Abstract
Background
We explored the relationship between major electrocardiogram (ECG) abnormalities (mECG) and 25‐hydroxy (25‐OH) vitamin D deficiency (VDD) and the effect of mECG abnormalities on all‐cause and cardiovascular mortality in a healthy cohort with 25‐OH vitamin D insufficiency and deficiency.
Hypothesis
Lower levels of serum 25‐OH vitamin D are associated with increased prevalence of mECG on resting ECG.
Methods
We identified 5108 individuals from the National Health and Nutrition Examination Survey‐III. mECG abnormalities included: major Q‐QS wave abnormalities, ST depression/elevation, negative T waves, Wolff‐Parkinson‐White pattern, and ventricular conduction defect. Our cohort was divided into 3 groups based on 25‐OH vitamin D levels: Group 1 (referent): >40 ng/mL; group 2 (insufficient): ≥20.01 to ≤40 ng/mL; and group 3 (deficient): ≤20 ng/mL. Logistic regression and Cox proportional hazards regression models were built.
Results
The prevalence of major ECG abnormalities across 25‐OH vitamin D sufficiency, insufficiency, and deficiency was 5.9%, 11%, and 13 %, respectively (P = 0.01). VDD was an independent predictor of mECG abnormalities after adjusting for traditional risk factors (continuous variable odds ratio [OR]: 0.98, 95% confidence interval [CI]: 0.97‐0.99, P = 0.007; categorical variable group 3 vs group 1 OR: 2.36, 95% CI: 1.1‐5.12, P = 0.03). Baseline major ECG abnormalities were predictive of long‐term all‐cause (hazard ratio [HR]:1.52, 95% CI: 1.23‐1.89), composite cardiovascular (HR: 1.7, 95% CI: 1.34‐2.15), cardiovascular (HR: 1.64, 95% CI: 1.27‐2.12), and ischemic heart disease mortality (HR: 1.98, 95% CI: 1.46‐2.69) in individuals with 25‐OH vitamin D levels ≤40 ng/mL.
Conclusions
VDD is associated with increased prevalence of major ECG abnormalities. Well‐structured trials are needed to assess progression/resolution of mECG abnormalities with vitamin D supplementation in deficient individuals.
Introduction
The overall prevalence of vitamin D deficiency in the United States is 41.6% and highest among African Americans (82.1%).1 Vitamin D deficiency has been associated with a spectrum of disorders such as diabetes,2 dysmetabolism, dyslipidemia,3 hypertension,4 atherosclerosis,5 endothelial dysfunction, peripheral vascular disease,6 congestive heart failure,7 myocardial infarction,8 stroke,9 left ventricular hypertrophy,10 and cardiovascular disease.11, 12 There is insufficient evidence to warrant supplementing vitamin D to prevent cardiovascular disease.13
Major and minor electrocardiogram (ECG) abnormalities have been demonstrated to be strong independent predictors of cardiovascular events and mortality.14, 15 Recently, it has been demonstrated that the addition of ECG abnormalities to the Framingham Risk Score resulted in an overall clinical net reclassification of 3.6% in the National Health and Nutrition Examination Survey‐III (NHANES‐III) cohort16 and 7.4% in an elderly cohort.14
Studies exploring the association of vitamin D with various ECG abnormalities are scarce.10, 17
To the best of our knowledge, the association between vitamin D levels and major ECG abnormalities has not been studied. The present study sought to assess the independent relationship between the 25‐hydroxy (25‐OH) vitamin D and major ECG abnormalities, and the effect of major ECG abnormalities in individuals with 25‐OH vitamin D insufficiency and deficiency on long‐term cardiovascular and all‐cause mortality in a healthy cohort.
Methods
Study Cohort
We queried the National Health and Nutrition Examination Survey‐III (NHANES‐III) database, an observational study of nationally representative individuals between years 1988 and 1994. Our study sample was selected from 8561 individuals over age 40 years who underwent a resting baseline 12‐lead ECG. In order to ensure a disease‐free cohort, we excluded diabetics (self‐reported use of insulin/oral hypoglycemic agents or hemoglobin A1c ≥ 6.5%) (n = 935); individuals with self‐reported leg pain as per the Edinburgh claudication questionnaire18 suggestive of intermittent claudication (n = 53); subjects with self‐reported angina (n = 239); and those with a past history of congestive heart failure (n = 198), myocardial infarction (n = 192), and stroke (n = 135). We also excluded individuals with a heart rate more than 100 bpm (n = 68) and subjects with missing data. Our final cohort consisted of 5108 healthy individuals, with a median follow‐up of 13.2 years and representative of 57 million people in the United States.
Vitamin D Deficiency
25‐OH vitamin D was measured in the NHANES‐III as a part of the nutrition biomarker component using the DiaSorin radioimmunoassay kit (DiaSorin, Stillwater, MN) at the National Center for Environmental Health (Centers for Disease Control and Prevention) in Atlanta, Georgia. The DiaSorin assay kit was reformulated in 1998. To assess the magnitude of change of the reformulated assay on the originally measured 25‐OH vitamin D in NHANES‐III, 150 samples representative of the entire NHANES‐III population were remeasured using the reformulated assay, and the results were regressed using the following equation: reformulated 25‐OH vitamin D = 0.8429 * original 25‐OH vitamin D + 2.5762 (nmol/L).19
The cutoff value used to define vitamin D deficiency has been subject to debate. We defined 25‐OH vitamin D deficiency and insufficiency as levels ≤20 ng/mL and 20‐40 ng/mL, respectively, as parathyroid hormone levels reach its nadir at 25‐OH vitamin D levels of 30 to 40 ng/mL.20 25‐OH vitamin D levels >40 ng/mL were considered sufficient.21
Major ECG Abnormalities
Subjects over the age of 40 years underwent a resting 12‐lead ECG using the Marquette MAC 12 (GE Healthcare, Little Chalfont, United Kingdom). The ECGs were interpreted by the Novacode ECG program and were classified as per the Minnesota Coding System. The details have been discussed elsewhere.22 Major ECG abnormalities include major Q and QS waves, ST‐segment depression/elevation, T‐wave inversion, and ventricular conduction defects (see Supporting Information, Table 1, in the online version of this article). Individuals without aforementioned abnormalities on ECG and those with minor ECG abnormalities were considered to have no major ECG abnormalities. Individuals with atrial flutter, atrial fibrillation, supraventricular tachycardia, and pacemakers were excluded from the study.
Table 1.
Baseline Characteristics Across 25‐OH Vitamin D Level Categories
| Vitamin D Deficiency, Range: 3.98–20 ng/mL, Mean ± SD: 15.04 ± 3.41 | Vitamin D Insufficiency, Range: 20.01–40 ng/mL, Mean ± SD: 27.32 ± 5 | Vitamin D Sufficiency, Range: 40.01–70.49 ng/mL, Mean ± SD: 45.83 ± 5.39 | P Value | |
|---|---|---|---|---|
| No. (%) | 229 (4.48%) | 2750 (53.84%) | 2129 (41.68%) | |
| Demographics | ||||
| Age, y | 55.65 ± 14.6 | 55.16 ± 11.22 | 53.53 ± 9.94 | 0.1 |
| Ethnic group | <0.001 | |||
| Caucasian, % | 76.91 | 94.17 | 96.4 | |
| African American, % | 17.71 | 3.82 | 1.51 | |
| Males, % | 33.92 | 51.02 | 55.1 | <0.001 |
| Anthropometry | ||||
| BMI, kg/m2 | 27.96 ± 7.36 | 26.58 ± 4.32 | 25.9 ± 3.6 | <0.001 |
| Comorbidities | ||||
| Smoker, % | 26.9 | 23.3 | 24.8 | 0.23 |
| Family history of MI at <50 years old, % | 15.7 | 14.3 | 16.9 | 0.52 |
| Hypertension , % | 35 | 29.4 | 26.6 | 0.009 |
| Systolic blood pressure, mm of Hg | 128.6 ± 21.2 | 126.9 ± 16.4 | 126.4 ± 13.3 | 0.06 |
| Diastolic blood pressure, mm of Hg | 76.4 ± 11.2 | 76.1 ± 8.8 | 76.9 ± 7.2 | 0.42 |
| Hypercholesterolemia, % | 57.7 | 54.7 | 57.2 | 0.5 |
| Total cholesterol, mg/dL | 216.2 ± 49.2 | 215.8 ± 37.1 | 214.8 ± 33.8 | 0. 9 |
| HDL cholesterol, mg/dL | 52.8 ± 18.8 | 51 ± 14.5 | 52.4 ± 13.8 | 0.02 |
| Chemistry | ||||
| Phosphorus, mg/dL | 3.47 ± .058 | 3.39 ± 0.44 | 3.39 ± 0.36 | <0.001 |
| Normalized calcium, mmol/L | 1.23 ± 0.06 | 1.24 ± 0.04 | 1.24 ± 0.03 | <0.001 |
| Potassium, mmol/L | 4.05 ± 0.38 | 4.07 ± 0.29 | 4.06 ± 0.25 | 0.24 |
| Creatinine, mg/dL | 0.84 ± 0.3 | 0.86 ± 0.17 | 0.89 ± 0.16 | 0.001 |
| eGFR, mL/min/1.73 m2 | 92.07 ± 25.75 | 89.61 ± 17.08 | 87.16 ± 13.8 | 0.002 |
| C‐reactive protein, mg/dL | 0.43 ± 0.73 | 0.37 ± 0.44 | 0.48 ± 0.61 | 0.02 |
| Homocysteine, µmol/L | 10.75 ± 8.88 | 9.6 ± 3.51 | 10.17 ± 3.3 | 0.007 |
| ECG abnormalities | ||||
| Major ECG abnormalities, % | 13.01 | 11 | 5.86 | 0.01 |
| Minor ECG abnormalities, % | 19.49 | 16.74 | 20.24 | 0.2 |
| LVH, % | 14.89 | 12.85 | 11.63 | 0.3 |
Abbreviations: 25‐OH, 25‐hydroxy; BMI, body mass index; ECG, electrocardiograph; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LVH, left ventricular hypertrophy; MI, myocardial infarction; SD, standard deviation.
Statistical Analysis
NHANES‐III has a complex multistage stratified sample design.23 Designated weighting specified in the NHANES‐III dataset were used to perform statistical analysis to minimize biases. We used the total NHANES‐III pseudostratum as our strata variable, the total NHANES‐III pseudo‐primary sampling units as our survey sampling units, and the total mobile exam center final weight as our sampling unit weight.24
We used the χ2 test for categorical variables and adjusted Wald's test for continuous variables to examine differences in baseline characteristics across 3 groups of 25‐OH vitamin D levels: group 1 (referent), >40 ng/m:, group 2, 20–40 ng/mL; and group 3, ≤20 ng/mL. Stepwise univariate and multivariate logistic regression models were built. 25‐OH vitamin D values (independent variable) were examined as both categorical and continuous values. Model 1 was adjusted for age, race and sex. Model 2 was further adjusted for cigarette smoking, systolic blood pressure, family history of premature myocardial infarction in relatives <50 years old, and ratio of total cholesterol to high‐density lipoprotein cholesterol in addition to covariates in model 1. Model 3 was adjusted for the following covariates, in addition to those mentioned in models 1 and 2: estimated glomerular filtration rate calculated as per the Modification of Diet in renal Disease Formula,25 body mass index, C‐reactive protein, and left ventricular hypertrophy (LVH). We performed a subgroup analysis of individuals without LVH (n = 4253), as 25‐OH vitamin D deficiency has been associated with LVH.10 LVH was defined using the Novacode measured left ventricular mass index of >115 grams/m2 and >130grams/m2 in females and males, respectively.26
We further characterized the utility of major ECG abnormalities in predicting long‐term all‐cause, composite cardiovascular (International Classification of Diseases, 10th Edition [ICD‐10] codes I00‐I99) (any cardiovascular cause), cardiovascular (ICD‐10 codes I20‐I25, I60‐I69, I70) (ischemic heart disease, atherosclerosis, and cerebrovascular causes), and ischemic heart disease mortality (ICD‐10 codes I20‐I25) in 25‐OH vitamin D sufficient and in the pooled insufficient and deficient groups. The end point for our cohort was ascertained using the NHANES‐III linked mortality file using ICD‐10 codes (up to December 31, 2006).27 Kaplan‐Meier survival curves were generated for univariate analysis. Step‐wise univariate and multivariate Cox‐proportional hazards regression models were built to estimate hazards ratio for the aforementioned end points. Models were adjusted for age, sex, race, cigarette smoking, systolic blood pressure, family history of premature myocardial infarction in relatives <50 years old, ratio of total cholesterol to high‐density lipoprotein cholesterol, estimated glomerular filtration rate calculated as per the Modification of Diet in renal Disease Formula,25 body mass index, C‐reactive protein, and serum phosphorus.
All covariates and 25‐OH vitamin D were visually and statistically examined for their distribution around the mean and were appropriately log‐transformed to minimize the influence of outlier values and maintain normal distribution. Covariates age, systolic blood pressure, ratio of total cholesterol to high‐density lipoprotein cholesterol, and body mass index were log‐transformed to achieve normal distribution. A P value of <0.05 was considered significant. Stata SE 11.1 (StataCorp LP, College Station, TX) was used for performing the statistical analysis.
Results
The average age of our study cohort was 58.4 years, 54.01% were females, and 88.97% belonged to Caucasian ethnicity. The prevalence of 25‐OH vitamin D insufficiency and deficiency in our study population was 53.8% and 4.5%, respectively. In the 25‐OH vitamin D deficient group, there were a significantly (P < 0.05) higher proportion of individuals of African American ethnicity, female sex, higher body mass index, and hypertension (Table 1).
The prevalence of major ECG abnormalities across 25‐OH vitamin D sufficiency, insufficiency, and deficiency was 5.86%, 11%, and 13.01%, respectively (P = 0.01). Serum 25‐OH vitamin D levels were a significant predictor of major ECG abnormalities after adjusting for possible confounders in the entire study data (continuous variable odds ratio [OR]: 0.98, 95% confidence interval [CI]: 0.97‐0.99, P = 0.007; categorical variable OR: 2.36, 95% CI: 1.1‐5.12, P = 0.03) and in individuals without LVH (continuous variable OR: 0.98, 95% CI: 0.96‐0.99, P = 0.004; categorical variable OR: 2.97, 95% CI: 1.1‐8.08, P = 0.03) (Table 2 and Table 3).
Table 2.
Univariate and Multivariate Odds Ratios for Major Electrogram Abnormalities as Predicted by Serum 25‐OH Vitamin D Levels in the Entire Cohort
| Odds Ratio | 95% Confidence Interval | P Value | ||
|---|---|---|---|---|
| Continuous variable | ||||
| Model 1 | 0.98 | 0.97‐0.99 | 0.007 | |
| Model 2 | 0.98 | 0.97‐0.99 | 0.01 | |
| Model 3 | 0.98 | 0.97‐0.99 | 0.007 | |
| Categorical variable | ||||
| Model 1 | Group 1 | Referent | ||
| Group 2 vs group 1 | 1.97 | 1.09‐3.55 | 0.03 | |
| Group 3 vs group 1 | 2.63 | 1.41‐4.92 | 0.003 | |
| Model 2 | Group 1 | Referent | ||
| Group 2 vs group 1 | 1.94 | 1.06‐3.56 | 0.03 | |
| Group 3 vs group 1 | 2.29 | 1.2‐4.36 | 0.01 | |
| Model 3 | Group 1 | Referent | ||
| Group 2 vs group 1 | 2.07 | 0.98‐4.37 | 0.06 | |
| Group 3 vs group 1 | 2.36 | 1.1‐5.12 | 0.03 | |
Abbreviations: 25‐OH, 25‐hydroxy.
Model 1 was adjusted for age, sex, race/ethnicity. Model 2 was adjusted for traditional cardiovascular risk factors cigarette smoking, systolic blood pressure, family history of premature myocardial infarction <50 years of age, and ratio of total cholesterol to high‐density lipoprotein cholesterol in addition to Model 1. Model 3 was adjusted for estimated glomerular filtration rate, body mass index, serum phosphorus, C‐reactive protein, and left ventricular hypertrophy in addition to Model 2. Age, systolic blood pressure, ratio of total cholesterol to high‐density lipoprotein cholesterol, and body mass index was log‐transformed to achieve normal distribution. Group 1: Referent, vitamin D >40 ng/mL. Group 2: vitamin D 20.01–40 ng/mL. Group 3: vitamin D ≤20 ng/mL.
Table 3.
Univariate and Multivariate Odds Ratios For Major Electrocardiogram Abnormalities as Predicted by Serum 25‐OH Vitamin D Levels in Subjects Without Left Ventricular Hypertrophy
| Odds Ratio | 95% Confidence Interval | P Value | ||
|---|---|---|---|---|
| Continuous variable | ||||
| Model 1 | 0.98 | 0.96‐0.99 | 0.005 | |
| Model 2 | 0.98 | 0.96‐0.99 | 0.007 | |
| Model 3 | 0.98 | 0.96‐0.99 | 0.004 | |
| Categorical variable | ||||
| Model 1 | Group 1 | Referent | ||
| Group 2 vs group 1 | 2.25 | 0.88‐5.74 | 0.09 | |
| Group 3 vs group 1 | 2.91 | 1.05‐8.06 | 0.04 | |
| Model 2 | Group 1 | Referent | ||
| Group 2 vs group 1 | 2.29 | 0.9‐5.79 | 0.08 | |
| Group 3 vs group 1 | 2.84 | 1.02‐7.87 | 0.04 | |
| Model 3 | Group 1 | Referent | ||
| Group 2 vs group 1 | 2.36 | 0.94‐5.9 | 0.07 | |
| Group 3 vs group 1 | 2.97 | 1.1‐8.08 | 0.03 | |
Abbreviations: 25‐OH, 25‐hydroxy.
Model 1 was adjusted for age, sex, race/ethnicity. Model 2 was adjusted for traditional cardiovascular risk factors cigarette smoking, systolic blood pressure, family history of premature myocardial infarction <50 years of age, and ratio of total cholesterol to high‐density lipoprotein cholesterol in addition to Model 1. Model 3 was adjusted for estimated glomerular filtration rate, body mass index, serum phosphorus, C‐reactive protein, and left ventricular hypertrophy in addition to Model 2. Age, systolic blood pressure, ratio of total cholesterol to high‐density lipoprotein cholesterol, and body mass index were log‐transformed to achieve normal distribution. Group 1: Referent, vitamin D >40 ng/mL. Group 2: vitamin D 20.01–40 ng/mL. Group 3: vitamin D ≤20 ng/mL. This analysis was conducted on individuals without left ventricular hypertrophy (n = 4253).
Our cohort had a mean follow‐up period of 13.2 years, and there was a total of 1410 (28.9%) deaths in individuals with 25‐OH vitamin D levels ≤40 ng/mL, of which 569 (11.7%) were due to composite cardiovascular mortality, 403 (8.3%) were due to cardiovascular mortality, and 301 (6.2%) were due to ischemic heart disease mortality. All‐cause mortality resulted in 329 (47%) deaths in individuals with major ECG abnormalities vs 1081 (25.9%) in those without major ECG abnormalities. Similarly, 148 (21.1%) vs 421 (10.1%), 103 (14.7%) vs 300 (7.2%), and 82 (11.7%) vs 219 (5.2%) deaths occurred in individuals with and without major ECG abnormalities secondary to composite cardiovascular, cardiovascular, and ischemic heart disease causes, respectively. In individuals with 25‐OH vitamin D levels ≤ 40 ng/mL, major ECG abnormalities were a significant predictor of long‐term all‐cause (hazard ratio [HR]: 1.52, 95% CI: 1.23‐1.89), composite cardiovascular (HR: 1.7, 95% CI: 1.34‐2.15), cardiovascular (HR: 1.64, 95% CI: 1.27‐2.12), and ischemic heart disease mortality (HR: 1.98, 95% CI: 1.46‐2.69) (Table 4) (Figure 1). Major ECG abnormalities did not predict long‐term all‐cause, cardiovascular, composite cardiovascular, and ischemic heart disease mortality in individuals with 25‐OH vitamin D levels >40 ng/mL.
Table 4.
Major Electrocardiogram Abnormalities and Mortality Across 25‐OH Vitamin D Groups
| Vitamin D Insufficiency and Deficiency | Vitamin D Sufficiency | |||
|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | Hazard Ratio | 95% Confidence Interval | |
| All‐cause mortality | ||||
| Model A | 2.57a | 2.01‐3.29 | 2.85a | 1.19‐6.56 |
| Model B | 1.53a | 1.24‐1.89 | 1.16 | 0.56‐2.42 |
| Model C | 1.52a | 1.23‐1.89 | 0.97 | 0.39‐2.43 |
| Composite cardiovascular mortality (ICD‐10 codes I00‐I99) | ||||
| Model A | 3.18a | 2.4‐4.21 | 4.89a | 1.61‐14.89 |
| Model B | 1.73a | 1.37‐2.19 | 1.95 | 0.72‐5.29 |
| Model C | 1.7a | 1.34‐2.15 | 1.51 | 0.36‐6.29 |
| Cardiovascular mortality (ICD‐10 codes I20‐I25, I60‐I69, I70) | ||||
| Model A | 3.11a | 2.31‐4.19 | 4.09 | 0.97‐17.17 |
| Model B | 1.67a | 1.3‐2.16 | 1.7 | 0.3‐9.67 |
| Model C | 1.64a | 1.27‐2.12 | 1.74 | 0.2‐15.06 |
| Ischemic heart disease mortality (ICD‐10 codes I20‐I25) | ||||
| Model A | 3.56a | 2.53‐5.01 | 5.95a | 1.26‐28.22 |
| Model B | 2.01a | 1.5‐2.7 | 2.59 | 0.49‐13.87 |
| Model C | 1.98a | 1.46‐2.69 | 2.9 | 0.42‐20.03 |
Abbreviations: 25‐OH, 25‐hydroxy; ICD‐10, International Classification of Diseases, 10th Edition.
Model A was unadjusted. Model B was adjusted for age, race, sex, cigarette smoking, systolic blood pressure, family history of premature myocardial infarction <50 years of age, and ratio of total cholesterol to high‐density lipoprotein cholesterol.
Model C was adjusted for estimated glomerular filtration rate, body mass index, serum phosphorus, and C‐reactive protein in addition to Model B. Age, systolic blood pressure, ratio of total cholesterol to high‐density lipoprotein cholesterol, and body mass index was log‐transformed to achieve normal distribution.
p = value <0.05.
Figure 1.
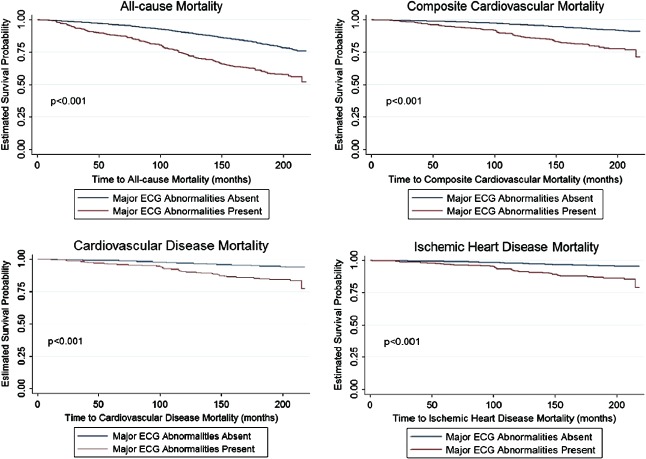
Kaplan‐Meier curves comparing survival in individuals with and without electrocardiogram (ECG) abnormalities (25‐hydroxy vitamin D levels ≤40 ng/mL).
Discussion
Our data suggest there is an independent association between low serum 25‐OH vitamin D levels and major ECG abnormalities in a nationally representative healthy cohort. The presence of major baseline ECG abnormalities in individuals with 25‐OH vitamin D levels ≤40 ng/mL were independent and statistically significant predictors of long‐term, all‐cause composite cardiovascular, cardiovascular, and ischemic heart disease mortality.
To the best of our knowledge, there has not been a prior study linking low 25‐OH vitamin D levels to an increased prevalence of major ECG abnormalities in a relatively healthy cohort. A study by Zhang et al examined the relationship between 25‐OH vitamin D and corrected QT interval and found no association.17 Vitamin D deficiency has been associated with LVH.10
The exact mechanisms by which 25‐OH vitamin D deficiency would result in an increased prevalence of major ECG abnormalities remains unknown. The major ECG abnormalities analyzed in our study were: Q‐QS abnormalities, ST‐depression/elevation, negative T waves, Wolff‐Parkinson‐White syndrome and ventricular conduction delay. The presence of Q waves on resting baseline ECG testing is suggestive of “silent” myocardial infarction in addition to myocarditis, amyloidosis, cardiomyopathy, pre‐excitation syndrome, and LVH.28 The presence of ST depression is suggestive of acute events like coronary ischemia, non–ST‐segment elevation myocardial infarction, posterior wall myocardial infarction, and reciprocal changes or could be indicative of left ventricular hypertrophy and digoxin intake.29 T‐wave abnormalities are a result of acute events like myocardial ischemia/infarction, pulmonary embolism, and cerebrovascular injury or assorted conditions such as ventricular overload patterns, digitalis effect, bundle branch block, and LVH.30 Vitamin D deficiency has been associated with incident cardiovascular disease in the Framingham cohort31 and with LVH.10 The association between vitamin D deficiency and incident cardiovascular disease was found at a level of <15 ng/mL.31 Our study findings demonstrate an association with increased prevalence of major ECG abnormalities on resting baseline ECG at a level of <40 ng/mL. The exact mechanisms linking ECG abnormalities with 25‐OH vitamin D deficiency remain unclear and should be an area for future research.
Vitamin D is being increasingly recognized as an indicator of poor health.32 In this context, our findings assume particular relevance as they were derived from a relatively healthy disease‐free cohort after rigorous exclusion of individuals with confounding comorbidities.
Limitations
Despite the benefit of a large, nationally representative, healthy dataset, and strict definitions for covariates and inclusion criteria, our study has some drawbacks. Technical issues related to ECG coding did not permit exclusion of the Wolf‐Parkinson‐White pattern from other major ECG abnormalities. No adjustments were made for medication use and vitamin supplementations, which could modify the disease process being studied or account for incident cardiovascular events and the interventions performed (medication regime intensification, lifestyle modification) during the follow‐up period. Our outcome measures were dependent on diagnoses mentioned on the death certificate. Although the accuracy of the cause of death could be a concern, vital status and date of death were accurately documented and have been used in other epidemiologic studies.33
Conclusion
Our study suggests an independent, statistically significant association between 25‐OH vitamin D levels and major ECG abnormalities. We demonstrated that presence of baseline ECG abnormalities in healthy individuals with 25‐OH vitamin D levels ≤40 ng/mL is associated with an increased risk of long‐term cardiovascular mortality.
Supporting information
TableS1: Minnesota Coding of Major ECG Abnormalities
Tushar A. Tuliani, MD and Maithili Shenoy, MD, contributed equally to this work. All authors have contributed to the analysis design and oversight, manuscript conception and drafting, statistical analysis, and/or editorial review of the manuscript, and they have all read and approved the final manuscript.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48–54. [DOI] [PubMed] [Google Scholar]
- 2. Gorham ED, Garland CF, Burgi AA, et al. Lower prediagnostic serum 25‐hydroxyvitamin D concentration is associated with higher risk of insulin‐requiring diabetes: a nested case‐control study. Diabetologia. 2012;55:3224–3227. [DOI] [PubMed] [Google Scholar]
- 3. Baker JF, Mehta NN, Baker DG, et al. Vitamin D, metabolic dyslipidemia, and metabolic syndrome in rheumatoid arthritis. Am J Med. 2012;125:1036.e1039‐1036 e1015. [DOI] [PubMed] [Google Scholar]
- 4. Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25‐hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. [DOI] [PubMed] [Google Scholar]
- 5. Carrelli AL, Walker MD, Lowe H, et al. Vitamin D deficiency is associated with subclinical carotid atherosclerosis: the Northern Manhattan study. Stroke. 2011;42:2240–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Melamed ML, Muntner P, Michos ED, et al. Serum 25‐hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008;28:1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zittermann A, Schleithoff SS, Tenderich G, et al. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105–112. [DOI] [PubMed] [Google Scholar]
- 8. Giovannucci E, Liu Y, Hollis BW, et al. 25‐hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poole KE, Loveridge N, Barker PJ, et al. Reduced vitamin D in acute stroke. Stroke. 2006;37:243–245. [DOI] [PubMed] [Google Scholar]
- 10. Achinger SG, Ayus JC. The role of vitamin D in left ventricular hypertrophy and cardiac function. Kidney Int Suppl. 2005:S37–S42. [DOI] [PubMed] [Google Scholar]
- 11. Lee HM, Liu M, Lee K, et al. Does low vitamin D amplify the association of COPD with total and cardiovascular disease mortality [published online ahead of print April 9, 2014]? Clin Cardiol. doi: 10.1002/clc.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cigolini M, Iagulli MP, Miconi V, et al. Serum 25‐hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2006;29:722–724. [DOI] [PubMed] [Google Scholar]
- 13. Desai CK, Huang J, Lokhandwala A, et al. The Role of vitamin supplementation in the prevention of cardiovascular disease events [published online ahead of print May 23, 2014]. Clin Cardiol. doi: 10.1002/clc.22299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Auer R, Bauer DC, Marques‐Vidal P, et al. Association of major and minor ECG abnormalities with coronary heart disease events. JAMA. 2012;307:1497–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Denes P, Larson JC, Lloyd‐Jones DM, et al. Major and minor ECG abnormalities in asymptomatic women and risk of cardiovascular events and mortality. JAMA. 2007;297:978–985. [DOI] [PubMed] [Google Scholar]
- 16. Badheka AO, Patel N, Tuliani TA, et al. Electrocardiographic Abnormalities and Reclassification of Cardiovascular Risk: Insights from NHANES‐III. Am J Med. 2013;126:319–326.e312. [DOI] [PubMed] [Google Scholar]
- 17. Zhang Y, Post WS, Dalal D, et al. Serum 25‐hydroxyvitamin D, calcium, phosphorus, and electrocardiographic QT interval duration: findings from NHANES III and ARIC. J Clin Endocrinol Metab. 2011;96:1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol. 1992;45:1101–1109. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). National Health and Nutrition Examination Survey. NHANES III. http://www.cdc.gov/nchs/nhanes/nh3data.htm. Accessed August 5, 2012.
- 20. Thomas MK, Lloyd‐Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–783. [DOI] [PubMed] [Google Scholar]
- 21. Lavie CJ, Lee JH, Milani RV. Vitamin D and cardiovascular disease will it live up to its hype? J Am Coll Cardiol. 2011;58:1547–1556. [DOI] [PubMed] [Google Scholar]
- 22. Wu CC, Yeh WT, Crow RS, et al. Comparison of electrocardiographic findings and associated risk factors between Taiwan Chinese and US White adults. Int J Cardiol. 2008;128:224–231. [DOI] [PubMed] [Google Scholar]
- 23. National Center for Health Statistics . Plan and operation of the third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat. 1994;1:1–416. [PubMed] [Google Scholar]
- 24. Mohadjer L, Montaquila JM, Waksberg J, et al. National Health and Nutrition Examination Survey III: Weighting and Estimation Methodology. Hyattsville, MD: Westat Inc. for National Center for Health Statistics; 1996. [Google Scholar]
- 25. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 26. Havranek EP, Emsermann CD, Froshaug DN, et al. Thresholds in the relationship between mortality and left ventricular hypertrophy defined by electrocardiography. J Electrocardiol. 2008;41:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Center for Health Statistics, Centers for Disease Control and Prevention . Analytic and Reporting Guidelines: The Third National Health and Nutrition Examination Survey, NHANES III (1988‐1994): Linked Mortality File Public‐use. 1996. http://www.cdc.gov/nchs/data/datalinkage/nh3_file_layout_public_2010.pdf. Accessed October 4, 2012.
- 28. Bonow R. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, PA: Saunders; 2011:157. [Google Scholar]
- 29. Pollehn T, Brady WJ, Perron AD, et al. The electrocardiographic differential diagnosis of ST segment depression. Emerg Med J. 2002;19:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hayden GE, Brady WJ, Perron AD, et al. Electrocardiographic T‐wave inversion: differential diagnosis in the chest pain patient. Am J Emerg Med. 2002;20:252–262. [DOI] [PubMed] [Google Scholar]
- 31. Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McGreevy C, Williams D. New insights about vitamin D and cardiovascular disease: a narrative review. Ann Intern Med. 2011;155:820–826. [DOI] [PubMed] [Google Scholar]
- 33. Calle EE, Terrell DD. Utility of the National Death Index for ascertainment of mortality among cancer prevention study II participants. Am J Epidemiol. 1993;137:235–241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TableS1: Minnesota Coding of Major ECG Abnormalities


