Abstract
Background
The plasma B‐type natriuretic peptide (BNP) level has been shown to be increased in patients with chronic atrial fibrillation (AF) independent of left ventricular ejection fraction (LVEF). The purpose of this study is to evaluate the relationship between the plasma BNP level and heart rate variation in patients with AF.
Hypothesis
The plasma BNP level is associated with heart rate variation in patients with AF.
Methods
A total of 102 patients with AF and preserved LVEF were included from 2 hospitals. The ambulatory electrocardiographic recording and measurement of plasma BNP levels were performed simultaneously. Echo‐Doppler parameters were measured as the average of 10 consecutive cardiac cycles.
Results
A difference in the mean heart rate between night and day (DIFF) and the standard deviation of the 5‐miniute mean R‐R interval (SDARR) were significantly associated with log‐transformed BNP levels (r = −0.411, P < 0.001 and r = −0.243, P = 0.049, respectively). In echocardiography, the ratio of E velocity to early diastolic velocity, which reflects left ventricular (LV) filling pressure, was significantly correlated with the DIFF and SDARR, along with the log‐transformed BNP level. Stepwise multiple linear regression analysis revealed that the DIFF and age were independent factors related with the BNP level (P < 0.01).
Conclusions
The reduced diurnal variation of heart rate was significantly associated with increased BNP, which is linked to LV diastolic dysfunction in patients with AF.
Introduction
B‐type natriuretic peptide (BNP) is a cardiac neurohormone secreted from the cardiac ventricles in response to ventricular volume expansion and pressure overload.1, 2 BNP levels are elevated in patients with symptomatic left ventricular (LV) dysfunction and correlate with the prognosis of heart failure3, 4; therefore, the plasma BNP test has been reported as a useful screening tool for LV dysfunction.5 It is also reported that individuals with atrial fibrillation (AF) had higher BNP levels than those with sinus rhythm6; however, the interpretation of BNP levels in patients with chronic AF and the usefulness of BNP measurements in these patients are unclear.
Heart rate change in a short time and diurnal variation in patients with sinus rhythm reflect the influence of the autonomic nervous system on the cardiovascular system and have been used to classify patients at risk for cardiovascular events.7, 8, 9, 10, 11, 12, 13 Recent studies have shown that reduced heart rate variation in AF patients with heart failure may predict an adverse prognosis.14, 15 Despite the irregular R‐R interval in patients with AF, the autonomic nervous system modifies ventricular response. For instance, there is a clear pattern of sympathovagal balance during the 24‐hour cycle, with vagal effects dominating at night and sympathetic nervous system activity dominating during the day.16 However, the relevance of time‐domain parameters and diurnal variation in assessing autonomic function in chronic AF remains to be elucidated. This study investigated the association of plasma BNP levels in patients with AF and preserved LV systolic function with heart rate variation determined by 24‐hour ambulatory electrocardiographic monitoring. In addition, echocardiographic variables were measured to evaluate the underling mechanisms regarding the relationship between the BNP level and the parameters of 24‐hour ambulatory electrocardiographic recording.
Methods
Study Patients
This study enrolled 102 consecutive outpatients referred to 2 hospitals (Sumitomo Besshi Hospital [n = 66] and Kagawa Saiseikai Hospital [n = 36]) with a diagnosis of atrial fibrillation and preserved LV systolic function. AF of all patients was confirmed on more than 2 consecutive electrocardiograms over a 3‐month period. Preserved LV systolic function was described by an echocardiogram, which showed more than 50% LV ejection fraction (LVEF) and no regional wall motion abnormality. Exclusion criteria were as follows: (1) patients with ischemic and nonischemic cardiomyopathy; (2) renal insufficiency (serum creatinine, more than 1.5 mg/mL); (3) patients with acute coronary syndrome; (4) patients with prosthetic valves, mitral valve stenosis, and aortic valve stenosis; (5) patients with mitral valve insufficiency and aortic valve insufficiency; (6) symptomatic heart failure (New York Heart Association class III or IV); and (7) patients with pulmonary hypertension or history of pulmonary embolism. Ambulatory electrocardiographic monitoring, echocardiography and hematological examination were performed simultaneously. This study protocol complied with the Declaration of Helsinki17 and was approved by the ethics committee of Sumitomo Besshi Hospital and Kagawa Saiseikai Hospital. Informed consent was obtained from all patients before the study.
Ambulatory Electrocardiographic Recording
Two‐channel, ambulatory, electrocardiographic recordings were obtained for 24 hours. Patients were advised to perform their routine daily activities. All electrocardiographic recordings were visually reviewed to confirm AF to ensure proper identification of abnormal QRS complexes and to delete artifacts. Data were analyzed with the Marquette system 8000 (Marquette Electronics, Milwaukee, WI). The system provided hourly and full‐duration data. It was decided not to analyze heart rate variability in the frequency domain because of the erratic ventricular response to random atrial impulses; therefore, heart rate variability was analyzed in the time domain. All beats considered supraventricular in origin regardless of their prematurity status were included in this analysis. All beats considered ventricular in origin were excluded. The following time domain measures were calculated: the standard deviation of all R‐R intervals in the entire 24‐hour electrocardiograph (ECG) recording (SDRR), the standard deviation (SD) of the mean of all R‐R intervals for all 5‐minute segments of 24‐hour ECG recording (SDARR), the root‐mean‐square difference of successive RR intervals (RMSSD), and the proportion of adjacent RR intervals varying by >50 ms (pRR50). Furthermore, the diurnal heart rate variation was quantified with the following measures: the difference of mean R‐R interval between night (0 am–am) and day (9 am to 7 pm) (DIFF). We also analyzed the maximum heart rate (max HR), minimum heart rate (min HR), and mean HR for 24 hours.
Transthoracic Echocardiography
Standard transthoracic M‐mode, 2‐dimensional, and Doppler echocardiograms were examined using commercially available equipment (iE33; Philips Healthcare, Andover, MA).18 Measurements were made according to the guidelines laid down by the American Society of Echocardiography,19 10 consecutive cardiac cycles were selected for all measurements, and the results were averaged. LVEF was measured using the modified Simpson rule algorithm. Left atrial volumes (LA) were measured in the apical 4‐chamber view using the multiple discs method. LV mass was calculated using the Penn convention.20 The LA volume index and LV mass index were calculated by dividing LA volume and LV mass by the body surface area, respectively. Peak early diastolic velocity (E velocity) and the deceleration time of E velocity were measured as the time interval from the E‐wave peak to the decline of velocity to baseline values. M‐mode recording was obtained by propagation of the early mitral inflow velocity into the left ventricle. A dimensionless ratio of E velocity by pulsed Doppler to propagation velocity was calculated. The isovolumic relaxation time was measured from the end of aortic flow to the onset of mitral inflow using continuous‐wave Doppler. Doppler tissue imaging of the mitral annulus was obtained from the apical (2‐ and 4‐chamber) views. A sample volume was placed sequentially at the lateral and septal mitral annulus, and early diastolic velocity (e′) was measured. We used the average value of e′ measured at septal and lateral sites. The ratio of E velocity to e′ of the medial mitral annulus (E/e′) was also calculated.
Statistical Analysis
Distribution of continuous variables was determined by the Kolmogorov‐Smirnov test, and all parameters except for the plasma BNP level were found to be parametric. Differences in characteristics across BNP tertiles were compared by 1‐way analysis of variance, followed by the Bonferroni post hoc test for continuous variables. Pearson correlation coefficients, linear regression, and multiple stepwise linear regressions were calculated. Values are presented as the mean ± SD. Differences at P < 0.05 were considered significant. Data were analyzed using SPSS 11.0 for Windows (SPSS Inc., Chicago, IL).
Results
Clinical Characteristics of the Subjects
The baseline characteristics of the subjects are presented in Table 1. In all subjects (N = 102), the mean age was 71 ± 12 years, and 67 (65.7%) were male. The average plasma BNP levels were 164.3 ± 123.8 pg/mL. Next, to evaluate factors affecting plasma BNP levels, all patients were classified into tertiles based on plasma BNP levels. Patients in the highest BNP tertile were significantly older than those in the lowest BNP tertile. Gender, body mass index, systolic blood pressure, and diastolic blood pressure did not differ among BNP tertiles. The prevalence of hypertension, diabetes, dyslipidemia, and medications were also similar among tertiles. In European Heart Rhythm Association classification,21 patients with class II in the highest BNP tertile were higher than those in the lowest BNP tertile. Regarding the parameters of 24‐hour ambulatory electrocardiography, maximum heart rates, the DIFF, and SDARR fell with BNP levels. Univariate linear regression analysis also showed that the log‐transformed BNP level was significantly correlated with the DIFF (r = −0.411, P < 0.001) and SDARR (r = −0.243, P = 0.049) (Figure, A, B, respectively).
Table 1.
Clinical Characteristics and Electrocardiographic Findings According to BNP Tertiles (N = 102)
| All, N = 102 | BNP Tertiles, pg/mL | P | |||
|---|---|---|---|---|---|
| T1 (4.8–95.5), n = 34 | T2 (95.8–200), n = 34 | T3 (202–659), n = 34 | |||
| Age, y | 71 ± 12 | 68 ± 14 | 71 ± 12 | 75 ± 7.0 | 0.035 |
| Male, n (%) | 67 (65.7) | 24 (70.6) | 22 (64.7) | 21 (61.8) | 0.885 |
| Body mass index, kg/m2 | 24.0 ± 3.3 | 24.4 ± 3.4 | 24.4 ± 3.4 | 23.1 ± 3.1 | 0.558 |
| EHRA classification, n | |||||
| I / II | 80/22 | 30/4 | 28/6 | 22/12 | 0.049 |
| SBP, mm Hg | 133 ± 16 | 131 ± 12 | 132 ± 12 | 138 ± 23 | 0.711 |
| DBP, mm Hg | 75 ± 11 | 76 ± 12 | 76 ± 11 | 76 ± 10 | 0.917 |
| Diabetes, n (%) | 17 (16.7) | 5 (14.7) | 6 (17.6) | 6 (17.6) | 0.889 |
| Hypertension, n (%) | 55 (53.9) | 19 (55.9) | 17 (50.0) | 19 (55.9) | 0.946 |
| Dyslipidemia, n (%) | 33 (32.4) | 12 (35.3) | 10 (29.4) | 11 (32.4) | 0.577 |
| Creatinine, mg/dL | 0.86 ± 0.21 | 0.84 ± 0.18 | 0.82 ± 0.19 | 0.91 ± 0.25 | 0.316 |
| Medication, n (%) | |||||
| Digitalis | 46 (45.1) | 18 (52.9) | 15 (44.1) | 13 (38.2) | 0.313 |
| CCBs | 22 (21.6) | 7 (20.6) | 10 (29.4) | 5 (14.7) | 0.441 |
| β‐Blockers | 38 (37.3) | 10 (29.4) | 13 (38.2) | 15 (44.1) | 0.543 |
| ACEI/ARBs | 47 (46.1) | 14 (41.2) | 15 (44.1) | 18 (52.9) | 0.768 |
| Diuretics | 23 (22.5) | 7 (20.6) | 6 (17.6) | 10 (29.4) | 0.622 |
| Ambulatory electrocardiogram | |||||
| Max HR, bpm | 142.0 ± 28.1 | 143.4 ± 30.4 | 147.5 ± 25.9 | 135 ± 27.3 | 0.047 |
| Mean HR, bpm | 76.3 ± 12.7 | 75.4 ± 15.1 | 78.3 ± 11.9 | 75.3 ± 11.0 | 0.783 |
| Min HR, bpm | 41.8 ± 12.5 | 41.1 ± 12.7 | 41.6 ± 12.4 | 42.5 ± 12.9 | 0.587 |
| DIFF, bpm | 18.2 ± 9.6 | 21.1 ± 8.8 | 20.7 ± 9.7 | 12.8 ± 8.1 | 0.007 |
| SDRR | 222.8 ± 63.1 | 238.2 ± 72.8 | 223.0 ± 60.2 | 207.3 ± 52.4 | 0.576 |
| SDARR | 129.6 ± 57.5 | 145.6 ± 66.5 | 130.3 ± 52.2 | 112.4 ± 48.5 | 0.049 |
| RMSSD | 90.3 ± 17.2 | 92.6 ± 22.0 | 88.4 ± 17.7 | 90.4 ± 12.8 | 0.995 |
| pRR50 | 65.2 ± 13.2 | 67.0 ± 16.3 | 63.4 ± 11.6 | 65.1 ± 10.2 | 0.766 |
Abbreviations: ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BNP, B‐type natriuretic peptide; CCBs, calcium channel blockers; DBP, diastolic blood pressure; DIFF, difference of mean R‐R interval between night and day; EHRA, European Heart Rhythm Association; Max HR, maximum heart rate; Mean HR, mean heart rate; Min HR, minimum heart rate; pRR50, proportion of adjacent R‐R intervals varying by >50 ms; RMSSD, root‐mean‐square difference of successive R‐R intervals; SBP, systolic blood pressure; SDARR, standard deviation of the mean of all R‐R intervals for all 5‐minute segments; SDRR, standard deviation of all R‐R intervals; T, tertile.
Values are expressed as numbers with percentages in parentheses or as the mean ± standard deviation.
Heart Rate Circadian Rhythm and Other Parameters
As the DIFF was significantly covariate related with the BNP level in patients with AF, we next assessed the clinical and electrocardiographic factors influencing the DIFF in all subjects (N = 102) (Table 2). When patients were divided into 3 groups according to DIFF tertiles, age and medications did not differ among tertiles. As to the parameters of ambulatory electrocardiography, the max HR, SDRR, and SDARR in patients with the highest DIFF tertile were significantly higher than in patients with the lowest DIFF tertile.
Table 2.
Clinical Characteristics and Electrocardiographic Findings According to DIFF Tertiles (N = 102)
| DIFF, bpm | P | |||
|---|---|---|---|---|
| T1 (−3.1–12.3), n = 34 | T2 (13.1–22.2), n = 34 | T3 (22.3–42.4), n = 34 | ||
| Age, y | 74 ± 8 | 74 ± 8 | 71 ± 7 | 0.976 |
| Male, n (%) | 19 (55.9) | 24 (75.0) | 24 (75.0) | 0.094 |
| Body mass index, kg/m2 | 23.6 ± 4.0 | 24.5 ± 2.7 | 23.9 ± 3.1 | 0.722 |
| Creatinine, mg/dL | 0.853 ± 0.249 | 0.899 ± 0.211 | 0.816 ± 0.140 | 0.622 |
| SBP, mm Hg | 130.7 ± 18.2 | 136.9 ± 19.6 | 132.0 ± 10.2 | 0.645 |
| DBP, mm Hg | 73.9 ± 11.3 | 79.5 ± 9.6 | 73.6 ± 11.2 | 0.209 |
| Medication, n (%) | ||||
| Digitalis | 15 (44.1) | 14 (41.2) | 17 (50.0) | 0.565 |
| CCBs | 7 (20.6) | 8 (23.5) | 7 (20.6) | 0.772 |
| β‐Blockers | 15 (44.1) | 13 (38.2) | 10 (29.4) | 0.440 |
| ACEI/ARBs | 14 (41.2) | 16 (47.1) | 17 (50.0) | 0.559 |
| Diuretics | 9 (26.5) | 9 (26.5) | 5 (14.7) | 0.480 |
| BNP, pg/mL | 247.1 ± 159.1 | 131.8 ± 76.6 | 114.0 ± 70.4 | <0.001 |
| Ambulatory electrocardiogram | ||||
| Max HR, bpm | 127.1 ± 26.8 | 139.8 ± 24.5 | 159.0 ± 23.9 | 0.004 |
| Mean HR, bpm | 74.4 ± 12.4 | 74.6 ± 12.9 | 80.0 ± 12.4 | 0.175 |
| Min HR, bpm | 44.5 ± 12.6 | 42.0 ± 12.9 | 38.8 ± 11.8 | 0.268 |
| SDRR | 200.3 ± 53.3 | 230.4 ± 68.2 | 238.8 ± 62.1 | 0.006 |
| SDARR | 97.4 ± 47.3 | 132.0 ± 51.0 | 162.3 ± 56.6 | 0.002 |
| RMSSD | 92.1 ± 14.6 | 92.4 ± 20.9 | 86.9 ± 16.8 | 0.955 |
| pRR50 | 67.8 ± 10.4 | 65.9 ± 15.5 | 61.5 ± 12.0 | 0.680 |
Abbreviations: ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BNP, B‐type natriuretic peptide; CCBs, calcium channel blockers; DBP, diastolic blood pressure; DIFF, difference of mean R‐R interval between night and day; Max HR, maximum heart rate; Mean HR, mean heart rate; Min HR, minimum heart rate; pRR50, proportion of adjacent R‐R intervals varying by >50 ms; RMSSD, root‐mean‐square difference of successive R‐R intervals; SBP, systolic blood pressure; SDARR, standard deviation of the mean of all R‐R intervals for all 5‐minute segments; SDRR, standard deviation of all R‐R intervals; T, tertile.
Values are expressed as numbers with percentages in parentheses or as the mean ± standard deviation.
Figure 1.
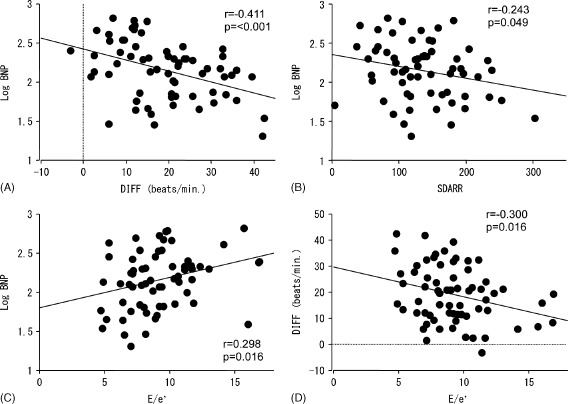
The correlation between the log‐transformed B‐type natriuretic peptide (BNP) level and the difference of mean R‐R interval between night and day (DIFF) (A), standard deviation of the mean of all R‐R intervals for all 5‐minute segments (SDARR) (B), and ratio of E velocity to early diastolic velocity (E/e′) (C) and the correlation between DIFF and E/e′ (D) in patients with atrial fibrillation.
Parameters Between Echocardiographic Measurements and Ambulatory Electrocardiography
To evaluate the potential mechanisms in the relation of heart rate variation to BNP level, we assessed the association between echocardiographic parameters and the parameters of ambulatory electrocardiography in subjects using data sets from Sumitomo Besshi Hospital (n = 66) (Table 3). The Figure, C and D, showed that E/e′ was significantly correlated with log‐transformed BNP and DIFF. As it is well known that LV diastolic function is affected by age, age‐adjusted analyses were performed. E correlated with max HR (r = −0.330, P = 0.018). E/e′ was significantly associated with the DIFF (r = −0.300, P = 0.016), SDARR (r = −0.258, P = 0.028), along with the log‐transformed BNP level (r = 0.298, P = 0.016).
Table 3.
Age‐Adjusted Correlation Between Parameters of Electrocardiography and Ambulatory Electrocardiogram (n = 66)
| LAVI | LVMI | E | DcT | IVRT | FPV/E | E/e′ | |
|---|---|---|---|---|---|---|---|
| Max HR | −0.011 | −0.200 | −0.330a | 0.117 | 0.088 | 0.021 | −0.198 |
| Mean HR | 0.077 | −0.369a | −0.122 | 0.047 | 0.012 | −0.067 | 0.001 |
| Min HR | 0.041 | −0.222 | −0.096 | −0.077 | −0.009 | −0.053 | 0.126 |
| DIFF | −0.062 | −0.079 | −0.050 | 0.258 | −0.017 | −0.097 | −0.300a |
| SDRR | −0.129 | −0.075 | 0.013 | 0.032 | 0.165 | −0.187 | −0.166 |
| SDARR | −0.119 | −0.103 | −0.144 | 0.193 | 0.249 | −0.127 | −0.258a |
| RMSSD | −0.087 | −0.037 | 0.071 | −0.082 | 0.019 | 0.148 | −0.104 |
| pRR50 | −0.110 | 0.017 | 0.054 | −0.156 | −0.054 | 0.128 | −0.114 |
| Log BNP | 0.034 | 0.040 | 0.069 | −0.065 | −0.007 | 0.055 | 0.298a |
Abbreviations: BNP, B‐type natriuretic peptide; DcT, deceleration time of E wave; DIFF, difference of mean R‐R interval between night and day; E, peak early diastolic velocity; E/e′, ratio of E velocity to early diastolic velocity; FPV/E, ratio of flow propagation velocity by E velocity; IVRT, isovolumic relaxation time; LAVI, left atrial volume index; LVMI, left ventricular mass index; Max HR, maximum heart rate; Mean HR, mean minimum heart rate; Min HR, minimum heart rate; pRR50, proportion of adjacent R‐R intervals varying by >50 ms; RMSSD, root‐mean‐square difference of successive R‐R intervals; SDARR, standard deviation of the mean of all R‐R intervals for all 5‐minute segments; SDRR, standard deviation of all R‐R intervals.
P < 0.05.
Assessments of Clinical, Ambulatory, Electrocardiographic Parameters and Echocardiographic Parameters Affecting Plasma BNP Levels
Stepwise multiple linear regression analyses in all subjects (N = 102) were performed using the following variables: age, gender, body mass index, max HR, mean HR, min HR, SDRR, SDARR, RMSSD, pRR50, and E/e′. Among variables, the DIFF and age were selected as independent correlates of BNP levels in patients with AF (Table 4).
Table 4.
Result of Stepwise Analysis (N = 102)
| Dependent Variable | Explanatory Variable | Standardized Regression Coefficient | t | P |
|---|---|---|---|---|
| Log BNP | DIFF | −0.390 | −3.484 | 0.001 |
| Age | 0.309 | 2.758 | 0.008 |
Abbreviations: BNP, B‐type natriuretic peptide; DIFF, difference of mean R‐R interval between night and day.
Multiple regression coefficient r = 0.515, P < 0.01.
Discussion
The present study demonstrated that the reduced diurnal variation of heart rate was independently associated with the increased BNP levels in patients with AF. The present finding suggests that the diurnal variation of the ventricular rate determined with 24‐hour ambulatory electrocardiographic recordings be involved in an elevation of LV filling pressure, which elevates the BNP level in patients with AF
Many reports investigated heart rate variability in patients with heart failure in sinus rhythm.8, 22, 23 Reduced variability is a consistent finding in patients with heart failure, and these reports suggested that heart rate variability might be useful for patient management. Although atrial fibrillation has been regarded as a limitation of this method, several studies have shown the usefulness of ambulatory electrocardiography in predicting an adverse outcome in patients with AF.15, 24 The presence of circadian rhythm in atrioventricular (AV) nodal conduction during AF has been shown, and the attenuation of circadian variation is associated with the progress of heart failure.25, 26 Our study showed a significant correlation between the diurnal heart rate variation and BNP level in AF patients, whereas Khand et al. reported that the circadian rhythm in the AV nodal functional refractory period was not significantly correlated with the BNP level in patients with AF.26 The discrepancy of the results may lie in the difference in LV systolic function in study populations. The average LVEF in a previous study was more reduced than in our study; therefore, LV systolic dysfunction itself might induce an increase in BNP level, which affects the relationship between heart rate variation and BNP level.
The precise mechanisms underlying the relationship between the circadian rhythm of the ventricular rate and BNP remain unclear. BNP is synthesized in the ventricular myocardium and released into the blood in response to ventricular dilatation and an elevation of LV filling pressure, which are affected by LV diastolic dysfunction. One explanation is that the interplay between LV diastolic dysfunction and reduced circadian rhythm may contribute to the increased BNP level. Only a few studies have reported the relationship between autonomic abnormalities and LV diastolic dysfunction in patients with sinus rhythm.7, 27, 28 In the present study, we demonstrated that the DIFF and SDARR correlated with E/e′ as a marker of LV filling pressure in patients with AF. Our data are consistent with previous findings in patients with sinus rhythm, although it is difficult to determine the causal relationship between the impairment of autonomic function and LV diastolic dysfunction. In the present study, we evaluated the relationship between left atrial volume index (LAVI) and parameters of electrocardiographic recording, because LV diastolic dysfunction is reported to be associated with LA size; however, no significant correlation was observed between them. Several experimental studies showed that the enlargement of atrial size induced reflex vagal excitation, and the subsequent atrial remodeling may affect the variation of the ventricular rate.29, 30, 31 LV diastolic dysfunction is a major determinant of LA enlargement in patients with sinus rhythm, whereas other factors, such as the duration of AF, may also have an effect on it in patients with AF. Although the present study showed that LAVI was not correlated with the DIFF and SDARR, it is difficult to explain the possibility that LA enlargement induced by LV diastolic dysfunctions caused the variation of ventricular response. Taking this into account, the reduced variation of the ventricular rate in AF patients may not be a consequence, but rather a mediator of LV diastolic dysfunction, although this hypothesis deserves further study.
Other possible mechanisms of the increase in BNP level in patients with reduced diurnal variation of ventricular rate are that β‐blockers are likely more frequently used in patients with a higher BNP level. The use of β‐blockers has been reported to be involved in the increase in BNP.9 In this study, multivariate analysis showed that β‐blockers were not an independent factor correlated with BNP level. On the other hand, in this study, patients with lower DIFF tended to use β‐blockers more frequently. Considering that the administration of β‐blockers has been shown to improve heart rate variability and diurnal variation of the ventricular rate in patients with cardiovascular disease,32, 33 we believe that β‐blockers did not attenuate the relationship between the BNP level and diurnal variation of the ventricular rate.
Limitations
There are several limitations of this study. First, because this is a cross‐sectional study, our data do not allow us to determine the causal relationship between the BNP level and the reduced diurnal variation seen in patients with AF, and a prospective study is required to clarify the mechanisms. Second, all patients were enrolled under medication expected to modify the ventricular response to AF. We cannot exclude the possible influence of such medication on AV conduction, although there are no significant associations of any drugs with the BNP level and the measurement of heart rate variability. Third, invasive hemodynamic measurement, including cardiac catheterization, was not performed, because patients were recruited from the outpatient cardiology clinic. To make up this for limitation, tissue Doppler echocardiographic assessment was performed. Emerging evidence indicated that E/e′, which has been used as a reliable and reproducible parameter of LV filling pressure in patients with sinus rhythm,2 could be applicable to patients with AF.34 Fourth, in this study, AF was defined on more than 2 consecutive electrocardiograms over a 3‐month period. Therefore, patients with paroxysmal AF cannot be excluded completely, whereas most patients in this study had persistent or long‐standing persistent AF according to medical records and electrocardiograms.
Conclusion
The present study demonstrated a significant relationship between diurnal heart rate variation and plasma BNP level in patients with AF. The reduction of heart rate variation in patients with AF may be involved in an increased LV filling pressure, which increases BNP levels. Parameters such as max HR, mean HR, and min HR derived from 24‐hour ambulatory electrocardiographic recording have been used for the most part to control the heart rate in patients with AF. Our results suggest the 24‐hour ambulatory electrocardiographic recording may have the potential to investigate the prognosis regarding heart failure in patients with AF.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Lubien E, DeMaria A, Krishnaswamy P, et al. Utility of B‐natriuretic peptide in detecting diastolic dysfunction: comparison with doppler velocity recordings. Circulation. 2002;105:595–601. [DOI] [PubMed] [Google Scholar]
- 2. Dokainish H, Zoghbi WA, Lakkis NM, et al. Optimal noninvasive assessment of left ventricular filling pressures: a comparison of tissue doppler echocardiography and B‐type natriuretic peptide in patients with pulmonary artery catheters. Circulation. 2004;109:2432–2439. [DOI] [PubMed] [Google Scholar]
- 3. Berger R, Huelsman M, Strecker K, et al. B‐type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002;105:2392–2397. [DOI] [PubMed] [Google Scholar]
- 4. McDonagh TA, Robb SD, Murdoch DR, et al. Biochemical detection of left‐ventricular systolic dysfunction. Lancet. 1998;351:9–13. [DOI] [PubMed] [Google Scholar]
- 5. Rodeheffer RJ. Measuring plasma b‐type natriuretic peptide in heart failure: good to go in 2004? J Am Coll Cardiol. 2004;44:740–749. [DOI] [PubMed] [Google Scholar]
- 6. Lee SH, Jung JH, Choi SH, et al. Determinants of brain natriuretic peptide levels in patients with lone atrial fibrillation. Circ J. 2006;70:100–104. [DOI] [PubMed] [Google Scholar]
- 7. Stein PK, Tereshchenko L, Domitrovich PP, et al. Diastolic dysfunction and autonomic abnormalities in patients with systolic heart failure. Eur J Heart Fail. 2007;9:364–369. [DOI] [PubMed] [Google Scholar]
- 8. Van Hoogenhuyze D, Weinstein N, Martin GJ, et al. Reproducibility and relation to mean heart rate of heart rate variability in normal subjects and in patients with congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1991;68:1668–1676. [DOI] [PubMed] [Google Scholar]
- 9. Davis ME, Richards AM, Nicholls MG, et al. Introduction of metoprolol increases plasma B‐type cardiac natriuretic peptides in mild, stable heart failure. Circulation. 2006;113:977–985. [DOI] [PubMed] [Google Scholar]
- 10. Stein KM, Borer JS, Hochreiter C, et al. Prognostic value and physiological correlates of heart rate variability in chronic severe mitral regurgitation. Circulation. 1993;88:127–135. [DOI] [PubMed] [Google Scholar]
- 11. Cripps TR, Malik M, Farrell TG, et al. Prognostic value of reduced heart rate variability after myocardial infarction: clinical evaluation of a new analysis method. Br Heart J. 1991;65:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abe Y, Tamura A, Nasu M. Relationship between heart rate variability and left ventricular remodeling after reperfused first anterior wall acute myocardial infarction. Circ J. 2003;67:225–228. [DOI] [PubMed] [Google Scholar]
- 13. Koyama J, Watanabe J, Yamada A, et al. Evaluation of heart‐rate turbulence as a new prognostic marker in patients with chronic heart failure. Circ J. 2002;66:902–907. [DOI] [PubMed] [Google Scholar]
- 14. Stein KM, Borer JS, Hochreiter C, et al. Variability of the ventricular response in atrial fibrillation and prognosis in chronic nonischemic mitral regurgitation. Am J Cardiol. 1994;74:906–911. [DOI] [PubMed] [Google Scholar]
- 15. Frey B, Heinz G, Binder T, et al. Diurnal variation of ventricular response to atrial fibrillation in patients with advanced heart failure. Am Heart J. 1995;129:58–65. [DOI] [PubMed] [Google Scholar]
- 16. Kienzle MG, Ferguson DW, Birkett CL, et al. Clinical, hemodynamic and sympathetic neural correlates of heart rate variability in congestive heart failure. Am J Cardiol. 1992;69:761–767. [DOI] [PubMed] [Google Scholar]
- 17. World Medical Association Declaration Of Helsinki . Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–926. [PubMed] [Google Scholar]
- 18. Kaji Y, Miyoshi T, Doi M, et al. Augmentation index is associated with B‐type natriuretic peptide in patients with paroxysmal atrial fibrillation. Hypertens Res. 2009;32:611–616. [DOI] [PubMed] [Google Scholar]
- 19. Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Soc Echocardiogr. 2003;16:1091–1110. [DOI] [PubMed] [Google Scholar]
- 20. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. [DOI] [PubMed] [Google Scholar]
- 21. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 22. Casolo G, Balli E, Taddei T, et al. Decreased spontaneous heart rate variability in congestive heart failure. Am J Cardiol. 1989;64:1162–1167. [DOI] [PubMed] [Google Scholar]
- 23. Stefenelli T, Bergler‐Klein J, Globits S, et al. Heart rate behaviour at different stages of congestive heart failure. Eur Heart J. 1992;13:902–907. [DOI] [PubMed] [Google Scholar]
- 24. Yamada A, Hayano J, Sakata S, et al. Reduced ventricular response irregularity is associated with increased mortality in patients with chronic atrial fibrillation. Circulation. 2000;102:300–306. [DOI] [PubMed] [Google Scholar]
- 25. Hayano J, Sakata S, Okada A, et al. Circadian rhythms of atrioventricular conduction properties in chronic atrial fibrillation with and without heart failure. J Am Coll Cardiol. 1998;31:158–166. [DOI] [PubMed] [Google Scholar]
- 26. Khand AU, Rankin AC, Cleland JG, et al. The assessment of autonomic function in chronic atrial fibrillation: description of a non‐invasive technique based on circadian rhythm of atrioventricular nodal functional refractory periods. Europace. 2006;8:927–934. [DOI] [PubMed] [Google Scholar]
- 27. Arora R, Krummerman A, Vijayaraman P, et al. Heart rate variability and diastolic heart failure. Pacing Clin Electrophysiol. 2004;27:299–303. [DOI] [PubMed] [Google Scholar]
- 28. Poulsen SH, Jensen SE, Moller JE, et al. Prognostic value of left ventricular diastolic function and association with heart rate variability after a first acute myocardial infarction. Heart. 2001;86:376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chorro FJ, Kirchhof CJ, Brugada J, et al. Ventricular response during irregular atrial pacing and atrial fibrillation. Am J Physiol. 1990;259:H1015–H1021. [DOI] [PubMed] [Google Scholar]
- 30. Kaseda S, Zipes DP. Contraction‐excitation feedback in the atria: a cause of changes in refractoriness. J Am Coll Cardiol. 1988;11:1327–1336. [DOI] [PubMed] [Google Scholar]
- 31. Ninomiya I. Direct evidence of nonuniform distribution of vagal effects on dog atria. Circ Res. 1966;19:576–583. [DOI] [PubMed] [Google Scholar]
- 32. Burger AJ, Kamalesh M. Effect of beta‐adrenergic blocker therapy on the circadian rhythm of heart rate variability in patients with chronic stable angina pectoris. Am J Cardiol. 1999;83:596–598, A598. [DOI] [PubMed] [Google Scholar]
- 33. Mortara A, La Rovere MT, Pinna GD, et al. Nonselective beta‐adrenergic blocking agent, carvedilol, improves arterial baroflex gain and heart rate variability in patients with stable chronic heart failure. J Am Coll Cardiol. 2000;36:1612–1618. [DOI] [PubMed] [Google Scholar]
- 34. Hadano Y, Murata K, Liu J, et al. Can transthoracic doppler echocardiography predict the discrepancy between left ventricular end‐diastolic pressure and mean pulmonary capillary wedge pressure in patients with heart failure? Circ J. 2005;69:432–438. [DOI] [PubMed] [Google Scholar]


