ABSTRACT
Background
The recent cholesterol guideline recommends high‐intensity statins in cardiovascular disease (CVD) patients. High‐intensity statins are associated with more frequent side effects. Therefore, it may be of concern that these recommendations might reduce statin adherence.
Hypothesis
High‐intensity statins are associated with lower adherence compared with low‐ to moderate‐intensity statins.
Methods
In a national database of 972 532 CVD patients from the Veterans Health Administration, we identified patients receiving statins between October 1, 2010, and September 30, 2011. We assessed statin adherence by calculating proportion of days covered (PDC) and determined whether high‐intensity statin therapy was independently associated with a lower PDC.
Results
Statins were prescribed in 629 005 (64.7%). Of those, 229 437 (36.5%) received high‐intensity statins. Mean PDC (0.87 vs 0.86, P < 0.0001) and patients with PDC ≥0.80 (76.3% vs 74.2%, P < 0.0001) were slightly higher for those receiving low‐ to moderate‐intensity compared with high‐intensity statins. In adjusted analyses, high‐intensity statin use was associated with a significant but modest PDC reduction compared with low‐ to moderate‐intensity statin use, whether PDC was assessed as a continuous (β‐coefficient: −0.008, P < 0.0001) or categorical (PDC ≥0.80 [odds ratio: 0.94, 95% confidence interval: 0.93‐0.96]) measure of statin adherence.
Conclusions
An approach of high‐intensity statin therapy will lead to a significant practice change, as the majority of CVD patients are not on high‐intensity therapy. However, this change may be associated with a very modest reduction in statin adherence compared with low‐ to moderate‐intensity therapy that is unlikely to be of clinical significance.
Introduction
The 2013 American College of Cardiology (ACC)/American Heart Association (AHA) guideline on treatment of blood cholesterol recommends high‐intensity statin use in most patients with cardiovascular disease (CVD).1 This is based on studies showing that statin therapy,2, 3, 4 and more important, high‐intensity statin therapy,5, 6, 7 is associated with an improvement in cardiovascular outcomes compared with low‐intensity statin therapy.
Although a number of studies have compared high‐intensity statins with low‐ or moderate‐intensity statins,5, 6, 7 most have focused on evaluating the effectiveness of these therapies in reducing CVD events and improving prognosis. Evaluation of safety data indicates that high‐intensity statin therapy is also accompanied by increased side effects and adverse events, posing a risk to adherence in CVD patients treated with high‐intensity statins. Although it is possible that a higher risk of side effects from high‐intensity statin therapy could affect medication adherence compared with low‐intensity statin therapy, this issue has not been well explored in the literature. This has implications as health care providers adopt the recent cholesterol guideline1 into clinical practice.
Therefore, using data from a national cohort of patients with CVD, we aimed to determine whether the use of high‐intensity statin therapy is associated with a lower adherence to statin medications compared with low‐ to moderate‐intensity statin therapy.
Methods
Cohort Development and Study Population
Using the Department of Veterans Affairs (VA) administrative data sources, we identified CVD (coronary heart disease [CHD], peripheral artery disease [PAD], ischemic stroke) patients with primary‐care clinic visits in the VA health care system between October 1, 2010, and September 30, 2011. These patients received primary care in 130 VA health care system facilities or their associated community‐based outpatient clinics. We identified patients as having CHD by using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnoses and procedure codes for unstable angina or myocardial infarction (MI), or current procedural terminology (CPT) codes for percutaneous coronary intervention or coronary artery bypass grafting (see Supporting Table 1 in the online version of this article). We included patients with ≥2 outpatient diagnosis codes or 1 inpatient diagnosis code for unstable angina, or 1 code for MI, percutaneous coronary intervention, or coronary artery bypass grafting.8 We also used ICD‐9‐CM diagnosis codes (see Supporting Table 1 in the online version of this article) to identify patients with PAD. We excluded patients with metastatic cancers and those receiving hospice care, as these patients are typically not considered candidates for quality measurement (Figure 1).9
Table 1.
Comparison of Baseline Characteristics Among CVD Patients on High‐Intensity and Low‐ to Moderate‐Intensity Statin Therapy
| Characteristic | High‐Intensity Statin Therapy, n = 229 437 | Low‐ to Moderate‐Intensity Statin Therapy, n = 399 568 | P Value |
|---|---|---|---|
| Age, y, mean/SD | 68.8/9.7 | 72/10.5 | <0.0001 |
| Female sex, n (%) | 2828 (1.23) | 4868 (1.22) | 0.60 |
| Race, n (%) | |||
| White | 196 006 (85.4) | 348 733 (87.3) | |
| Black | 20 833 (9.1) | 32 051 (8.0) | |
| Other | 3944 (1.7) | 6610 (1.6) | |
| Unknown | 8654 (3.8) | 12 174 (3.1) | <0.0001 |
| DM, n (%) | 110 405 (48.1) | 399 568 (63.5) | <0.0001 |
| Hypertension, n (%) | 191 636 (83.5) | 331 630 (83) | <0.0001 |
| History of CHD, n (%)a | 220 562 (96.1) | 377 380 (94.4) | <0.0001 |
| History of MI, n (%) | 104 843 (45.7) | 172 050 (43.1) | <0.0001 |
| History of PAD only, n (%) | 8875 (3.9) | 22 188 (5.5) | <0.0001 |
| Diagnostic Cost Group RRS, mean/SD | 1.77/2.52 | 1.94/2.85 | <0.0001 |
| Receiving primary care from a physician provider, n (%)b | 173 851 (78) | 300 610 (77.5) | <0.0001 |
| Receiving care at a teaching facility | 96 939 (42.3) | 166 728 (41.7) | <0.0001 |
| Number of primary‐care visits in prior 12 months, mean/SD | 4.89/4 | 4.86/4 | 0.006 |
| Receiving nonstatin lipid‐lowering medication(s), n (%)c | 36 434 (15.9) | 44 037 (11) | <0.0001 |
Abbreviations: CHD, coronary heart disease; CVD, cardiovascular disease; DM, diabetes mellitus; MI, myocardial infarction; PAD, peripheral artery disease; RRS, relative risk score; SD, standard deviation.
History of CHD with or without PAD.
Percentage calculated from among those with available data.
Nonstatin lipid‐lowering medications include bile acid sequestrants, ezetimibe, niacin therapy, fish oil, or fibrate use.
Figure 1.
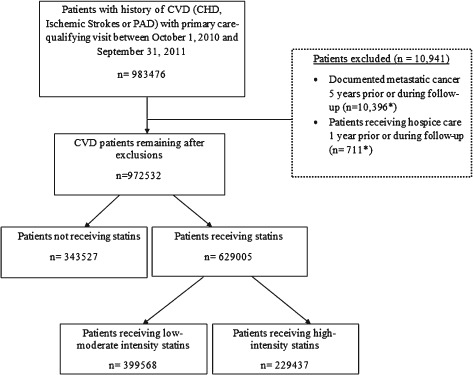
Flowchart of the study cohort identification and exclusion. *Total adds up to >10 941 as some patients met >1 exclusion criteria. Abbreviations: CHD, coronary heart disease; CVD, cardiovascular disease; PAD, peripheral artery disease.
Using VA administrative pharmacy data sources, we identified patients who received any statin prescription within 100 days prior to or 14 days following their most recent primary‐care visit during the study interval. The statins studied included atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, simvastatin, or pitavastatin. Among those receiving statin, we further subdivided the cohort into those receiving low‐ to moderate‐intensity therapy and those receiving high‐intensity therapy, based on their most recent statin fill. The definition of high‐intensity statin therapy was derived based on the recent ACC/AHA cholesterol guidelines1 as daily statin intensity associated with approximately ≥50% low‐density lipoprotein cholesterol (LDL‐C) reduction (atorvastatin dose of 40–80 mg/d, rosuvastatin 20–40 mg/d, or simvastatin >40 mg/d).
We assessed statin adherence in each CVD patient receiving statin therapy by calculating the proportion of days covered (PDC), a well‐studied measure of a patient's adherence to medications.10, 11, 12, 13, 14, 15 The PDC for statins for each CVD patient was defined as the total number of days supplied for each statin fill (eg, 30, 60, 90) divided by the observation time interval from their most recent primary‐care visit in the study interval. The observational time interval started on the day of the first dispensed prescription in 365 days prior to the index primary‐care visit for a patient started on a statin medication or the farthest prescription within 365 days from the index primary‐care visit for a patient already on a statin. Proportion of days covered was calculated as both a continuous outcome and as a dichotomous outcome, with patients having a statin PDC ≥0.80 classified as adherent and those with statin PDC <0.80 classified as nonadherent. These thresholds are consistent with prior literature.11, 12, 15
Using the VA administrative datasets, we assessed several patient characteristics, including age, race, and history of diabetes mellitus (DM) or hypertension (see Supporting Table 1 for codes). To assess the impact of a patient's illness burden on statin adherence, we calculated a mean Diagnostic Cost Group (DCG) relative risk score (RRS) for each CVD patient. The DCG RRS is a ratio of predicted to the mean cost and has been used as a measure of illness burden.16, 17, 18, 19, 20 For example, a patient with an RRS of 2 is expected to be twice as costly, with an illness burden twice as high as an “average” patient. We also assessed several facility, provider, and system‐of‐care characteristics. These included whether the facility was a teaching facility, the type of provider (physician or nonphysician primary‐care provider, such as a nurse practitioner [NP] or a physician assistant [PA]), and the number of primary‐care visits received by a CVD patient in the 12 months prior to their most recent primary‐care visit.
Outcomes and Analyses
We first compared patient, facility, provider, and system‐of‐care characteristics between patients receiving high‐intensity and low‐ to moderate‐intensity statin therapy. We then compared statin adherence (mean PDC and proportion of patients with PDC ≥0.80) between the 2 groups using the t test and χ2 test, respectively.
Our outcome was statin adherence. We performed multivariate hierarchical regression to determine whether receipt of high‐intensity statin therapy was independently associated with lower PDC. Two sets of analyses were conducted. We first performed hierarchical linear regression using PDC as a continuous variable (scaled to a 5% PDC change). β‐Coefficients for these models including high‐intensity statin therapy (adjusting for covariates described below) were calculated. These β‐coefficients can be interpreted as unit change in PDC associated with high‐intensity statin therapy adjusting for covariates. We then performed multivariate hierarchical logistic regression to determine the association between high‐intensity statin use and PDC ≥0.80. For both analyses, covariates used for adjustment included patient characteristics (age, race, sex, history of DM or hypertension, DCG RRS, history of CHD vs PAD), facility and provider characteristics (receipt of care at a teaching or nonteaching facility, from a physician or nonphysician [NP or PA]), and system‐of‐care characteristics (number of primary‐care visits in 12 months prior to the index visit). Because the random variance in care could differ secondary to clustering of patients between facilities, we also adjusted for clustering of patients at the facility level in our regression models using generalized linear latent and mixed models (GLLAMM) in STATA.
As the recent ACC/AHA guideline allows the use of moderate‐intensity statin therapy in CVD patients unable to tolerate high‐intensity statin therapy,1 we also performed exploratory analyses comparing statin adherence among patients receiving low‐intensity statin therapy vs those receiving moderate‐ to high‐intensity statin therapy. Low‐intensity statin therapy was defined as per the ACC/AHA cholesterol management guideline1 as the daily dose of statin that would lower LDL‐C on average by <30%.
We conducted the analyses with SAS version 9.1.3 (SAS Institute Inc., Cary, NC) and STATA version 11 (StataCorp, College Station, TX). The protocol was approved by the institutional review boards at Baylor College of Medicine and the Michael E. DeBakey VA Medical Center.
Results
The flowchart of the study population is shown in the Figure 1. From the initial 983 476 CVD patients, we excluded 10 941 (1.1%) patients due to documented metastatic cancer or receipt of hospice care. Among those remaining, 343 527 (35.3%) were not receiving a statin and 629 005 patients (64.7% of eligible) were receiving a statin. Of these 629 005 CVD patients, 399 568 (63.5%) were receiving low‐ to moderate‐intensity statins and 229 437 (36.5%) were receiving high‐intensity statins. Of the total CVD population of 972 532 after exclusions, only 23.6% were receiving high‐intensity statin therapy.
A comparison of the baseline characteristics of patients receiving high‐intensity and low‐ to moderate‐intensity statin therapy is shown in Table 1. The CVD patients receiving high‐intensity statins were younger, more likely to have hypertension and CHD, more likely to have prior history of MI, more likely to receive primary care from a physician provider (as opposed to NPs or PAs), and more likely to receive care at a teaching facility and to receive nonstatin lipid‐lowering medications compared with those on low‐ to moderate‐intensity statins. Patients receiving high‐intensity statins were less likely to be white, had a lower prevalence of DM and PAD, and had a lower illness burden compared with patients on low‐ to moderate‐dose statins.
Table 2 describes the mean PDC and percentage of patients with PDC ≥0.80 among the low‐ to moderate‐ and high‐intensity statin therapy groups. Mean PDC was statistically significantly higher among CVD patients receiving low‐ to moderate‐intensity statins (0.87) compared with those receiving high‐intensity statins (0.86, P < 0.0001); however, the differences were numerically small. The percentage of CVD patients with PDC ≥0.80 was also slightly higher among those receiving low‐ to moderate‐intensity statins (76.3%) compared with those receiving high‐intensity statins (74.2%, P < 0.0001). Results remained consistent after excluding patients on 80 mg of simvastatin (see Supporting Table 2 in the online version of this article). In the overall cohort (irrespective of the statin dose), 154 187 CVD patients (24.5%) had PDC >0.80. Nonadherent patients (see Supporting Table 3 in the online version of this article) were younger, more likely to be female or black, and were more likely to have PAD only (vs CHD). Nonadherent patients also had a higher overall illness burden compared with adherent patients and higher LDL‐C and non–high‐density lipoprotein cholesterol levels.
Table 2.
Comparison of Statin Adherence Between CVD Patients Receiving High‐Intensity and Low‐ to Moderate‐Intensity Statin Therapy
| Statin Adherence | High‐Intensity Statin Therapy, n = 229 435a | Low‐ to Moderate‐Intensity Statin Therapy, n = 399 563a | P Value |
|---|---|---|---|
| PDC, mean (SD) | 0.86 (0.18) | 0.87 (0.18) | <0.0001 |
| Patients with PDC ≥0.80, n (%) | 170 150 (74.2) | 304 661 (76.3) | <0.0001 |
Abbreviations: CVD, cardiovascular disease; PDC, proportion of days covered; SD, standard deviation.
Number of patients with available PDC for statins in each of the groups. PDC is missing for 2 patients in the high‐intensity therapy group and 5 patients in the low‐ to moderate‐intensity therapy group.
Table 3.
Association Between Receipt of High‐Intensity Statin Therapy and Proportion of Days Covered After Multivariate Regression Analyses
| Outcome(s) | Unadjusted β‐Coefficient (P Value)a | Model 1 β‐Coefficient (P Value)a | Model 2 β‐Coefficient (P Value)a |
|---|---|---|---|
| PDC as a continuous measure (low‐moderate intensity as referent category)b | −0.02 (<0.0001) | −0.008 (<0.0001) | −0.008 (<0.0001) |
| Unadjusted OR (95% CI)c | Model 1 OR (95% CI)c | Model 2 OR (95% CI)c | |
| PDC ≥0.8 (low‐moderate intensity as referent category) | 0.89 (0.88‐0.91) | 0.95 (0.93‐0.96) | 0.94 (0.93‐0.96) |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; DM, diabetes mellitus; OR, odds ratio; PAD, peripheral artery disease; PDC, proportion of days covered; RRS, relative risk score.
Model 1: Adjusted for age, race (white vs others), sex, history of DM, hypertension, Diagnostic Cost Group RRS (marker of a patient's illness burden). Model 2: Model 1 plus provider type (physicians vs nonphysician providers [ie, nurse practitioners or physician assistants]), teaching vs nonteaching facility, number of primary‐care visits in the prior 12 months, history of CHD vs PAD.
β‐Coefficient can be defined as the unit change in PDC associated with high‐intensity statin therapy compared with low‐ to moderate‐intensity statin therapy adjusting for covariates of interest.
PDC modeled as per‐5% change.
OR for PDC ≥0.80 associated with high‐dose statin therapy (low‐moderate dose used as the referent category) adjusting for covariates of interest.
In adjusted analyses (Table 3), high‐intensity statin therapy was associated with a significant but modest reduction in PDC compared with low‐ to moderate‐intensity statin therapy (β‐coefficient: −0.008, P < 0.0001). Similarly, high‐intensity statin therapy was associated with a significant but modest 6% lower likelihood of PDC ≥0.80 compared with low‐ to moderate‐intensity statin therapy (odds ratio: 0.94, 95% confidence interval: 0.93‐0.96).
In exploratory analyses, we compared mean PDC and proportion of CVD patients with PDC ≥0.80 among those receiving low‐intensity statin therapy vs moderate‐ to high‐intensity statin therapy (see Supporting Table 4 in the online version of this article). Mean PDC (0.82 vs 0.87 for the low‐intensity vs moderate‐ to high‐intensity statins, respectively, P < 0.0001) and the proportion of CVD patients with PDC ≥0.80 (68.5% vs 75.7% for the low‐intensity vs moderate‐ to high‐intensity statins, respectively, P < 0.0001) were higher for the moderate‐ to high‐intensity group compared with the low‐intensity group. We also performed exploratory analyses (see Supporting Table 5 in the online version of this article) comparing low‐, moderate‐, and high‐intensity statin therapy. The results of these analyses were consistent with the main analyses and did not show a lower adherence for the moderate‐ or high‐intensity statin therapy compared with low‐intensity statin therapy. In fact, the adherence to statin therapy was lowest for CVD patients on low‐intensity statin therapy. Results remained consistent when analyses were performed after excluding patients age >75 years (see Supporting Table 6 in the online version of this article).
Discussion
Our results indicate that although high‐intensity statin therapy was associated with a statistically significant lower adherence whether continuous PDC or categorical PDC ≥0.80 was used as a measure of statin adherence, these differences were numerically modest in the adjusted models and unlikely to be clinically significant. Importantly, almost one‐quarter (24.5%) of the patients were not adherent to their statin therapy (PDC <0.80), irrespective of the dose of the statin that they were prescribed.
Prior studies comparing low‐ to moderate‐intensity vs high‐intensity statins have shown that the use of high‐intensity statins has been associated with higher rates of statin discontinuation. The IDEAL Study (High‐Dose Atorvastatin vs Usual‐Dose Simvastatin for Secondary Prevention After Myocardial Infarction)6 compared the effects of high‐intensity atorvastatin (80 mg/d) and low‐ to moderate‐dose simvastatin (20 mg/d) on the occurrence of major coronary events. Results indicated that patients in the atorvastatin group had significantly higher rates of drug discontinuation due to nonserious adverse events compared with patients in the simvastatin group (9.6% vs 4.2%, respectively, P < 0.001). Furthermore, 14% of patients in the atorvastatin group and 7% of patients in the simvastatin group permanently discontinued medication by the end of the study. The Treating to New Targets (TNT) study7 showed significantly higher rates of adverse events in the patients receiving 80 mg of atorvastatin, compared with those receiving 10 mg of atorvastatin (8.1% vs 5.8%, respectively, P < 0.001). The respective rates of discontinuation due to treatment‐related adverse events were 7.2% and 5.3%, respectively (P < 0.001). Based on these studies, it is conceivable that among CVD patients who receive high‐intensity vs low‐ to moderate‐intensity statins, the adherence to statins could be lower. Our results indicate that although this might be the case, the reduction in statin adherence for high‐intensity compared with low‐ to moderate‐dose statin therapy is very modest and likely not clinically significant.
Our results are reassuring given the emphasis put on the use of high‐intensity statin therapy in CVD patients in the recently released 2013 ACC/AHA cholesterol management guideline. Although our results cannot account for initial discontinuation of statin therapy when CVD patients are put on low‐ to moderate‐intensity or high‐intensity statins, these results should provide reassurance to the providers that the long‐term adherence to statins is minimally affected by prescribing high‐intensity statins among patients with established atherosclerosis, a group shown to derive the most benefit from statin therapy.21, 22 The minor differences in statin adherence between the high‐intensity and low‐ to moderate‐intensity groups are a reflection of the large sample size of our study population. Therefore, differences in statin adherence, although statistically significant, are likely not clinically meaningful.
Our results also show that implementation of these guidelines will require a substantial change in practice patterns, as only 23.6% of patients with CVD were on high‐intensity statins. In addition, close to 35% of the patients were not receiving statins. These results indicate a substantial gap in clinical practice and identify a need to align practice with clinical guidelines.
The ACC/AHA guidelines on cholesterol management1 indicate that a moderate‐dose statin therapy can be used if a CVD patient is not able to tolerate high‐intensity statin therapy due to side effects. Our results (see Supporting Table 4 in the online version of this article) indicate that when this tailored approach of prescribing moderate‐ to high‐intensity statin therapy is followed, adherence is indeed improved compared with low‐intensity statin therapy. On the other hand, our results showing that the adherence to low‐intensity statin therapy was lower compared with moderate‐ and high‐intensity statin therapy could indicate that, in CVD patients who are nonadherent to their medication regimen, the providers might be trying a low‐intensity statin approach to ensure that patients are on at least some statin therapy.
Our results indicate that almost one‐quarter of patients with CVD are not adherent to statin therapy (PDC <0.80), irrespective of the dose of statin used. These results are consistent with prior studies16, 23, 24 and indicate a need to target specific reasons leading to medication nonadherence in this high‐risk secondary‐prevention population.
Our study has several limitations that should be kept in mind when interpreting these results. Given the observational nature of our analyses, residual confounding cannot be completely excluded. However, the fact that these data were obtained outside of the clinical‐trial setting greatly enhances generalizability to routine practice. We did not have data on patients' level of physical activity, which could also alter their propensity to develop musculoskeletal side effects from various statin doses, which in turn could possibly affect statin adherence. These results indicate experience from the VA health care system, with a predominantly male population, and therefore the findings may not be generalizable to other health care settings, especially given that the side‐effect profile and, therefore, adherence to statin therapy, could vary by sex. On the other hand, our analyses included 7696 females. In addition, the frequency and the dynamics of primary care for CVD could be different outside the VA health care system, limiting the generalizability of our findings. Lastly, our analyses did not account for medication affordability or copay, which could affect adherence23; although, given the relatively universal health care coverage afforded to veterans receiving care in the VA health care system, this may not be a major determinant of adherence in the VA health care system.
Conclusion
An approach of high‐intensity statin therapy use in patients with established CVD, as suggested by the recent ACC/AHA cholesterol management guidelines,1 is associated with a significant but very modest reduction in statin adherence. Almost one‐quarter of patients with established CVD are nonadherent to their statin therapy, irrespective of the dose of statin used. Future quality‐improvement initiatives should target these CVD patients.
Disclosures
Dr. Virani is supported by a Department of Veterans Affairs Health Services Research and Development (HSR&D) Service Career Development Award (09‐028), American Heart Association Beginning Grant‐in‐Aid, and the American Diabetes Association Clinical Science and Epidemiology award. This work was also supported by the Houston VA HSR&D Center for Innovations grant (grant HFP 90‐020). Dr. Ballantyne has received grant/research support from the following institutions (all significant and all paid to the institution, not the individual): Abbott, Amarin, Amgen, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi‐Synthelabo, National Institutes of Health, American Heart Association. He also has served as a consultant for Abbott, Aegerion, Amarin, Amgen, Cerenis, Esperion, Genzyme, Kowa, Merck, Novartis, Pfizer, Resverlogix, Regeneron, Roche, and Sanofi‐Synthelabo.
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Supporting information
Table S1. Table of International Classification of Diseases, 9th Revision, Clinical Modification and Current Procedural Terminology Codes used in the current analyses
Table S2. Comparison of statin adherence between CVD patients receiving high‐intensity and low to moderate intensity statin therapy (after excluding patients on 80 mg of simvastatin)
Table S3. Comparison of baseline characteristics among of patients with PDC >0.8 and those with PDC <0.8
Table S4. Comparison of statin adherence between CVD patients receiving low‐intensity and moderate to high intensity statin therapy
Table S5. Comparison of statin adherence between CVD patients receiving high, moderate, and low‐intensity statin therapy
Table S6. Comparison of statin adherence between CVD patients receiving high, moderate, and low‐intensity statin therapy (after excluding patients with age >75 years)
Acknowledgments
The authors thank Mark Kuebeler, MS, for his programming effort on this article.
A complete list of all author disclosures can be found at the end of this article.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol. 2014;63(25 part B):3024–3025]. J Am Coll Cardiol. 2014;63(25 part B):2889–2934. [DOI] [PubMed] [Google Scholar]
- 2. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA. 1998;279:1615 1622. [DOI] [PubMed] [Google Scholar]
- 3. Heart Protection Study Collaborative Group . MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet. 2002;360:7–22.12114036 [Google Scholar]
- 4. Scandinavian Simvastatin Survival Study Group . Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 5. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 6. Pedersen TR, Faergeman O, Kastelein JJ, et al. High‐dose atorvastatin vs usual‐dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. [DOI] [PubMed] [Google Scholar]
- 7. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 8. Solberg LI, Engebretson KI, Sperl‐Hillen JM, et al. Are claims data accurate enough to identify patients for performance measures or quality improvement? The case of diabetes, heart disease, and depression. Am J Med Qual. 2006;21:238–245. [DOI] [PubMed] [Google Scholar]
- 9. Woodard LD, Landrum CR, Urech TH, et al. Treating chronically ill people with diabetes mellitus with limited life expectancy: implications for performance measurement. J Am Geriatr Soc. 2012;60:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ho PM, Magid DJ, Shetterly SM, et al. Importance of therapy intensification and medication nonadherence for blood pressure control in patients with coronary disease. Arch Intern Med. 2008;168:271–276. [DOI] [PubMed] [Google Scholar]
- 11. Steiner JF, Koepsell TD, Fihn SD, et al. A general method of compliance assessment using centralized pharmacy records: description and validation. Med Care. 1988;26:814–823. [DOI] [PubMed] [Google Scholar]
- 12. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. [DOI] [PubMed] [Google Scholar]
- 13. Vigen R, Shetterly S, Magid DJ, et al. A comparison between antihypertensive medication adherence and treatment intensification as potential clinical performance measures. Circ Cardiovasc Qual Outcomes. 2012;5:276–282. [DOI] [PubMed] [Google Scholar]
- 14. Yang Y, Thumula V, Pace PF, et al. Predictors of medication nonadherence among patients with diabetes in Medicare Part D programs: a retrospective cohort study. Clin Ther. 2009;31:2178–2188. [DOI] [PubMed] [Google Scholar]
- 15. Karve S, Cleves MA, Helm M, et al. Good and poor adherence: optimal cut‐point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25:2303–2310. [DOI] [PubMed] [Google Scholar]
- 16. Virani SS, Woodard LD, Chitwood SS, et al. Frequency and correlates of treatment intensification for elevated cholesterol levels in patients with cardiovascular disease. Am Heart J. 2011;162:725–732. [DOI] [PubMed] [Google Scholar]
- 17. Virani SS, Woodard LD, Landrum CR, et al. Institutional, provider, and patient correlates of low‐density lipoprotein and non–high‐density lipoprotein cholesterol goal attainment according to the Adult Treatment Panel III guidelines. Am Heart J. 2011;161:1140–1146. [DOI] [PubMed] [Google Scholar]
- 18. Virani SS, Wang D, Woodard LD, et al. Is simple reporting of non–high‐density‐lipoprotein cholesterol levels associated with improved non‐HDL‐C goal attainment in primary care setting? J Clin Lipidol. 2012;6:545–552. [DOI] [PubMed] [Google Scholar]
- 19. Woodard LD, Urech T, Landrum CR, et al. Impact of comorbidity type on measures of quality for diabetes care. Med Care. 2011;49:605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petersen LA, Pietz K, Woodard LD, et al. Comparison of the predictive validity of diagnosis‐based risk adjusters for clinical outcomes. Med Care. 2005;43:61–67. [PubMed] [Google Scholar]
- 21. Cholesterol Treatment Trialists' (CTT) Collaboration , Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wenger NK. Prevention of cardiovascular disease: highlights for the clinician of the 2013 American College of Cardiology/American Heart Association guidelines. Clin Cardiol. 2014;37:239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choudhry NK, Avorn J, Glynn RJ, et al. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365:2088–2097. [DOI] [PubMed] [Google Scholar]
- 24. Rodriguez F, Cannon CP, Steg PG, et al; REACH Registry Investigators . Predictors of long‐term adherence to evidence‐based cardiovascular disease medications in outpatients with stable atherothrombotic disease: findings from the REACH Registry. Clin Cardiol. 2013;36:721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Table of International Classification of Diseases, 9th Revision, Clinical Modification and Current Procedural Terminology Codes used in the current analyses
Table S2. Comparison of statin adherence between CVD patients receiving high‐intensity and low to moderate intensity statin therapy (after excluding patients on 80 mg of simvastatin)
Table S3. Comparison of baseline characteristics among of patients with PDC >0.8 and those with PDC <0.8
Table S4. Comparison of statin adherence between CVD patients receiving low‐intensity and moderate to high intensity statin therapy
Table S5. Comparison of statin adherence between CVD patients receiving high, moderate, and low‐intensity statin therapy
Table S6. Comparison of statin adherence between CVD patients receiving high, moderate, and low‐intensity statin therapy (after excluding patients with age >75 years)


