Abstract
Background
Data regarding the associations between sleep duration and clinical cardiovascular (CV) events are limited. We aimed to analyze any associations between self‐reported sleep duration and CV events.
Hypothesis
Methods
This is a cross‐sectional analysis of nationally representative population of noninstitutionalized US civilians recruited in the 2007 to 2008 National Health and Nutrition Examination Survey. This is a questionnaire‐based study including only those subjects who answered questions on sleep duration and CV events. The main outcome measures were prevalence of congestive heart failure, myocardial infarction, stroke, coronary artery disease, and angina.
Results
After logistic regression analysis, significant associations between sleep duration and prevalence of stroke, myocardial infarction, congestive heart failure, coronary artery disease, and angina were found. There was a statistically significant increase in stroke in those with <6 hours of sleep (odds ratio [OR]: 2.0111, 95% confidence interval [CI]: 1.4356‐2.8174), in myocardial infarction in those with <6 hours of sleep (OR: 2.0489, 95% CI: 1.4878‐2.8216), in congestive heart failure in those with <6 hours of sleep (OR: 1.6702, 95% CI: 1.1555 to 2.4142), in coronary artery disease in those with >8 hours of sleep (OR: 1.1914, 95% CI: 1.0712‐3.4231), and in angina in those with >8 hours of sleep (OR: 2.0717, 95% CI: 1.0497‐4.0887).
Conclusions
The results of this cross‐sectional analysis suggest that sleep duration may be associated with the prevalence of various CV events.
Introduction
The associated costs with disturbed sleep in terms of sick days, treatment, and many other unsaid effects on society are extensive.1, 2 Apart from cardiovascular (CV) diseases, shorter or longer sleep durations have also been associated with respiratory disorders, obesity, and poor self‐rated health.3, 4, 5 Studies have even reported the duration of sleep to be a significant predictor of mortality.6, 7
Very few studies have looked at association of sleep duration with prevalence of CV events, and they found varying results. In a previous meta‐analysis, longer duration of sleep was associated with a greater risk of developing cardiovascular disease (CVD) or dying from it.8 The mechanisms explaining the associations of sleep patterns and prevalence of CV diseases are not well understood. This study retrospectively analyzes the association between sleep duration and the prevalence of CV events using nationally representative data from the National Health and Nutrition Examination Surveys database (NHANES).
Methods
The National Health and Nutrition Examination Survey is a database aimed at assessing the health and nutrition status of children and adults in the United States.9 The database consists of a nationally representative probability sample of noninstitutionalized US civilians. This study uses the 2007 to 2008 NHANES database, the most recent and complete dataset of NHANES. The database and its methods were reviewed and approved by the National Center for Health Statistics Ethics Review Board.
Only patients with data reported for sleep duration and ≥1 of the CV endpoints being analyzed were included in this retrospective study. Sleep duration was extracted from a questionnaire and was classified into 1 of 3 categories: <6 hours, 6 to 8 hours, or >8 hours. Data regarding the CV endpoints of congestive heart failure (CHF), myocardial infarction (MI), stroke, coronary artery disease (CAD), and angina were extracted from questionnaires with responses of yes or no. Associations were adjusted for sex, age, body mass index, total cholesterol level, high‐density lipoprotein cholesterol, smoking status, systolic blood pressure, history of sleep apnea, and family history of heart attack.
Baseline patient characteristics were analyzed using the ANOVA test for continuous variables and χ2 analysis for categorical variables to assess for any significant difference among different groups. Mann–Whitney U tests were used for comparisons demonstrating a skewed distribution, and independent samples t test was used for comparisons demonstrating a normal distribution. Impact of sleep duration on the prevalence of CV events was assessed using logistic regression analysis for adjusting the confounding variables. Odds ratios (OR) with 95% confidence intervals (CI) were calculated for all 3 groups. Adjusted ORs were then calculated using the 6 to 8 hours of sleep group as a reference. A P value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY).
Results
Out of a total of 10 149 subjects surveyed, 6538 subjects reported sleep duration on the questionnaire. Average sleep duration of the total population who reported sleep duration in the survey was 6.85 ± 1.49 hours. Of these 6538 subjects, not all reported CV events. The following numbers of patients were finally available for each reported event: 3011 for CHF, 3019 for MI, 3015 for stroke, 3012 for angina, and 3014 for CAD. Significant differences in age at screening, smoking status, and ethnicity were noted between those in various sleep‐duration classifications (Table 1). Mean age at screening increased along with sleep duration, and the percentage of smokers decreased as sleep duration increased. There was also a statistically significant difference in the ethnic diversity of the different sleep classifications. Baseline mean sleep durations were also compared within each CV event with respect to the presence or absence of the event (Table 2), and no statistically significant differences were noted.
Table 1.
Baseline Patient Characteristics With Respect to Different Sleep‐Duration Groups
| Sleep Duration <6 Hours | Sleep Duration 6 to 8 Hours | Sleep Duration >8 Hours | P Value | |
|---|---|---|---|---|
| No. of patients | 599 | 2282 | 138 | NA |
| Age, y | 60.6 ± 9.8 | 61.0 ± 9.6 | 64.9 ± 10.1 | <0.001a |
| Male sex | 297 (49.6) | 1009 (44.2) | 69 (49.8) | 0.451 |
| Smokers | 150 (25) | 437 (19.1) | 23 (16.7) | <0.001a, b |
| BMI, kg/m2 | 30.1 ± 7.1 | 29.3 ± 6.4 | 29.4 ± 5.0 | 0.202b |
| TC, mg/dL | 203.3 ± 44.2 | 201.5 ± 42.1 | 200.43 ± 46.3 | 0.506 |
| SBP, mm Hg | 131.6 ± 19.7 | 130.6 ± 20.0 | 133.0 ± 24.9 | 0.556 |
| Ethnicity | ||||
| Mexican American | 72 (12.0) | 358 (15.7) | 26 (18.8) | 0.031a |
| Other Hispanic | 87 (14.5) | 256 (11.2) | 9 (6.5) | |
| Non‐Hispanic Black | 203 (33.9) | 422 (18.5) | 23 (16.7) | |
| Non‐Hispanic White | 214 (35.8) | 1162 (50.9) | 77 (55.8) | |
| Other or multiracial | 23 (3.8) | 87 (3.7) | 3 (2.2) |
Abbreviations: BMI, body mass index; MI, myocardial infarction; NA, not applicable; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol.
Data are given as n (%) or mean ± standard deviation. This table reports the baseline characteristics of individuals reporting MI because this was the largest studied group (n = 3019).
Denotes a statistically significant difference.
Mann–Whitney U test was used due to skewed distribution.
Table 2.
Comparison of Baseline Sleep Duration Within Each Studied CV Event
| CV Event | Event Present | Sleep Duration, Mean (SD) | P Value |
|---|---|---|---|
| CHF | Y | 6.6913 (1.93788) | 0.743 |
| N | 6.7441 (1.44400) | ||
| MI | Y | 6.5215 (1.89511) | 0.098 |
| N | 6.7571 (1.44181) | ||
| Stroke | Y | 6.7771 (1.95687) | 0.811 |
| N | 6.7411 (1.43902) | ||
| CAD | Y | 6.7912 (1.71452) | 0.779 |
| N | 6.8700 (3.76004) | ||
| Angina | Y | 6.7800 (1.41121) | 0.797 |
| N | 6.7415 (1.47119) |
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; CV, cardiovascular; MI, myocardial infarction; N, no; SD, standard deviation; Y, yes.
Compared with those sleeping for 6 to 8 hours, individuals reporting <6 hours of sleep had significant increases in prevalence of stroke (OR: 2.0111, 95% CI: 1.4356‐2.8174, P = 0.037), MI (OR: 2.0489, 95% CI: 1.4878‐2.8216, P = 0.042), and CHF (OR: 1.6702, 95% CI: 1.1555‐2.4142, P < 0.001). Individuals who reported >8 hours of sleep were found to have statistically significant increases in prevalence of CAD (OR: 1.1914, 95% CI: 1.0712‐3.4231, P < 0.001) and angina (OR: 2.0717, 95% CI: 1.0497‐4.0887, P = 0.008) when compared with those reporting to be sleeping 6 to 8 hours. The ORs and corresponding P values for each CV event in the 3 groups are depicted in Table 3 and Figure 1. Polynomial trend lines revealed almost U‐shaped curves for CHF, stroke, and CAD. After further breakdown of sleep duration by a difference of 1 hour each, the percentages of various CV events showed similar trends (Figure 2).
Table 3.
Odds Ratios and 95% Confidence Intervals for Cardiovascular Events With Respect to Sleep Duration
| Sleep Duration | Sleep Duration | Sleep Duration | |
|---|---|---|---|
| <6 Hours | 6 to 8 Hours | >8 Hours | |
| CHF | 1.6702 (1.1555‐2.4142), P < 0.001 | 1.000 (Ref) | 1.3248 (0.6313‐2.7801), P = 0.071 |
| MI | 2.0489 (1.4878‐2.8216), P = 0.042 | 1.000 (Ref) | 1.3838 (0.7089‐2.7013), P = 0.451 |
| Stroke | 2.0111 (1.4356‐2.8174), P = 0.037 | 1.000 (Ref) | 1.7102 (0.8972‐3.2600), P < 0.087 |
| CAD | 1.2598 (0.8752‐1.8134), P = 0.206 | 1.000 (Ref) | 1.1914 (1.0712‐3.4231), P < 0.001 |
| Angina | 1.2181 (0.7766‐1.9105), P < 0.642 | 1.000 (Ref) | 2.0717 (1.0497‐4.0887), P = 0.008 |
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; MI, myocardial infarction; Ref, reference.
Figure 1.
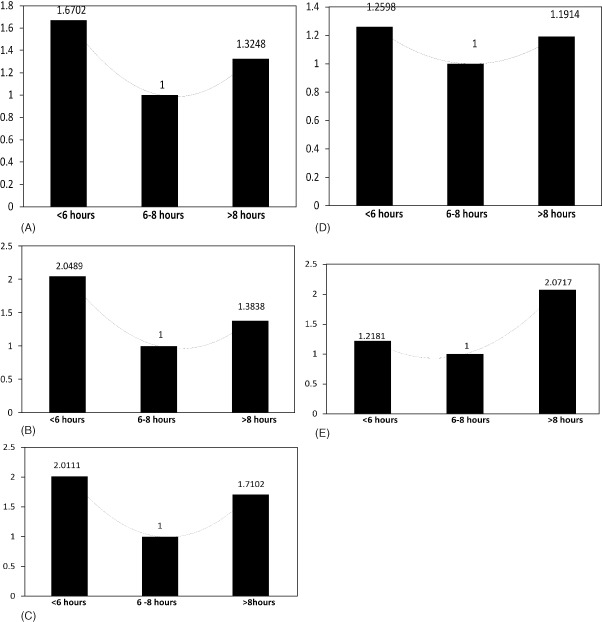
Odds ratios for each outcome in different sleep‐duration groups when compared with 6–8 hours of sleep duration as reference.
Figure 2.
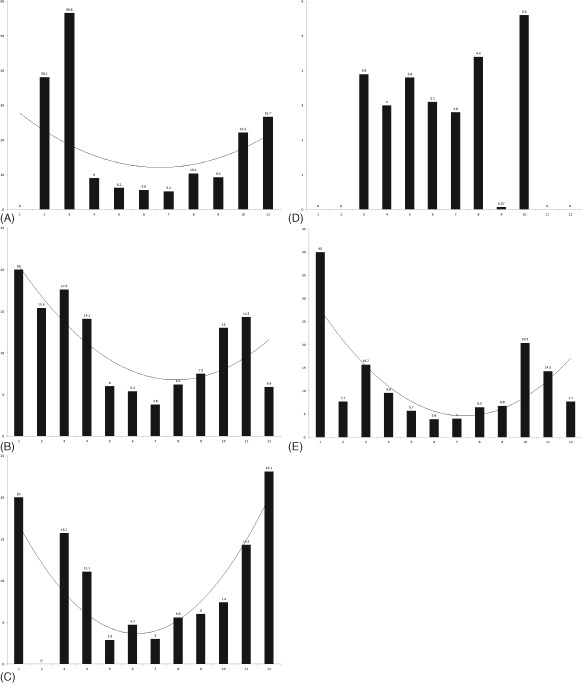
Frequencies of cardiovascular events (A) coronary artery disease, (B) myocardial infarction, (C) congestive heart failure, (D) angina, and (E) stroke when divided by individual sleep hours.
Discussion
The results of this cross‐sectional study show that there in fact may be an association between duration of sleep and the prevalence of MI, CHF, stroke, CAD, and angina, even after accounting for confounding variables.
Cappuccio et al performed a meta‐analysis of 15 prospective studies and determined that short sleep duration was not significantly associated with a greater risk of developing CVD or dying from it. However, long duration of sleep was associated with a greater risk of developing CVD or dying from it.8 As a limitation, the authors noted that the studies included in the meta‐analysis did not exclude obstructive sleep apnea. Some other studies not included in the meta‐analysis have noted an association between short sleep duration and increased risk of CVD.10, 11, 12, 13, 14, 15, 16, 17
An international symposium, Sleep as Restitution, was organized in August 2002. A conclusion of the symposium was that sleep is a state of altered metabolism, and disturbances of sleep patterns have far‐reaching effects on endocrinology, immunology, and metabolism.18 Shorter sleep duration has a variety of effects, including hyperactivation of the sympathetic nervous system, glucose intolerance, increased cortisol levels, increased blood pressure, decreased variability in heart rate, disruption of the hypothalamic axis, and a general increase in inflammatory markers.19, 20, 21, 22, 23, 24 Both short and long sleep durations have been found to be associated with a high serum triglyceride level or a low high‐density lipoprotein cholesterol level in women.25
The mechanisms causing longer sleep duration to be associated with a higher prevalence of CVD are also still to be delineated. Residual confounding and comorbidities have been implicated as one mechanism. Depressive symptoms, low socioeconomic status, physical inactivity, and subclinical diseases have been associated with long duration of sleep and may confound the associations with CVD.26, 27 Longer sleep duration may signify a risk of CV diseases, the risk being reversible, or may even be a consequence of chronic comorbidity.8, 26
Qureshi et al have previously used NHANES I follow‐up data to show that individuals reporting >8 hours of sleep were at significantly increased risk of stroke (relative risk: 1.5, 95% CI: 1.1‐2.1).28 In subjects reporting <6 hours of sleep, risk of stroke did not reach statistical significance. Both sleep durations of <6 and >8 hours were not associated with any significantly increased risk for CAD. In another study, the authors noted that subjects reporting sleep durations of ≤5 hours or ≥9 hours were significantly more likely to have incident diabetes mellitus over the follow‐up period, after controlling for covariates.29 The U‐shaped curves seen in our study with sleep duration and prevalence of various CV events are in accordance with previous studies that have demonstrated U‐shaped relationships between sleep duration and risk of mortality.30, 31, 32
This study has several limitations. First is its cross‐sectional nature, which allows only for an analysis of association and prevalence at a given point, rather than causality and incidence. As in any cross‐sectional study, caution should be exercised when interpreting the direction of association. Long duration of follow‐ups in large population sizes will be needed to achieve any significant outcomes in prospective studies. Second, most of the study data were gathered via self‐reported cross‐sectional questionnaires, which may only be representative of one's sleep habits for a limited amount of time, and effects of sustained sleep patterns could not be assessed in our study. Although questionnaires may introduce reporting or recall bias, various studies have reported high correlations between sleep diaries and sleep studies with that of subjective estimates of sleep duration.33, 34 Furthermore, assessments of sleep duration in the primary‐healthcare setting can be done readily from self‐reported data. Third, the individuals answering the questionnaires may not differentiate time asleep from time lying in bed awake, which has been previously reported to create bias. The NHANES 2007 to 2008 database had a separate self‐reported data point recording how long it took a person to go to sleep, in minutes. This may have helped reduce the bias introduced by time asleep vs time lying awake in bed. Fourth, the reasons for short or long sleep durations could not be assessed in this analysis due to the nature of the database.
There are many strengths of this study. Use of the NHANES database is advantageous, as this patient population is representative of the US population and has been used previously in a number of studies to assess for national disease prevalence and deduce associations.9 Rigorous quality control and standardized protocols employed by the US Centers for Disease Control and Prevention for collecting information are highlights of NHANES surveys and add to the strengths of the study. The availability of data for multiple CV events also makes this study of particular value. To our knowledge, this is the first study to report association of sleep duration with prevalence of 5 different CV endpoints. Multiple confounders were identified in and adjusted for in our analysis. All the risk factors for CVD routinely used to compute the Framingham Risk Score were adjusted. As mentioned above, many studies that have analyzed the associations between sleep patterns and CVD have not excluded individuals with sleep apnea.8 Sleep apnea has been shown to be associated with significant CV morbidity and mortality.35, 36 Sleep apnea also has been shown to be highly prevalent in subjects with obesity in a previous NHANES survey study, making the adjustment essential because their combination may have even more pronounced CV effects.37 The results of our study have been adjusted for sleep apnea, thereby implicating closer associations between sleep duration and prevalence of CV diseases than may have been reported previously. However, it should be noted that sleep apnea remains undiagnosed in the majority of the population and adjustment may not have been complete.
We believe that the additional analyses lend encouraging support to our findings. The current sleep‐stratification schema utilizing 6 to 8 hours of sleep as a reference value in this study seems to be the most appropriate for future analysis. This value was utilized as the reference group because it has been previously reported to be associated with the least risk of CVD.29 Second, the additional analysis breaking down sleep duration by each hour demonstrated that similar trends remained true for all CV events. This supports the finding that people whose sleep duration is at the extremes are at the highest risk of CV events, and the risk decreases as the number reaches a middle point. Although sleep durations were found to be associated with the prevalence of CV events in the initial analysis, the baseline t tests performed initially found no significant differences in baseline average sleep duration between those reporting a particular CV event and those without it.
Conclusion
This cross‐sectional analysis of NHANES 2007 to 2008 database demonstrated that shorter sleep duration is associated with greater prevalence of stroke, MI, and CHF. Longer sleep duration was associated with higher prevalence of CAD and angina.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Leger D. The cost of sleep‐related accidents: a report for the National Commission on Sleep Disorders Research. Sleep. 1994;17:84–93. [DOI] [PubMed] [Google Scholar]
- 2. Leger D, Guilleminault C, Bader G, et al. Medical and socio‐professional impact of insomnia. Sleep. 2002;25:625–629. [PubMed] [Google Scholar]
- 3. Bliwise DL. Sleep‐related respiratory disturbances. J Gerontol. 1984;39:255. [DOI] [PubMed] [Google Scholar]
- 4. Cappuccio FP, Taggart FM, Kandala NB, et al. Meta‐analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steptoe A, Peacey V, Wardle J. Sleep duration and health in young adults. Arch Intern Med. 2006;166:1689–1692. [DOI] [PubMed] [Google Scholar]
- 6. Cappuccio FP, D'Elia L, Strazzullo P, et al. Sleep duration and all‐cause mortality: a systematic review and meta‐analysis of prospective studies. Sleep. 2010;33:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heslop P, Smith GD, Metcalfe C, et al. Sleep duration and mortality: the effect of short or long sleep duration on cardiovascular and all‐cause mortality in working men and women. Sleep Med. 2002;3:305–314. [DOI] [PubMed] [Google Scholar]
- 8. Cappuccio FP, Cooper D, D'Elia L, et al. Sleep duration predicts cardiovascular outcomes: a systematic review and meta‐analysis of prospective studies. Eur Heart J. 2011;32:1484–1492. [DOI] [PubMed] [Google Scholar]
- 9. Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465–477. [DOI] [PubMed] [Google Scholar]
- 10. Eguchi K, Pickering TG, Schwartz JE, et al. Short sleep duration as an independent predictor of cardiovascular events in Japanese patients with hypertension. Arch Intern Med. 2008;168:2225–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eguchi K, Hoshide S, Ishikawa S, et al. Short sleep duration is an independent predictor of stroke events in elderly hypertensive patients. J Am Soc Hypertens. 2010;4:255–262. [DOI] [PubMed] [Google Scholar]
- 12. Grandner MA, Jackson NJ, Pak VM, et al. Sleep disturbance is associated with cardiovascular and metabolic disorders. J Sleep Res. 2012;21:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamazaki Y, Morikawa Y, Nakamura K, et al. The effects of sleep duration on the incidence of cardiovascular events among middle‐aged male workers in Japan. Scand J Work Environ Health. 2011;37:411–417. [DOI] [PubMed] [Google Scholar]
- 14. Kronholm E, Laatikainen T, Peltonen M, et al. Self‐reported sleep duration, all‐cause mortality, cardiovascular mortality and morbidity in Finland. Sleep Med. 2011;12:215–221. [DOI] [PubMed] [Google Scholar]
- 15. Laugsand LE, Vatten LJ, Platou C, et al. Insomnia and the risk of acute myocardial infarction: a population study. Circulation. 2011;124:2073–2081. [DOI] [PubMed] [Google Scholar]
- 16. Liu Y, Tanaka H; Fukuoka Heart Study Group. Overtime work, insufficient sleep, and risk of non‐fatal acute myocardial infarction in Japanese men. Occup Environ Med. 2002;59:447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sabanayagam C, Shankar A. Sleep duration and cardiovascular disease: results from the National Health Interview Survey. Sleep. 2010;33:1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Åkerstedt T, Nilsson PM. Sleep as restitution: an introduction. J Intern Med. 2003;254:6–12. [DOI] [PubMed] [Google Scholar]
- 19. Tochikubo O, Ikeda A, Miyajima E, et al. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27:1318–1324. [DOI] [PubMed] [Google Scholar]
- 20. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. [DOI] [PubMed] [Google Scholar]
- 21. Kato M, Phillips BG, Sigurdsson G, et al. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35:1173–1175. [DOI] [PubMed] [Google Scholar]
- 22. Meier‐Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C‐reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. [DOI] [PubMed] [Google Scholar]
- 23. Lusardi P, Mugellini A, Preti P, et al. Effects of a restricted sleep regimen on ambulatory blood pressure monitoring in normotensive subjects. Am J Hypertens. 1996;9:503–505. [DOI] [PubMed] [Google Scholar]
- 24. Shearer WT, Reuben JM, Mullington JM, et al. Soluble TNF‐α receptor 1 and IL‐6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–170. [DOI] [PubMed] [Google Scholar]
- 25. Kaneita Y, Uchiyama M, Yoshiike N, et al. Associations of usual sleep duration with serum lipid and lipoprotein levels. Sleep. 2008;31:645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stranges S, Dorn JM, Shipley MJ, et al. Correlates of short and long sleep duration: a cross‐cultural comparison between the United Kingdom and the United States. Am J Epidemiol. 2008;168:1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krueger PM, Friedman EM. Sleep duration in the United States: a cross‐sectional population‐based study. Am J Epidemiol. 2009;169:1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qureshi AI, Giles WH, Croft JB, et al. Habitual sleep patterns and risk for stroke and coronary heart disease: a 10‐year follow‐up from NHANES I. Neurology. 1997;48:904–911. [DOI] [PubMed] [Google Scholar]
- 29. Gangwisch JE, Heymsfield SB, Boden‐Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kojima M, Wakai K, Kawamura T, et al. Sleep patterns and total mortality: a 12‐year follow‐up study in Japan. J Epidemiol. 2000;10:87–93. [DOI] [PubMed] [Google Scholar]
- 31. Kripke DF, Garfinkel L, Wingard DL, et al. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. [DOI] [PubMed] [Google Scholar]
- 32. Ferrie JE, Shipley MJ, Cappuccio FP, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30:1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Signal TL, Gale J, Gander PH. Sleep measurement in flight crew: comparing actigraphic and subjective estimates to polysomnography. Aviat Space Environ Med. 2005;76:1058–1063. [PubMed] [Google Scholar]
- 34. Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8:175–183. [DOI] [PubMed] [Google Scholar]
- 35. Marin JM, Carrizo SJ, Vicente E, et al. Long‐term cardiovascular outcomes in men with obstructive sleep apnoea‐hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. [DOI] [PubMed] [Google Scholar]
- 36. Shahar E, Whitney CW, Redline S, et al. Sleep‐disordered breathing and cardiovascular disease: cross‐sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. [DOI] [PubMed] [Google Scholar]
- 37. Li C, Ford ES, Zhao G, et al. Prevalence of self‐reported clinically diagnosed sleep apnea according to obesity status in men and women: National Health and Nutrition Examination Survey, 2005–2006. Prevent Med. 2010;51:18–23. [DOI] [PubMed] [Google Scholar]


