, et al. Cardiac tumors: classification and epidemiology In: Basso C, Valente M, Thiene G, eds. Cardiac Tumor Pathology. New York, NY: Springer Science + Business Media; 2013:23–30. [Google Scholar]
- 5. Agaimy A, Rosch J, Weyand M, et al. Primary and metastatic cardiac sarcomas: a 12‐year experience at a German heart center. Int J Clin Exp Pathol. 2012;5:928–938. [PMC free article] [PubMed] [Google Scholar]
- 6. Carrel T, Erdös G, Eberle B, et al. Surgical treatment of cardiac tumours—an overview and presentation of interesting cases. Cardiovasc Med. 2011;12:242–257. [Google Scholar]
- 7. Kumar N, Agarwal S, Ahuja A, et al. Spectrum of cardiac tumors excluding myxoma: experience of a tertiary center with review of the literature. Pathol Res Pract. 2011;207:769–774. [DOI] [PubMed] [Google Scholar]
- 8. Patel J, Sheppard M. Pathological study of primary cardiac and pericardial tumors in a specialist UK centre: surgical and autopsy series. Cardiovasc Pathol. 2010;19:343–352. [DOI] [PubMed] [Google Scholar]
- 9. Matebele M, Peters P, Mundy J, et al. Cardiac tumors in adults: surgical management and follow‐up of 19 patients in an Australian tertiary hospital. Interact Cardiovasc Thorac Surg. 2010;10:892–895. [DOI] [PubMed] [Google Scholar]
- 10. Elbardissi A, Dearani A, Daly R, et al. Survival after resection of primary cardiac tumors: a 48‐year experience. Circulation. 2008;118(14 suppl):S7–S15. [DOI] [PubMed] [Google Scholar]
- 11. Yu K, Liu Y, Wang H, et al. Epidemiological and pathological characteristics of cardiac tumors: a clinical study of 242 cases. Interact Cardiovasc Thorac Surg. 2007;6:636–639. [DOI] [PubMed] [Google Scholar]
- 12. Bossert T, Gummert JF, Battellini R, et al. Surgical experience with 77 primary cardiac tumors. Interact Cardiovasc Thorac Surg. 2005;4:311–315. [DOI] [PubMed] [Google Scholar]
- 13. Agarwal V, Agarwal S, Srivastava AK, et al. Primary cardiac tumors: surgical experience and follow up. Indian Heart J. 2003;55:632–636. [PubMed] [Google Scholar]
- 14. Grande A, Ragni T, Viganò M. Primary cardiac tumors: a clinical experience of 12 years. Tex Heart Inst J. 1993;20:223–230. [PMC free article] [PubMed] [Google Scholar]
- 15. Burke A, Jeudy J Jr, Virmani R. Cardiac tumors: an update. Heart. 2008;94:117–123. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka Y, Yamabe H, Yamasaki H, et al. A case of reversible ventricular tachycardia and complete atrioventricular block associated with primary cardiac B‐cell lymphoma. Pacing Clin Electrophysiol. 2009;32:816–819. [DOI] [PubMed] [Google Scholar]
- 17. Nagano M, Uike N, Suzumiya J, et al. Successful treatment of a patient with cardiac lymphoma who presented with a complete atrioventricular block. Am J Hematol. 1998;59:171–174. [DOI] [PubMed] [Google Scholar]
- 18. Grebenc ML, Rosado de Christenson ML, Burke AP, et al. Primary cardiac and pericardial neoplasms: radiologic‐pathologic correlation. Radiographics. 2000;20:1073–1103. [DOI] [PubMed] [Google Scholar]
- 19. O'Donnell DH, Abbara S, Chaithiraphan V, et al. Cardiac tumors: optimal cardiac MR sequences and spectrum of imaging appearances. AJR Am J Roentgenol. 2009;193:377–387. [DOI] [PubMed] [Google Scholar]
- 20. Hoey E, Ganeshan A, Nader K, et al. Cardiac neoplasms and pseudotumors: imaging findings on multidetector CT angiography. Diagn Interv Radiol. 2012;18:67–77. [DOI] [PubMed] [Google Scholar]
- 21. Sparrow PJ, Kurian JB, Jones TR, et al. MR imaging of cardiac tumors. Radiographics. 2005;25:1255–1276. [DOI] [PubMed] [Google Scholar]
- 22. Frank H. Evaluation of congenital heart disease and cardiac masses by magnetic resonance imaging. J Kardiol. 2003;10:19–25. [Google Scholar]
- 23. Puppala S, Hoey ET, Mankad K, et al. Primary cardiac angiosarcoma arising from the interatrial septum: magnetic resonance imaging appearances. Br J Radiol. 2010;83:e230–e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buckley O, Madan R, Kwong R, et al. Cardiac masses part 2: key imaging features for diagnosis and surgical planning. AJR Am J Roentgenol. 2011;197:W842–W851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rahbar K, Seifarth H, Schäfers M, et al. Differentiation of malignant and benign cardiac tumors using 18 F‐FDG PET/CT. J Nucl Med. 2012;53:856–863. [DOI] [PubMed] [Google Scholar]
- 26. Mayer F, Aebert H, Rudert M, et al. Primary malignant sarcomas of the heart and great vessels in adult patients: a single‐center experience. Oncologist. 2007;12:1134–1142. [DOI] [PubMed] [Google Scholar]
- 27. Strina C, Zannoni M, Parolin V, et al. Bone metastases from primary cardiac sarcoma: case report. Tumori. 2009;95:251–253. [DOI] [PubMed] [Google Scholar]
- 28. Llombart‐Cussac A, Pivot X, Contesso G, et al. Adjuvant chemotherapy for primary cardiac sarcomas: the IGR experience. Br J Cancer. 1998:1624–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77:107. [DOI] [PubMed] [Google Scholar]
- 30. Zhang PJ, Brooks JS, Goldblum JR, et al. Primary cardiac sarcomas: a clinicopathologic analysis of a series with follow‐up information in 17 patients and emphasis on long‐term survival. Hum Pathol. 2008;39:1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vander Salm TJ. Unusual primary tumors of the heart. Semin Thorac Cardiovasc Surg. 2000;12:89–100. [DOI] [PubMed] [Google Scholar]
- 32. Parissis H, Akbar MT, Young V. Primary leiomyosarcoma of the right atrium: a case report and literature update. J Cardiothorac Surg. 2010;5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Donsbeck AV, Ranchere D, Coindre JM, et al. Primary cardiac sarcomas: an immunohistochemical and grading study with long‐term follow‐up of 24 cases. Histopathology. 1999;34:295–304. [DOI] [PubMed] [Google Scholar]
- 34. Ceresoli GL, Ferreri AJ, Bucci E, et al. Primary cardiac lymphoma in immunocompetent patients: diagnostic and therapeutic management. Cancer. 1997;80:1497–1506. [DOI] [PubMed] [Google Scholar]
- 35. Randhawa K, Ganeshan A, Hoey ET. Magnetic resonance imaging of cardiac tumors: part 2, malignant tumors and tumor‐like conditions. Curr Probl Diagn Radiol. 2011;40:169–179. [DOI] [PubMed] [Google Scholar]
- 36. Lee JC, Platts DG, Huang YT, et al. Position emission tomography combined with computed tomography as an integral component in evaluation of primary cardiac lymphoma. Clin Cardiol. 2010;33:E106–E108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaiuma S, Masai T, Yamauchi T, et al. Primary malignant mesothelioma presenting as pericardial constriction. Ann Thorac Cardiovasc Surg. 2008;14:396–398. [PubMed] [Google Scholar]
- 38. Patel J, Sheppard MN. Primary malignant mesothelioma of the pericardium. Cardiovasc Pathol. 2011;20:107–109. [DOI] [PubMed] [Google Scholar]
- 39. Basso C, Valente M, Poletti A, et al. Surgical pathology of primary cardiac and pericardial tumors. Eur J Cardiothorac Surg. 1997;2:730–738. [DOI] [PubMed] [Google Scholar]
- 40. Talbot SM, Taub RN, Keohan ML, et al. Combined heart and lung transplantation for unresectable primary cardiac sarcoma. J Thorac Cardiovasc Surg. 2002;124:1145–1148. [DOI] [PubMed] [Google Scholar]


