Abstract
Background
Elderly patients are at high risk of mortality when they present with ST‐elevation myocardial infarction (STEMI). However, few data exist about prognostic factors in this sub‐group when treated with primary percutaneous coronary intervention (pPCI).
Hypothesis
To assess outcome and predictors of mortality among patients aged >80 years treated with pPCI.
Methods
We evaluated 139 consecutive patients (age 85.1 ± 3.9 years, 43.2% males) who underwent pPCI for STEMI.
Results
Male patients were younger and were more likely to have a history of coronary artery disease. Overall 30‐day and 1‐year mortality rates were 20.9% and 28.1%, respectively. Thrombolysis in Myocardial Infarction (TIMI) flow 3 was achieved in 82% of patients. There was a pPCI success rate in male patients. At univariable analysis, older age, diabetes mellitus, Killip class >III, left ventricular ejection fraction (LVEF) <40%, no use of stent, failure of pPCI, systolic blood pressure (SBP) <100 mm Hg, and infarct‐related artery (left anterior descending vs others) were associated with higher 1‐year mortality. Multivariate analysis identified LVEF <40% (hazard ratio: [HR] = 3.70; 95% confidence interval [CI]: 1.30‐7.87; P = 0.0001), age (1‐year step, HR: 1.13; 95% CI: 1.04‐1.23; P = 0.007), failure of pPCI (HR: 2.93; 95% CI: 1.44‐5.98; P = 0.0001), Killip class ≥III (HR: 2.29; 95% CI: 1.03‐5.4; P = 0.04) and SBP <100 mm Hg (HR: 2.64; 95% CI: 1.22‐5.19; P = 0.01) to be independently associated with increased 1‐year mortality.
Conclusions
Our data show that elderly patients with STEMI have a high risk of mortality, which is particularly high in the first 30 days. Older age, LVEF <40% at admission, hemodynamic instability (higher Killip class or low SBP), and postinterventional TIMI flow <3 were independent predictors of mortality in our population.
Introduction
The population is progressively aging and cardiovascular prevention is improving in Western countries. Thus, the age of patients presenting with ST‐elevation myocardial infarction (STEMI) is expected to rise in the next decades. In elderly patients, the outcome after STEMI is worse than in younger people, but they are less likely to receive reperfusion therapies.1, 2 Primary percutaneous coronary intervention (pPCI) is recommended as the treatment of choice in patients with STEMI when feasible, regardless of age. Cardiologists are often hesitant in undertaking pPCI in very elderly patients, even if they are eligible for invasive reperfusion, because of a perception of poor procedure results, high procedure‐related complication rates, and a high prevalence of comorbidities in this subgroup.3
Moreover, there is paucity of evidence‐based data about safety and efficacy of pPCI in very elderly patients due to their exclusion and under‐representation in clinical trials.4 Therefore, it has become important to investigate invasive treatment modalities and outcomes in this subgroup in the real world.
The aim of this study was to assess the outcome and predictors of mortality among patients aged ≥80 years with STEMI, treated with pPCI, who were consecutively admitted to our hospital.
Methods
Between January 2008 and November 2012, a total of 728 consecutive and unselected patients were admitted to our hospital with STEMI. One hundred sixty‐five (22.8%) patients were ≥80 years old. From this subgroup, 139 patients who were treated with pPCI were included in the analysis.
Baseline characteristics and procedural data of all patients were entered prospectively into a dedicated database at the time of procedure by qualified cardiac catheterization laboratory personnel and doctors. Mortality data were collected retrospectively from either a review of hospital records or telephone contacts with patients or their referring physicians. Our prospective database was approved by the ethics committee, and patients signed informed consent.
Acute STEMI was diagnosed when symptoms of an acute myocardial infarction (MI) lasting 30 minutes to 12 hours was present, accompanied by an electrocardiogram with ST‐segment elevation >1 mm (0.1 mV) in 2 or more contiguous leads other than V2 or V3 (where 0.15 mV was required) or a new left bundle branch block.
Patients were transferred to the cardiac catheterization laboratory and underwent emergency coronary angiography to perform pPCI. Procedural decisions, including device selection and adjunctive pharmacotherapy, such as glycoprotein IIb/IIIa inhibitors, were made at the discretion of the operator. All patients were treated with intravenous (IV) heparin (5000 IU) or bivalirudin and aspirin (250 mg IV). Clopidogrel (300 or 600 mg) was administered orally before pPCI.
A successful procedure was defined as an infarct‐related artery stenosis <20% associated with Thrombolysis in Myocardial Infarction (TIMI) grade 3 flow. Failure of pPCI was defined as resulting in TIMI grade 0 to 2 flow, regardless of the residual stenosis.
Transthoracic 2‐dimensional echocardiography was performed in the intensive coronary care unit (CCU) before catheterization when feasible or when diagnosis was uncertain, and within 2 hours after catheterization in any case. Unstable patients (ie, hemodynamic instability, heart failure, shock) underwent echocardiography in the catheterization laboratory to assess left ventricular ejection fraction (LVEF) and exclude mechanical complications. After the procedure, those who survived underwent another echocardiography upon CCU admission within 2 hours.
Post‐pPCI antiplatelet therapy included lifelong aspirin (75–150 mg qid) and clopidogrel (75 mg qid) for 12 months, unless contraindicated.
Primary Outcome and Follow‐up
The primary end point of the present study was all‐cause 30‐day and 1‐year mortality. Clinical follow‐up data were obtained by either a review of hospital records or telephone contacts with patients or their referring physicians. When a patient reported a clinical event, it was confirmed by contacting the referring physician whenever possible. No patients were lost during follow‐up.
Statistical Methods
Data are presented as mean ± standard deviation for continuous data or as number and percent for discrete variables, unless otherwise specified. Continuous variables were shown as median and interquartile range (IQR) when they had a skewed distribution. Distributions of continuous data variables were compared using either the t test or the Mann–Whitney test, depending on whether the data followed a normal distribution. We compared distributions of categorical variables, expressed as frequency (percentage) using the χ2 test.
Cumulative event rates of all‐cause death were estimated using the Kaplan‐Meier method. The log‐rank statistic was used to test for significant differences in mortality between groups. Multivariate predictors of 30‐day death were analyzed by backward stepwise Cox proportional hazards regression. A Cox proportional regression multivariable model was constructed from variables having significant association with outcome at univariable analysis using backward selection, with a 0.05 significance level for keeping and 0.10 for removal. A P value of <0.05 was considered to be statistically significant. The proportional hazard assumption was tested for each variable included in the final model through graphical assessment. A Log‐minus‐log plot of survival and a Schoenfeld residual and time plot were used for categorical variables and continuous variables, respectively. Proportional hazard assumption was met for each variable.
Results
Baseline Characteristics
From January 2007 to November 2012, a total of 728 patients were admitted with a diagnosis of STEMI. There were 165 (22.7%) patients with age ≥80 years, and they were less likely to undergo pPCI than younger patients (81.3% vs 94.2%, P = 0.0001). A total of 139 patients (81.3% of the octogenarian cohort) underwent urgent coronary angiography for pPCI. The baseline characteristics of the octogenarian cohort including cardiovascular risk factors, previously known cardiovascular disease, comorbidities, and clinical presentation are summarized in Table 1. Patients treated with pPCI were slightly younger (85.1 ± 3.9 vs 87.6 ± 5, P = 0.007) and were less likely to have Killip class ≥ III (P = 0.0001) compared with patients treated conservatively.
Table 1.
Demographic and Clinical Characteristics of the Patients
| Characteristic | pPCI, n = 139 | Conservative, n = 26 | P |
|---|---|---|---|
| Age, y, median (IQR) | 85 (82–88) | 87 (84–91) | 0.004 |
| Female gender, n (%) | 79/139 (56.8) | 16/26 (61.4) | 0.41 |
| Hypertension, n (%) | 95/139 (68.3) | 17/26 (65.4) | 0.42 |
| Diabetes mellitus, n (%) | 30/139 (21.6) | 4/26 (15.4) | 0.38 |
| Dyslipidemia, n (%) | 27/139 (19.4) | 3/26 (11.5) | 0.26 |
| Smoke, n (%) | 8/139 (5.8) | 2/26 (7.7) | 0.49 |
| Previous MI or revascularization, n (%) | 30/139 (21.6) | 4/26 (15.4) | 0.34 |
| Previous antiplatelet therapy, n (%) | 42/139 (30.2) | 6/26 (23.1) | 0.31 |
| Left ventricular ejection fraction at admission, median % (IQR) | 40 (35–50) | 37.5 (30–45) | 0.36 |
| Systolic blood pressure <100 mm Hg at admission, n (%) | 28/139 (20.1) | 6/26 (23.1) | 0.47 |
| Heart rate >100 bpm at admission, n (%) | 29/139 (20.8) | 9/26 (34.6) | 0.09 |
| Killip class, n (%) | |||
| I | 82 (59) | 10/26 (38.5) | |
| II | 36 (25.9) | 4/26 (15.4) | |
| III | 12 (8.6) | 7/26 (26.9) | |
| IV | 9 (6.5) | 5/26 (19.2) | |
| Anterior MI, n (%) | 63/139 (45.3) | 11/26 (42.3) | 0.43 |
Abbreviations: IQR, interquartile range; MI, myocardial infarction; pPCI, primary percutaneous coronary intervention.
Among patients treated with pPCI, the mean age was 85.1 ± 3.9 years, and the oldest patient who underwent pPCI was 98 years old. There was a prevalence of female patients (79/139, 56.8%), and females were slightly older than males (85.7 vs 84.4 years, P = 0.047). LVEF at admission was 40.4 ± 10.2%. The majority of patients (82/139, 59%) were in Killip class I at admission, whereas 21 patients (15.1%) were Killip class III or IV. Sixty‐three patients (45.3%) presented with anterior STEMI. Thirty patients had a previous history of MI or revascularization (pPCI or coronary artery bypass grafting [CABG]), and 42 (30.2%) were on chronic antiplatelet therapy. Male patients were more likely to have a history of previous MI or revascularization (P = 0.006) and were more likely to be on chronic antiplatelet therapy at the time of admission (P = 0.005). There were no gender differences in cardiovascular risk factors (hypertension, dyslipidemia, smoking, or diabetes mellitus), LVEF at admission, and Killip class.
Procedural Characteristics
Procedural characteristics are summarized in Table 2. The majority of patients (82.7%) underwent catheterization through a radial approach, 7 (5.0%) patients had both a radial and femoral approach, and 17 (12.2%) had a femoral‐only approach. The culprit lesion was most often found in the left anterior descending artery. (48.2%). About one‐third of the patients (35.3%) had multivessel disease. Preprocedural TIMI flow 0 was documented in 69.8% of the patients. Success of the procedure (i.e, post‐procedural TIMI 3 flow) was achieved in 114 (82%) patients. Stents were used during pPCI in 76.3% of procedures. In 99/106 patients (93.4%), bare‐metal stents (BMSs) were placed. Of the 33 patients who did not received a stent during the procedure, 4 died before stent placement, in 2 patients only the guidewire crossed the lesion because of anatomy complexity, 9 patients received plain old balloon angioplasty (POBA) because of a failure to deliver the stent, 7 received POBA because of distal lesions with small‐diameter vessels, and 11 patients had massive thrombosis and were treated with thrombectomy, glycoprotein IIb/IIIa inhibitors, and POBA. These patients underwent a coronary angiography after 7 days of anticoagulation, and 9 of them received a stent during the second angiography; 4 received drug‐eluting stents (DESs) and 5 received BMSs. Manual thrombectomy was used among 71 (51.1%) patients. Glycoprotein IIb/IIIa inhibitors were used in 68.3% of the procedures. Procedural data did not differ significantly between males and females, except for a higher pPCI success rate in male patients (90.0% vs 75.9%, P = 0.04). The median time from onset of symptoms to balloon was 3.5 hours (IQR, 2.3–6.4). Door‐to‐balloon time was 38 minutes (IQR, 28–49 minutes). Bleeding complications were relatively low in our patients and not significantly higher in older patients. Intra‐aortic balloon counterpulsation was used in 4 patients (3.6%), all of whom had cardiogenic shock and anterior MI. There were 8 (5.8%) patients with in‐hospital bleeding requiring transfusion and 1 (0.7%) intracranial hemorrhage (Table 2).
Table 2.
Procedural Characteristics
| Characteristic | Value |
|---|---|
| Infarct‐related artery, n (%) | |
| Left main | 3/139 (2.2) |
| Left anterior descending artery | 67/139 (48.2) |
| Left circumflex artery | 16/139 (11.5) |
| Right coronary artery | 46/139 (33.1) |
| Saphenous vein graft | 3/139 (2.2) |
| Multivessel disease, n (%) | 49/139 (35.3) |
| Preprocedural TIMI flow, n (%) | |
| 0 | 97/139 (69.8) |
| 1 | 27/139 (19.4) |
| 2 | 8/139 (5.7) |
| 3 | 7/139 (5) |
| Postprocedural TIMI flow, n (%) | |
| 0 | 11/139 (7.9) |
| 1 | 3/139 (2.2) |
| 2 | 11/139 (7.9) |
| 3 | 114 (82.0) |
| Use of stent during pPCI, n (%) | 106 (76.3) |
| Use of glycoprotein IIb/IIIa inhibitors, n (%) | 95 (68.3) |
| Bivalirudin, n (%) | 19 (13.7) |
| Thrombus aspiration, n (%) | 71 (51.1) |
| Vascular access, n (%) | |
| Radial | 115 (82.8) |
| Femoral | 17 (12.2) |
| Both radial and femoral | 7 (5.1) |
| Use of IABP, n (%) | 5 (3.6) |
| In‐hospital bleeding requiring blood transfusion, n (%) | 8 (5.8) |
| In‐hospital definite stent thrombosis, n (%) | 2 (1.4) |
Abbreviations: IABP, intra‐aortic balloon counterpulsation; pPCI, primary percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
Clinical Outcome
Thirty‐day and 1‐year follow‐up were completed for all 139 patients treated with pPCI. Overall mortality at 30 days was 20.1% (28/139). One‐year mortality was 28.1% (39/139 patients). At univariable analysis, older age (P = 0.004), diabetes mellitus (P = 0.003), Killip class >3 (P = 0.001), LVEF <40% (P = 0.0001), use of stent (P = 0.003), failure of pPCI (TIMI flow <3, P = 0.0001), systolic blood pressure <100 mm Hg (P = 0.05), and infarct‐related artery (left anterior descending [LAD] or left main vs others, P = 0.009) were associated with a higher mortality rate at 1 year. Multivariate Cox proportional hazards analysis identified impaired LVEF (<40%) at admission (hazard ratio [HR]: 3.70; 95% confidence interval [CI]: 1.38‐7.87; P = 0.0001), age (1‐year step, HR: 1.13; 95% CI: 1.04‐1.23; P = 0.007), failure of pPCI (defined as TIMI flow post‐pPCI <3, HR: 2.93; 95% CI: 1.44‐5.98; P = 0.0001), Killip class ≥3 (HR: 2.29; 95% CI: 1.03‐5.4; P = 0.04,), and SBP <100 mm Hg (HR: 2.64; 95% CI: 1.22‐5.19; P = 0.01,) to be independently associated with an increased risk of mortality at 1 year (Table 3). Figure 1A shows mortality according to age tertiles. Failure to achieve TIMI 3 flow and LVEF <40% at admission were the strongest predictors of mortality (Figure 1B,C).
Table 3.
Predictors of 1‐Year Mortality: Univariable Analysis and Multivariable Cox Proportional Stepwise Regression Analysis
| Variable | Univariable HR (95% CI) | Univariable P Value | Multivariable HR (95% CI) | Multivariable P Value |
|---|---|---|---|---|
| Age (1‐year step) | 1.11 (1.04‐1.19) | 0.004 | 1.13 (1.04‐1.23) | 0.004 |
| Diabetes | 2.12 (1.11‐4.06) | 0.003 | ||
| Killip class (≥III) | 2.87 (1.43‐5.75) | 0.001 | 2.29 (1.03‐5.40) | 0.04 |
| SBP <100 mm Hg (admission) | 2.00 (1.00‐3.95) | 0.05 | 2.64 (1.22‐5.19) | 0.01 |
| LVEF <0.40 (admission) | 3.89 (1.92‐7.87) | <0.0001 | 3.70 (1.38‐7.87) | 0.0001 |
| Use of stent | 0.39 (0.21‐0.73) | 0.003 | ||
| TIMI flow 3 postprocedure | 3.87 (2.04‐7.40) | <0.0001 | 2.93 (1.44‐5.98) | 0.001 |
| Infarct‐related artery (LAD or left main vs other) | 1.67 (1.13‐2.40) | 0.009 |
Abbreviations: CI, confidence interval; HR, hazard ratio; LAD, left anterior descending; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; TIMI, Thrombolysis in Myocardial Infarction.
Figure 1.
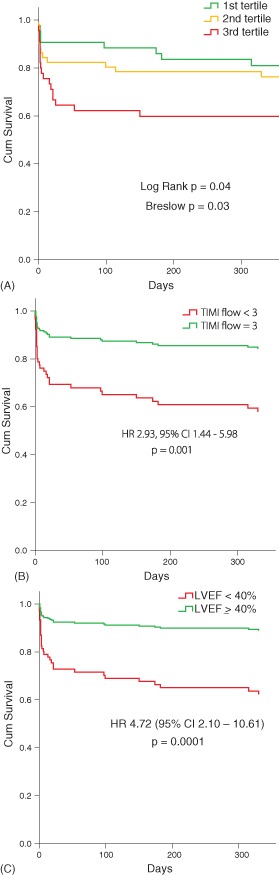
(A) Kaplan‐Meier survival analysis in regard to total mortality during 1 year of follow‐up according to age tertiles. Lowest age tertile is 80 to 82 years, middle age tertile is 83 to 86 years, and the highest age tertile is >87 years (unadjusted). (B) Adjusted survival analysis according to postprocedural Thrombolysis in Myocardial Infarction (TIMI) flow in the infarct‐related artery. Adjusted for age, sex, postprocedural TIMI flow, systolic blood pressure (SBP), diabetes, Killip class, use of stent, infarct‐related artery. (C) Adjusted survival analysis according to left ventricular ejection fraction (LVEF) at admission. Adjusted for age, sex, LVEF, SBP, diabetes, Killip class, use of stent, infarct‐related artery. Abbreviations: CI, confidence interval; HR, hazard ratio.
Among the 26 patients who were treated conservatively during the same period, the mortality at 30 days was 50.0%, and the mortality at 1 year was 57.7%. Figure 2 shows unadjusted survival curves between patients treated with pPCI with procedural success, patients treated with pPCI without procedural success (TIMI flow <3), and patients treated conservatively.
Figure 2.
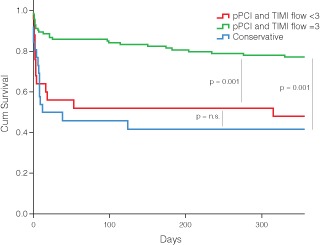
Unadjusted survival curves stratified by patients treated with primary percutaneous coronary intervention (pPCI) with procedural success, patients treated with pPCI without procedural success (TIMI flow <3), and patients treated conservatively. Abbreviations: n.s., not significant; TIMI, Thrombolysis in Myocardial Infarction.
Medical Therapy at Discharge
One hundred eighteen patients treated with pPCI and 15 patients treated conservatively survived to discharge. Patients treated with pPCI were more likely to receive secondary prevention medications at discharge. All pPCI patients were on dual antiplatelet therapy (DAPT) compared to 73.3% of patient treated conservatively (P = 0.001). However, 93.3% of conservatively treated patients were either on aspirin or P2Y12 inhibitors. Patient treated with pPCI were more likely to be on statins (94.9 vs 66.7%, P = 0.003). Use of β‐blockers were comparable between the 2 groups (pPCI: 74.4% vs 73.3% for conservative, P not significant), whereas there was a no significant trend toward less angiotensin‐converting enzyme inhibitors/angiotensin II receptor blocker prescriptions among patients treated conservatively (76.9% vs 53.3%, P = 0.053). Dual antiplatelet therapy continuation was prescribed for 12 month unless contraindicated.
Discussion
This observational study from a single center was conducted in a cohort of 139 patients age ≥ 80 years undergoing pPCI and outlines a real‐world experience for invasive treatment of STEMI in the very elderly. Our experience confirms that these patients account for a significant proportion of patients with acute MI. In fact, 1 out of 5 STEMI patients admitted to our hospital was an octogenarian or older. This proportion is higher than others reported in the past, but is consistent with the increasing trend of elderly patients with STEMI observed in the last years.5
Our data demonstrate that pPCI in the very elderly is feasible and has a high likelihood of success, although it is lower than in younger populations. Procedural success was 82%, which is comparable to other reports in similar populations.5, 6, 7 More complex anatomy, calcifications, and tortuosity of the vessels may account for lower procedural success in these patients. Incidence of bleeding requiring transfusion was 5.8%, a rate slightly higher than those reported in younger populations but similar to other studies among the elderly.6, 7 The failure to achieve TIMI 3 flow after pPCI was a strong predictor of early mortality in our population, with a 3‐fold increased risk of death. Interestingly, we observed similar mortality between patients treated conservatively and patients treated invasively who had an unsuccessful procedure. Although this observation was unadjusted for important biases (patients treated conservatively were more likely to be sicker), it suggests that restoration of TIMI 3 flow in elderly STEMI patients is critical to improve survival.
Benefits of reperfusion therapy have been attributed to the prompt restoration of normal blood flow in the infarct‐related artery, and the aim of all interventions is restoration of normal TIMI 3 flow within the epicardial coronary vessels. In fact, successful angiographic reperfusion after pPCI is associated with improved survival.8
It is worth noting that we found a trend toward higher procedural failure in females. This finding is consistent with a study of Brener et al. 8 that reported lesser degrees of epicardial reperfusion in women after pPCI. However, these data may be due to chance and need further investigation. Furthermore, no difference in mortality between males and females was found in our study.
Relative risk reduction provided by pPCI has been found to be similar in elderly and younger patients.9 However, because elderly patients are at higher risk of mortality they could benefit more from successful pPCI.
This subgroup is characterized by a high mortality rate, which is remarkably high in the first 30 days after hospitalization. Among our patients, higher age, reduced LVEF, low SBP at admission, high (III to IV) Killip class, and failure of pPCI were independently associated with higher mortality rates.
The 30‐day and 1‐year mortality observed in our population is consistent with those reported in recent studies among octogenarian and nonagenarian STEMI patients.5, 10, 11, 12, 13 In populations similar to ours, Antonsen et al.,10 Claessen et al.,5 Showkathali et al.,13 and Shah et al. 11 reported 30‐day mortality of 18%, 20%, 21%, and 25%, respectively. Newell et al. 12 reported a 1‐year mortality of 28.9% among patients age ≥80 years. Mortality in the first month accounts for roughly 70% to 75% of total mortality observed in the first year of follow‐up.
The causes of such high mortality are thought to be multifactorial. Previous studies have shown that age alone is a strong predictor for short‐ and long‐term mortality after pPCI.14 Furthermore, a higher prevalence of comorbidities and previous ischemic heart disease with subsequent left ventricular dysfunction may contribute to the unfavorable prognosis.2, 7
Late presentation after the onset of symptoms has been suggested as a possible cause of poor outcome in this subgroup. Elderly patients with STEMI most often have atypical symptoms causing prolonged patient delay (time of first symptom until first medical contact) and a subsequent delayed reperfusion, which is known to be less effective.15 It has been reported that approximately 25% of elderly patients with STEMI wait >6 hours before seeking help.11 In our population, time from onset of symptoms to balloon was 3.5 hours, which is comparable to the time observed in other studies among octogenarians,10 and longer than delays observed in younger populations.7 Longer ischemic time is associated with lower likelihood of TIMI 3 flow and greater myocardial necrosis. This could be a reason for the worse outcome in elderly patients.
Finally, when evaluating the high mortality rate in our population, it is important to emphasize that we treated the majority of our elderly STEMI cohort with pPCI (i.e., 81.3%). A recent study of Medina et al. among octogenarian patients with STEMI from the Get With The Guidelines–Coronary Artery Disease program reported a lower mortality in pPCI‐treated patients. However, in that registry only 44.9% of STEMI patients were treated with pPCI. Patients with comorbidities such as low LVEF, heart failure, renal impairment, pulmonary disease, previous MI, and arrhythmias were less likely to be treated with reperfusion therapy.16 Therefore, there could be a risk–treatment paradox when selecting therapies for elderly patients with STEMI. Higher risk patients are less likely to receive evidence base treatment. In our study, we included almost all eligible patients regardless of comorbidities, and therefore our pPCI population had a higher overall risk, which resulted in higher mortality.
In previous studies, cardiogenic shock and hemodynamic instability have been observed to occur more often in elderly STEMI patients, and have been indicated as strong predictors for in‐hospital and short‐term mortality2, 5, 6 Compared with these previous studies, the rate of cardiogenic shock in the present study was lower (6.5% of patients in Killip class IV). However, both higher Killip class (III–IV) and low blood pressure at admission resulted as independent predictors of mortality in our population, thus confirming the negative impact of hemodynamic instability on outcome.
Our population was treated mostly with BMSs. The lower utilization of DESs among our elderly patients reflects widespread perception of the higher risk of bleeding in elderly patients and the reluctance of operators to commit elderly patients to DAPT for a prolonged period without a detailed clinical history, which is often not available for patients presenting for pPCI. Although elderly patients are less likely to receive DESs, other authors reported a higher use of DESs than ours.10, 13
Limitations
The current study is an analysis of a cohort of consecutive patients admitted for STEMI and evaluated and treated by the investigators. The treatment was left to the discretion of the physician. This may have introduced a selection bias, which cannot be fully eliminated even by using multivariate analysis. Only patients undergoing pPCI were evaluated prospectively, which might reflect a selection bias toward the more fit elderly who had been considered for interventional treatment by the treating physician. Only all‐cause mortality was evaluated. Therefore, the exact causes of the high fatality rates remain unknown. Apart from the demographics presented in this article, other factors contributing to the increased mortality in elderly patients (such as renal dysfunction, anemia, frailty index, and noncardiac atherosclerosis) were not analyzed in this study.
Conclusion
These data show that very elderly patients with STEMI have considerably high mortality, which is particularly high in the first 30 days after hospitalization. Older age, LVEF <40% at admission, hemodynamic instability (higher Killip class or low blood pressure at admission), and failure to achieve complete reperfusion (TIMI flow 3) were independent predictors of mortality in our population. These findings suggest that achieving a successful reperfusion is crucial to reduce mortality in elderly patients with STEMI.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Gharacholou SM, Alexander KP, Chen AY, et al. Implications and reasons for the lack of use of reperfusion therapy in patients with ST‐segment elevation myocardial infarction: findings from the CRUSADE initiative. Am Heart J. 2010;159:757–763. [DOI] [PubMed] [Google Scholar]
- 2. Alexander KP, Newby LK, Cannon CP, et al. Acute coronary care in the elderly, part I: non‐ST‐segment‐elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2549–2569. [DOI] [PubMed] [Google Scholar]
- 3. Forman DE, Chen AY, Wiviott SD, et al. Comparison of outcomes in patients aged <75, 75 to 84, and ≥85 years with ST‐elevation myocardial infarction (from the ACTION Registry‐GWTG). Am J Cardiol. 2010;106:1382–1388. [DOI] [PubMed] [Google Scholar]
- 4. Lee PY, Alexander KP, Hammill BG, et al. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA. 2001;286:708–713. [DOI] [PubMed] [Google Scholar]
- 5. Claessen BE, Kikkert WJ, Engstrom AE, et al. Primary percutaneous coronary intervention for ST elevation myocardial infarction in octogenarians: trends and outcomes. Heart. 2010;96:843–847. [DOI] [PubMed] [Google Scholar]
- 6. Bueno H, Betriu A, Heras M, et al. Primary angioplasty vs. fibrinolysis in very old patients with acute myocardial infarction: TRIANA (TRatamiento del Infarto Agudo de miocardio eN Ancianos) randomized trial and pooled analysis with previous studies. Eur Heart J. 2011;32:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gharacholou SM, Lopes RD, Alexander KP, et al. Age and outcomes in ST‐segment elevation myocardial infarction treated with primary percutaneous coronary intervention: findings from the APEX‐AMI trial. Arch Intern Med. 2011;171:559–567. [DOI] [PubMed] [Google Scholar]
- 8. Brener SJ, Moliterno DJ, Aylward PE, et al. Reperfusion after primary angioplasty for ST‐elevation myocardial infarction: predictors of success and relationship to clinical outcomes in the APEX‐AMI angiographic study. Eur Heart J. 2008;29:1127–1135. [DOI] [PubMed] [Google Scholar]
- 9. Andersen HR, Nielsen TT, Rasmussen K, et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349:733–742. [DOI] [PubMed] [Google Scholar]
- 10. Antonsen L, Jensen LO, Terkelsen CJ, et al. Outcomes after primary percutaneous coronary intervention in octogenarians and nonagenarians with ST segment elevation myocardial infarction from the western denmark heart registry. Catheter Cardiovasc Interv. 2013;81:912–919. [DOI] [PubMed] [Google Scholar]
- 11. Shah P, Najafi AH, Panza JA, et al. Outcomes and quality of life in patients ≥85 years of age with ST‐elevation myocardial infarction. Am J Cardiol. 2009;103:170–174. [DOI] [PubMed] [Google Scholar]
- 12. Newell MC, Henry JT, Henry TD, et al. Impact of age on treatment and outcomes in ST‐elevation myocardial infarction. Am Heart J. 2011;161:664–672. [DOI] [PubMed] [Google Scholar]
- 13. Showkathali R, Boston‐Griffiths E, Parker M, et al. Should primary percutaneous coronary intervention be the routine reperfusion strategy in octogenarians presenting with ST elevation myocardial infarction? J Cardiovasc Med (Hagerstown). 2014;15:53–59. [DOI] [PubMed] [Google Scholar]
- 14. de Boer MJ, Ottervanger JP, Suryapranata H, et al. Old age and outcome after primary angioplasty for acute myocardial infarction. J Am Geriatr Soc. 2010;58:867–872. [DOI] [PubMed] [Google Scholar]
- 15. Brodie BR, Stone GW, Cox DA, et al. Impact of treatment delays on outcomes of primary percutaneous coronary intervention for acute myocardial infarction: analysis from the CADILLAC trial. Am Heart J. 2006;151:1231–1238. [DOI] [PubMed] [Google Scholar]
- 16. Medina HM, Cannon CP, Fonarow GC, et al. Reperfusion strategies and quality of care in 5339 patients age 80 years or older presenting with ST‐elevation myocardial infarction: analysis from get with the guidelines‐coronary artery disease. Clin Cardiol. 2012;35:632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]


