Abstract
Heart transplantation is the only curative therapy for chronic heart failure, and it plays an important role in the treatment of chronic heart failure with a survival rate of approximately 50% of all patients after 10 years. This has to be kept in mind when alternative therapies enter into our daily routine in treating this patient population. However, the shortage of appropriate donor organs and the expanding pool of patients waiting for heart transplantation have led to growing interest in alternative strategies, particularly in left ventricular assist device (LVAD) therapy. With growing clinical experience and continued technical advances, continuous‐flow pumps are evolving as a bridge to transplantation or as a destination therapy for advanced heart failure. Nevertheless, the importance of this new indication of chronic cardiac support compared to heart transplantation is still completely open and the object of controversial ongoing discussion. This review (1) describes the clinical use and long‐term outcome of a currently available miniaturized LVAD in the context to the standard of care—heart transplantation, (2) provides an outlook of the ongoing process of further optimization of LVADs, and (3) comments on the challenges with assist devices as alternatives to transplantation with a 5‐year outlook.
Introduction
The incidence of heart failure worldwide continues to increase and with it so does the number of treatment options to prevent or to delay the onset of end‐stage heart failure. The last decade has seen fundamental advances in medical and surgical therapy for patients with severe heart failure. Although heart transplantation remains the only curative strategy for patients with end‐stage heart failure, the rate of transplantation has remained relatively steady over the past 20 years.1, 2 This has resulted in a major imbalance between supply and demand, leading to a 20% mortality rate among heart transplant waiting‐list candidates.3 However, even significant scientific progress and numerous innovations in the field of heart transplantation (eg, xenotransplantation or stem cell therapy) cannot solve the current eminent problems of cardiac transplantation, namely a continuously increasing number of heart failure patients facing a dramatic decrease in suitable donor organs. As a consequence of the persistent donor organ shortage, there has been growing interest for alternative strategies, in particular in mechanical circulatory support not only as bridge to transplantation but also as a destination therapy.
Over the past 20 years, major advances have been made in the development of new mechanical technologies, both to support the failing heart until a transplant occurs and to serve as permanent cardiac support when cardiac transplantation is not an option.4 The landmark REMATCH (Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure) study in the late 1990s demonstrated improved survival and quality of life in patients implanted with a pulsatile‐flow left ventricular assist device (LVAD) compared to patients managed medically.5 Since that time, newer more durable devices have been developed that have further advanced the mechanical circulatory support field.6, 7, 8 Continuous‐flow pumps appear to have an advantage over the larger, bulkier, pulsatile‐flow assist devices, both in survival and complication rates.8, 9 Improvements in patient selection and management over the years have contributed to a marked increase in survival in LVAD patients.10, 11, 12, 13 Continuous‐flow LVADs also appear to enhance functional capacity and quality of life.14 Thus, the ongoing trend of further miniaturization of these devices and the demand and supply of acceptable donor hearts have led to innovative thinking about less‐invasive mechanical strategies for long‐term circulatory support.
Heart Transplantation Today
Heart transplantation was the ground‐breaking news worldwide in December 1967, when Dr. Christiaan Barnard performed the first human‐to‐human heart transplant in South Africa. However, initial enthusiasm for the procedure was soon restrained when it became evident that survival rates were usually measured in days or weeks. The 12‐ and 36‐month survival rates of the first 82 patients of the Stanford group were 48% and 25%, respectively.15 This initial poor survival was mainly due to inadequate immunosuppression leading to acute graft rejection, until the introduction of small drug molecules of different mechanisms of action over the last decades as the cornerstone of the antirejection therapy, beginning with cyclosporin A in the early 1980s. Nowadays, heart transplantation is the treatment of choice for well‐selected patients with advanced heart failure, with over 85 000 procedures having been performed worldwide during the last 4 decades. On average, more than 5000 heart transplants are undertaken every year, in more than 225 centers worldwide.16
However, avoiding complications in the long‐term follow‐up seems to be the challenge in heart transplantation. The future is likely to also hold improvements in the quality and length of life for heart‐transplant recipients, as research in areas such as vascular biology and immunology steadily translates into clinical reality. Overcoming the challenge of relentless allograft vasculopathy and the occurrence of infections and malignancies will most probably prolong many lives. In addition, adverse side effects of immunosuppressive drugs, such as nephrotoxicity, cardiovascular side effects (hypertension, diabetes mellitus, dyslipidemia), or cosmetic sides effects, have significant impact on patients' quality of life. Nonetheless, the ability to achieve long‐term survival of patients after heart transplantation are derived from 2 main advances—the performance and interpretation of myocardial biopsies.
Despite the increasing numbers of heart failure programs, the number of heart transplants worldwide has remained static. The pool of patients awaiting a heart transplant is much higher than the donor supply. This has resulted in a major imbalance between supply and demand, and around 20% of patients die while on the waiting list.17 However, even significant scientific progress and numerous innovations in the field of heart transplantation cannot solve the current eminent problems of cardiac transplantation—a continuously increasing number of heart failure patients facing a dramatic decrease in suitable donor organs. As a consequence of the persistent donor organ shortage, there has been growing interest for alternative strategies, in particular in mechanical circulatory support not only as bridge to transplantation but also as a destination therapy.
Continuous‐Flow Pumps—Mechanical Alternative?
Over the last 5 years, 2 different continuous‐flow pumps—the HeartMate II (Thoratec Corp., Pleasanton, CA) and the HeartWare Ventricular Assist Device (HVAD) (HeartWare, Inc., Miami Lakes, FL) have enjoyed clinical success in the alternative treatment of patients with end‐stage heart disease, not only as bridge to transplant but also for chronic support (Figures 1 and 2).
Figure 1.

HeartMate II left ventricular assist device and cross‐sectional internal view. (Illustration reprinted with permission from the Thoratec Corp.)
Figure 2.
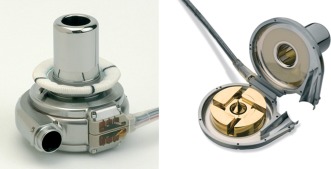
External (left) and internal (right) view of the third‐generation continuous‐flow rotary left ventricular assist device, the HeartWare Ventricular Assist Device. (Illustration reprinted with permission HeartWare, Inc.)
In the beginning, the engineering of continuous‐flow rotary pump technology represented a milestone and novel design concept for LVADs. These devices have the advantage of a smaller design and potential for greater long‐term mechanical reliability by eliminating the reservoir chamber and valves needed with first‐generation pulsatile pumps.6 The second‐generation rotary blood pumps are designed typically with an axial blood flow path and have an internal rotor within the blood flow path that is suspended by contact bearings. The HeartMate II is the most successful second‐generation implantable LVAD, with over 10 000 implants worldwide and 2000 implants in Europe. The device is US Food and Drug Administration‐approved as a bridge to transplant and as a destination therapy.7, 8, 9, 14
The reported survival rates have improved as experienced was gained from the initial clinical trial results to the postapproval study from 89% to 96% (30 days), 75% to 90% (6 months), and 68% to 85% (1 year).6, 18 Additionally, results from clinical studies have also shown early improvements followed by long‐term stability of renal and hepatic function, as well as limited adverse effects on neurocognitive performance.4, 5, 6, 7, 8, 14 These improvements have led to increased acceptance of LVAD therapy for long‐term support. The incidence of thromboembolic events is relatively low for the HeartMate II and ranges from 3 to 6 events per 100 patient‐years.5 The incidence of driveline and pump infection is remarkable, ranging from 13% to 27%.4, 5, 6, 7, 8, 13
Five years ago, the first HeartWare Ventricular Assist System was implanted in Austria. Currently, more than 1800 patients have been supported on the HeartWare system worldwide.
At the core of the HeartWare system is the HVAD pump, a continuous‐flow, centrifugal LVAD with an integrated titanium inflow cannula that can be inserted within the pericardial space directly. This design enables flexibility in implant technique that reduces the invasiveness, surgical complication rates, and patient recovery time. The pump features a wide‐bladed, hybrid, hydromagnetic impeller suspension system and has a displacement volume of 50 mL, weighs 160 g, and has a range of fixed speeds (1800–4000 rpm) providing up to 10 L/min of flow. The newly designed sewing ring revolutionizes and facilitates the positioning of the inflow cannula to the left ventricle, which makes it extremely versatile. The diaphragmatic approach to HVAD implantation is an alternative implantation technique that appears to be particularly suitable for patients with small lateral thoracic dimensions (eg, pediatric patients and/or an unusually enlarged heart).19, 20 Implantation via thoracotomy with 2 small incisions avoiding invasive sternotomy is most recently being utilized and shows promising results.21, 22 An anticoagulation regimen consisting of warfarin, with a target international normalized ratio from 1.7 to 2.3, and antiplatelet therapy of aspirin or clopidogrel, are recommended. In a recently published multicenter evaluation of the HVAD by Strueber and colleagues, the authors showed an actuarial overall survival at 6, 12, and 24 months of 90%, 84%, and 79%, respectively.23 The majority of patients (60%) remained on pump support at 12 months, yet the aggregate adverse event rate portfolio remained low compared with previous clinical trials, where the number of patients on pump support at 12 months was much lower. Only 20% of patients experienced bleeding requiring surgery after implant, and no (0%) pump pocket infections were documented, confirming that pericardial implant has another potential advantage. The Seattle Heart Failure Model was used retrospectively as a virtual control, and the estimated and predicted survival of this entire cohort if managed with optimal medical therapy was 73%, 58%, and 40%, respectively. Additionally, the health‐related quality of life, as measured by the Kansas City Cardiomyopathy Questionnaire, and neurocognitive function were enhanced significantly. Interestingly, the largest improvements were observed within the first 30 days after HVAD pump placement. Data collected retrospectively showed that although pump speeds were maintained at a constant value, HVAD pump flow and power consistently declined at night and increased in the morning. This finding was more pronounced 30 days after implantation compared to the 7‐day postoperative baseline value. Such a return to the circadian rhythms may be beneficial on cardiac function, as it may promote myocardial repair and regeneration.24
Several centers have published on biventricular application of the HVAD pump. Hetzer and associates described biventricular implantation techniques and reported on 5 patients implanted simultaneously with an LVAD and a right ventricular assist device (RVAD), and an additional 3 patients were implanted with an RVAD after initial LVAD implantation. All 5 patients with simultaneous LVAD and RVAD implantation survived and were discharged home.25 Strueber and colleagues reported on 1 patient who received an RVAD after initial LVAD implantation.26 The patient was awaiting transplantation at the time of publication. Loforte and colleagues described a new technique for a biventricular assist device implantation in a small (1.6 m2) patient.27 In addition, they reported a midterm follow‐up on this patient.28
Reality and Clinical Perspective
Table 1 compares the current status of transplantation and LVAD therapy, the challenges, and further directions. In Table 2, the current status and clinical developments of LVADs are highlighted.
Table 1.
Questions and Challenges in a 5‐Year Perspective in the Biological and Mechanical Treatment of Severe Heart Failure
| Feature | Left Ventricular Assist Devices | Heart Transplantation |
|---|---|---|
| Availability | Immediately | Waiting list |
| Survival benefit | ||
| To 2 years | ∼80% [4,5] | ∼80% [1] |
| To 5 years | ∼40% [4,5] | ∼70% [1] |
| Quality of life and functional capacity | Proven up to 2 years | Proven |
| Showering and bathing | Very limited | Unrestricted |
| Long‐term stability | Proven | Proven |
| Complications | Thromboembolic events, bleedings, drive‐line infections | Drug toxicities, allograft vasculopathy, malignancies |
| Drug monitoring | Yes, anticoagulation, different protocols | Yes, immunosuppression, different protocols |
| Myocardial biopsy | No | Yes, different protocols |
| Infrastructure, outstanding, and hospital care | Under construction and development | Available |
| Team and coordinator | Yes | Yes |
| Remote monitoring | In development | Not available |
| Organ supply | — | Limited, despite newer conservation technique and organ care systems |
| VAD development and new technique, peripherals | Stringent development | — |
| Reimbursement | Not clearly defined | Defined |
| Public policy | Insurance and costs | Insurance and costs |
| Ethical aspects | Not defined, decision making: which patient gets which device (eg, considering age, comorbidities, palliative care) | Regulated by the different societies |
Abbreviations: VAD, ventricular assist device.
Table 2.
Reality and Progress in the Modern Era of Left Ventricular Assist Device Therapy
| Heart Failure and Left Ventricular Assist Devices |
|---|
| Reality |
| Chronic heart failure is of increasing interest and the number 1 cause of death in the Western world. |
| Stable but rapidly increasing number of implanted LVADs with a clear trend from pulsatile to continuous‐flow pumps. |
| Improved survival rates, end‐organ function, neurocognitive benefits, and quality of life in bridge‐to‐transplant and destination therapy population. |
| The 2‐year survival of miniaturized rotary blood pumps is similar to that of heart transplantation. |
| Progress and developments down the road |
| Newer, miniaturized, continuous‐flow LVADs undergoing testing to may have a tremendous impact on solving the problem of organ shortage. |
| Ongoing miniaturization of rotary blood pumps suitable for biventricular support and for pediatrics. |
| Minimally invasive and off‐pump (without cardiopulmonary bypass) implantation is possible. |
| Risk stratifying using different virtual modalities (eg, Seattle Heart Failure Model). |
| Necessity |
| Randomization to best pharmacological treatment and/or rotary blood pumps to establish superiority of LVAD therapy (if appropriate). |
Abbreviations: LVAD, left ventricular assist device.
Summary
Heart failure presents an increasing public burden of morbidity and mortality even though mortality from coronary artery disease is decreasing. Heart failure, the number 1 cause of death in the Western world, accounts for 1 death every 30 seconds. Although effective pharmacological and electrophysiological therapies have improved outcomes, the need for mechanical circulatory support is well defined and rapidly growing. The recent decades have seen a vast improvement in the field of ventricular assist devices (VAD) and the associated clinical outcomes and patient quality of life. Additionally, the newer design offers reliability, portability, and ease of use for out‐of‐hospital patients. Furthermore, the miniaturized pumps show promise as a good treatment option for pediatric patients and for intrathoracic biventricular assist device (BVAD) support. Ongoing and future studies will provide additional insight into the use of the device for these applications as well as for its viability as a treatment option for destination therapy patients. It is a reality that VAD therapy is being used in more than one‐third of our patients (increasingly strong) eligible for heart transplantation, and this trend is the same for the elderly and heart failure patients who are not transplantable. There is also a tremendous shift in the indication of assist device therapy from bridge to transplant and to destination therapy. However, there are a significant number of patients who are not eligible for LVAD therapy (eg, diastolic heart failure, hypertrophic cardiomyopathy, complex congenital pathologies, severe right heart failure). These patients should be referred preferably to the transplant waiting list. With the anticipated progress in assist device therapy an the lack of appropriate donor hearts, it may be that in the future, heart transplantation will be prioritized to patients who cannot receive a VAD or who have complications while under VAD therapy.
The vision for the foreseeable future is that destination therapy will become an increasingly more important treatment option for patients suffering terminal heart failure and should be concentrated in expert centers. LVAD implantation as destination therapy is an expensive therapy, and the costs of device implantation are comparable to heart transplantation. Therefore, these procedures should only be performed in large centers with significant expertise in device implantation and a running heart transplant program, or in centers with a close collaboration to these centers. Along this line, studies have shown that there appears to be an important relationship between the device implant costs, the improved selection of operative candidates, the surgeons' experience, and the quality of medical care.29 These reductions have been linked to shorter length of hospital stay, lower complication rates, and the introduction of multidisciplinary clinical care of destination therapy recipients in specialized centers. Despite the improved safety profile and survival benefit of VAD therapy compared with optimal medical management of end‐stage heart failure, there are still certain ethical aspects to be considered. LVADs also alter the classic end‐of‐life picture. When is the patient actually dead? In addition, caregivers of recipients may experience significant physical as well as psychological stress from destination therapy with LVADs. There are also social and financial problems for recipients and their families. We therefore advocate an early outline of prerequisite conditions so that consenting to the use of an LVAD as a destination therapy is a well‐informed process. Such conditions include direct participation of a multidisciplinary care team, including psychologists, a precise plan of care for anticipated device‐related complications, advanced‐care planning for anticipated end‐of‐life situations, and timing of device deactivation. However, with an increasing number of implants, the community is faced with a new area of medical care. Despite the technical and surgical challenges, the outstanding postoperative care of this new patient population is also challenging. To fulfill these challenges, heart failure teams have to be assembled in every hospital to provide the best out‐patient care for VAD patients. These teams should be built using an interdisciplinary approach including cardiac surgeons, cardiologists, VAD coordinators, and specialized VAD nurses who work in close cooperation with the practitioners.
The salient questions in the era of modern LVAD are: Is there a real survival advantage, can we show a improved quality of life and quality of life‐adjusted life years, and finally can we handle serious adverse events and potentially rehospitalization within a period of 5‐years in comparison to heart transplants. Questions about ethics and economic aspects have to be addressed.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty‐seventh official adult heart transplant report—2010. J Heart Lung Transplant. 2010;29:1089–1103. [DOI] [PubMed] [Google Scholar]
- 2. Francis GS, Greenberg BH, Hsu DT, et al. ACCF/AHA/ACP/HFSA/ISHLT 2010 Clinical competence statement on management of patients with advanced heart failure and cardiac transplant. J Am Coll Cardiol. 2010;56:424–453. [DOI] [PubMed] [Google Scholar]
- 3.German organ transplantation fondation (DSO); http://www.dso.de/uploads/tx_dsodl/dso_jb2011_e_www.pdf.
- 4. Kirklin JK, Naftel DC, Kormos RL, et al. Third INTERMACS annual report: the evolution of destination therapy in the United States. J Heart Lung Transplant. 2011;30:115–123. [DOI] [PubMed] [Google Scholar]
- 5. Rose EA, Gelijns AC, Moskowitz AJ, et al. Long‐term use of a left ventricular assist device for end‐stage heart failure. N Engl J Med. 2001;345:1435–1443. [DOI] [PubMed] [Google Scholar]
- 6. Miller LW, Pagani FD, Russell SD, et al. Use of a continuous‐flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. [DOI] [PubMed] [Google Scholar]
- 7. Pagani FD, Miller LW, Russell SD, et al. Extended mechanical circulatory support with a continuous‐flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312–321. [DOI] [PubMed] [Google Scholar]
- 8. Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous‐flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. [DOI] [PubMed] [Google Scholar]
- 9. Aggarwal S, Pagani FD. Bridge to transplantation: current outcomes. J Card Surg. 2010;25:455–461. [DOI] [PubMed] [Google Scholar]
- 10. Lietz K, Miller LW. Patient selection for left‐ventricular assist devices. Curr Opin Cardiol. 2009;24:246–251. [DOI] [PubMed] [Google Scholar]
- 11. Wilson SR, Mudge GH, Stewart GC, et al. Evaluation for a ventricular assist device. Circulation. 2009;119:2225–2232. [DOI] [PubMed] [Google Scholar]
- 12. Warner Stevenson L, Pagani FD, Young JB, et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant. 2009;28:535–541. [DOI] [PubMed] [Google Scholar]
- 13. Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous‐flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29:S1–S39. [DOI] [PubMed] [Google Scholar]
- 14. Rogers JG, Aaronson KD, Boyle AD, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advance heart failure patients. J Am Coll Cardiol. 2010;55:1826–1834. [DOI] [PubMed] [Google Scholar]
- 15. Hunt SA. Taking heart—cardiac transplantation past, present, and future. N Engl J Med. 2006;355:231–235. [DOI] [PubMed] [Google Scholar]
- 16. Stehlik J, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: twenty‐eighth adult heart transplant report—2011. J Heart Lung Transplant. 2011;30:1078–1094. [DOI] [PubMed] [Google Scholar]
- 17. Komoda T, Hetzer R, Lehmkuhl HB. Influence of new Eurotransplant heart allocation policy on outcome of heart transplant candidates in Germany. J Heart Lung Transplant. 2008;27:1108–1114. [DOI] [PubMed] [Google Scholar]
- 18. Starling RC, Naka Y, Boyle AJ, et al. Results of the post‐U.S. Food and Drug Administration‐approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol. 2011;10;57:1890–1898. [DOI] [PubMed] [Google Scholar]
- 19. Gregoric ID, Cohn WE, Frazier OH. Diaphragmatic implantation of the HeartWare ventricular assist device. J Heart Lung Transplant. 2011;30:467–470. [DOI] [PubMed] [Google Scholar]
- 20. Griffith BP, Kormos RL, Borovetz HS, et al. HeartMate II left ventricular assist system: from concept to first clinical use. Ann Thorac Surg. 2001;71(3 suppl):S116–S120. [DOI] [PubMed] [Google Scholar]
- 21. Cheung A, Lamarche Y, Kaan A, et al. Off‐pump implantation of the HeartWare HVAD Left Ventricular Assist Device through minimally invasive incisions. Ann Thorac Surg. 2011;91:1294–1296. [DOI] [PubMed] [Google Scholar]
- 22. Loforte A, Monica PLD, Montalto A, et al. HeartWare third‐generation implantable continuous flow pump as biventricular support: mid‐term follow‐up. Interact Cardiovasc Thorac Surg. 2011;12:458–460. [DOI] [PubMed] [Google Scholar]
- 23. Strueber M, O'Driscoll G, Jansz P, et al.; HeartWare Investigators. Multicenter evaluation of an intrapericardial left ventricular assist system. J Am Coll Cardiol. 2011;57:1375–1382. [DOI] [PubMed] [Google Scholar]
- 24. Slaughter MS, Ising MS, Tamez D, et al. Increase in circadian variation after continuous‐ flow ventricular assist device implantation. J Heart Lung Transplant. 2010;29:695–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hetzer R, Krabatsch T, Stepanenko A, et al. Long‐term biventricular support with the HeartWare implantable continuous flow pump. J Heart Lung Transplant. 2010;29:822–824. [DOI] [PubMed] [Google Scholar]
- 26. Strueber M, Meyer AL, Malehsa D, et al. Successful use of the HeartWare HVAD rotary blood pump for biventricular support. J Thorac Cardiovasc Surg. 2010;140:936–937. [DOI] [PubMed] [Google Scholar]
- 27. Loforte A, Montalto A, Monica PLD, et al. Biventricular support with the HeartWare implantable continuous flow pump: an additional contribution. J Heart Lung Transplant. 2010;29:1443–1444. [DOI] [PubMed] [Google Scholar]
- 28. Casas F, Reddy SN, Shambaugh C, et al. The MVAD impeller: design elements. ASAIO J. 2011;57:81. [Google Scholar]
- 29. Lietz K. Destination therapy: patient selection and current outcomes. J Card Surg. 2010;25:462–471. [DOI] [PubMed] [Google Scholar]


