Abstract
Background
Atrial fibrillation (AF) is associated with inflammation. Increased serum C‐reactive protein (CRP) levels are important representatives of an inflammatory state of AF. A variety of studies have evaluated whether increased CRP levels have an association with AF recurrence after catheter ablation. However, the results remain inconsistent, therefore, this meta‐analysis was conducted to offer suggestions.
Hypothesis
Increased baseline CRP have an association with AF recurrence after catheter ablation.
Methods
Electronic databases including PubMed, Embase, Medline, ISI Web of Knowledge, and ScienceDirect were searched until December 31, 2012 for any CRP‐associated studies. Overall and subgroup analyses were performed. Standardized mean difference (SMD) and 95% confidence interval (CI) were used to evaluate the associations between CRP levels and postablation AF recurrence. Statistical analysis was performed with Review Manager 5.2 and Stata 11.0.
Results
Seven available studies were identified, which included 526 patients (179 recurrence vs 347 no recurrence). Overall, increased baseline CRP levels had significant positive association with postablation AF recurrence. The SMD in the CRP levels was 0.65 units (95% CI: 0.30‐0.99), and the z‐score for overall effect was 3.70 (P = 0.0002). The heterogeneity test showed that there were moderate differences between individual studies (P = 0.006, I 2 = 67%). Metaregression revealed that different sample sizes of studies possibly accounted for the heterogeneity. Positive associations were also found in subgroup analyses based on sample size. When stratifying for ethnicity, similarly significant associations were found in both European (Caucasian) and Asian populations.
Conclusions
Investigations demonstrate that baseline CRP levels are greater in patients with postablation AF recurrence. Further studies with larger sample size and delicate design for CRP should be conducted.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia encountered in clinical practice, and approximately one‐third of all cardiac arrhythmia‐related hospitalizations are due to AF. The prevalence of AF is approximately 1% in the general population, and as high as 9% in individuals by the age of 80 years.1 In light of the ageing population, the prevalence of AF is expected to double within the next 50 years.2 Importantly, AF could lead to increased rates of death, stroke, and other thromboembolic events; heart failure and hospitalizations; degraded quality of life; reduced exercise capacity; and left ventricular dysfunction.3
Current treatments of AF patients mainly include pharmacotherapy, catheter ablation and surgical ablation. Owing to increased adverse drug reactions and high AF recurrence rate, as well as surgical ablation being complex, and with the risk of mortality and significant complications, Pharmacotherapy and surgical ablation consequently have been sparsely adopted.3 Catheter ablation has been shown to be more efficacious in restoring sinus rhythm in patients with AF4, 5 and has been recommended as a class I recommendation supported with level A evidence in American College of Cardiology Foundation/American Heart Association/Heart Rhythm Society practice guidelines in 2011.6 Although catheter ablation has emerged as a curative therapy for patients with paroxysmal or persistent AF after failure (or intolerance) of one antiarrhythmic drug, it is still associated with a substantial AF recurrence rate.7 Rates of success for maintaining sinus rhythm varied widely, ranging from l<30% to 85%.8, 9 Determinants of catheter ablation success or freedom from arrhythmia depend on diverse clinical factors such as enlarged left atrial diameter (LAD), AF history, age, and cardiac function.5, 10 In addition, increasing evidence has demonstrated that inflammation plays an important role in the genesis and perpetuation of AF.11, 12, 13, 14 Serum C‐reactive protein (CRP), as an important representative of inflammatory state, is related to left atrial size and dysfunction.15 Thus, we can assume that increased CRP levels may also increase the risk of AF recurrence after catheter ablation.
To date, a variety of observational studies have focused on the associations between baseline CRP and postablation AF recurrence. However, the results of different studies were inconsistent. To better clarify the associations of CRP with postablation AF recurrence, we conducted a comprehensive meta‐analysis by collecting and analyzing previously published studies.
Methods
Because of inherent biases and differences in observational study designs, our meta‐analysis presents particular challenges. Consequently, the study strictly complies with the guidelines of the Meta‐analysis of Observational Studies in Epidemiology Group (MOOSE).16
Search Strategy
We carefully searched PubMed, Embase, Medline, ISI Web of Knowledge, and ScienceDirect databases for original articles published before December 31, 2012 to evaluate the associations between CRP and AF recurrence after catheter ablation. The medical subject headings and key words used for search were “C‐reactive protein'' and ''atrial fibrillation.” The references of all identified publications were hand searched for additional studies. Abstracts, unpublished reports, and non‐English language articles were not included.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) the study was a prospective observational study design, (2) the study evaluated the potential association between CRP levels before catheter ablation and postablation AF recurrence, 3) the study used AF recurrence rates as an outcome, and (4) the period of follow‐up was ≥3 months. The exclusion criteria were: (1) patients undergoing electrical shock or cardioversion, surgical operation or without any treatment (including catheter ablation); and (2) baseline CRP is not available in the sufficient follow‐up time period.
To minimize the bias and improve the reliability, two researchers (Z.J. and L.D.) extracted data with the inclusion and exclusion criteria independently and reached a consensus.
Quality Assessment and Data Extraction
Quality scoring is usually controversial, therefore we assessed it earnestly. Two reviewers (Z.J. and L.D.) independently evaluated each article included in our analysis according to a critical review checklist of the Dutch Cochrane Centre which was proposed by MOOSE16 (the key points of quality assessment are listed online in Supporting Table 1). If a study could not manage any of that, we regarded it as not having been performed, and consequently, there was a possibility for underestimation of the reported characteristics.
Data were extracted independently from each study based on the inclusion criteria. Agreements were reached after discussion for conflicting data. The following data were extracted from each eligible study: (1) publication details: first author's last name, publication year, and origin of the studied population; (2) characteristics of the studied population: sample size, age, gender, diagnoses, and methods of CRP measurement; (3) ablation method; (4) the rate of AF recurrence, methods of AF detection, and mean follow‐up time; and (5) mean and standard deviatioin (SD) of CRP in each group. If one study involved both early recurrence and late recurrence, data of the late recurrence were adopted.
In studies reporting the median and quartiles, the median was assumed to most accurately represent the central tendency and was treated as the mean. The distribution was assumed to be normal, with a z‐value of ±0.68 corresponding to the reported 25th and 75th percentiles. In this manner, the standard deviation was calculated.17
Statistical Analysis
Meta‐analyses
To accommodate differences in the way in which CRP is measured and reported across various laboratories, the association strength between CRP and postablation AF recurrence was measured by standardized mean difference [SMD] and 95% confidence interval [CI]. The significance of pooled SMD was tested by z‐test (P < 0.05 was considered significant). In addition to overall comparison, we performed subgroups analyses based on sample size and ethnicity of study population. To further appraise the impact of potential baseline confounders on the reported relative risk estimates within the included studies, we performed a metaregression analysis using inverse variance weighting and tested the following variables: ethnicity, measurement of CRP, the ablation method, publication year, study population, mean age, gender, and arrhythmia duration.
Exploring Heterogeneity
For each meta‐analysis, the Cochrane Q statistic and the I 2 value were used to check the statistical heterogeneity between studies, and the heterogeneity was considered significant when P < 0.05. The fixed‐effects model and random‐effects model (based on the Inverse Variance method) were used to pool the data from different studies. The random‐effects model was used when there was significant heterogeneity, which was used to take into account within‐study and between‐study variance; otherwise, the fixed‐effects model was used.18 I 2 was a value that could describe the percentage of variation across studies, where 0% to 25% indicated no observed heterogeneity, and larger values showed increasing heterogeneity, with 25% to 50% regarded as low, 50% to 75% as moderate, and 75% to 100% as high. To explore sources of heterogeneity, we performed metaregression and subgroup analyses.
Publication Bias
Any potential publication bias was evaluated by inspecting funnel plots and the Egger's test,19 and all statistical analyses were conducted using Review Manager 5.2 (The Cochrane Collaboration, Copenhagen, Denmark) and Stata 11.0 software (StataCorp, College Station, TX).
To ensure the credibility and accuracy of the results, 2 researchers (Z.J. and L.D.) entered the data into the software program independently and reached a consensus.
Results
Study Characteristics
The search strategy yielded 401 publications, of which 340 were not eligible for inclusion based on title and abstract review because they were either laboratory studies, review articles, or irrelevant to the current analysis. The remaining 61 studies were further evaluated. After carefully evaluating the quality of the 61 remaining articles, we excluded 50 studies, of which 39 included electrical cardioversion (21 studies excluded), surgical operation (14 studies removed), or nothing (4 studies excluded). Of the 11 remaining studies that evaluated drug therapy. After detailed review, 4 studies without grouping according to clinical outcome (AF recurrence and no AF recurrence) or data of baseline CRP available with enough follow‐up time were excluded. Finally, 7 relevant studies20, 21, 22, 23, 24, 25, 26 addressed the association between CRP and postablation AF recurrence (Figure 1). Eventually, there were 526 patients selected for the meta‐analysis: 179 in the AF‐recurrence group, and 347 in the no AF‐recurrence group. The follow‐up period varied between 3 months and 14 months. The quality assessment of the 7 studies is presented online in Supporting Table 1, whereas the characteristics of each study are depicted online in Supporting Table 2.
Figure 1.
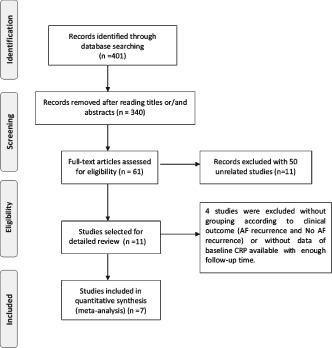
Flow diagram of study identification.
Quantitative Data Synthesis
Four studies 21, 22, 23, 25 showed that greater baseline CRP was related to AF recurrence than those without, whereas CRP levels did not show a significant difference between the 2 groups in 3 other studies.20, 24, 26 Overall, there was significant positive association between CRP concentration and AF recurrence. The SMD in the CRP levels between the patients with and those without AF recurrence was 0.65 units (95% CI: 0.30‐0.99), and the z‐score for overall effect was 3.70 (P = 0.0002). Forest plots on the basis of the meta‐analysis are shown in Figure 2.
Figure 2.
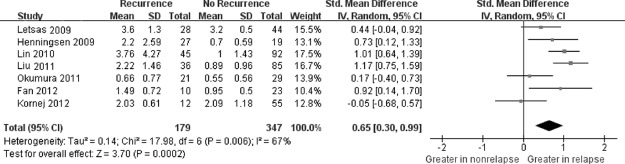
Forest plot of atrial fibrillation recurrence risk associated with increased baseline C‐reactive protein levels. A random‐effects model and Inverse Variance(IV) method were used. The squares and horizontal line represent the study‐specific standardized mean difference (SMD) and 95% confidence interval (CI). The diamond represents the pooled results of SMD and 95% CI. Abbreviations: SD, standard deviation.
Heterogeneity Analysis
The heterogeneity test showed that there were moderate differences between individual studies (P = 0.006; I2 = 67%). We subsequently performed sensitivity analyses and metaregression to find the origin of this heterogeneity. However, the change of heterogeneity did not make sense after excluding any article. Interestingly, after divided the sample size into 2 groups (n < 100 vs n > 100). The outcome of the metaregression indicated that sample size contributed to the heterogeneity (P = 0.025). Then, we underwent subgroup analyses according to sample size, which indicated that CRP concentration was greater in patients with AF recurrence in both groups and no heterogeneity existed. We could see in the first group (n < 100) that the SMD was 0.40 units (95% CI: 0.14‐0.66), whereas in the second group (n > 100) it was 1.08 units (95% CI: 0.80‐1.36). Therefore, the larger sample size showed the more obvious trend (Figure 3). Moreover, the ethnicity of the study population (P = 0.212), the measurement of CRP (high‐sensitive vs standard) (P = 0.256), the ablation method (P = 0.160), publication year (P = 0.827), study population (P = 0.546), mean age (P = 0.076), gender (P = 0.309), and arrhythmia duration (P = 0.993) were not statistically associated with heterogeneity and had almost no effect on our results.
Figure 3.
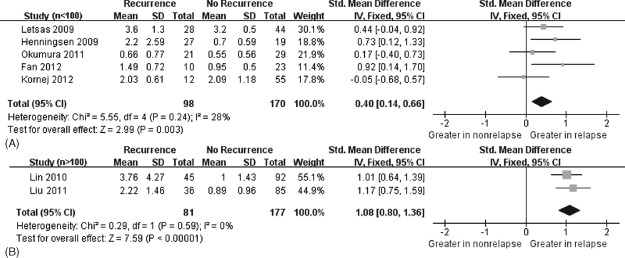
Forest plot of atrial fibrillation recurrence risk associated with increased baseline C‐reactive protein levels under the sample size (n < 100 vs n > 100). Fixed‐effects model and IV method were used. (A) The results by sample size n < 100. (B) The results by sample size n > 100. The squares and horizontal line represent the study‐specific standardized mean difference (SMD) and 95% confidence interval (CI). The diamond represents the pooled results of SMD and 95% CI.
On the subgroup analyses by ethnicity of population, greater CRP concentration also had association with postablation AF recurrence in Europeans (Caucasians),20, 21, 26 which covered 185 patients, and Asians,22, 23, 24, 25 which covered 341 patients (Figure 4). Consequently, this conclusion can be applied to Europeans (Caucasians) and Asians. Further studies of Blacks should be conducted.
Figure 4.
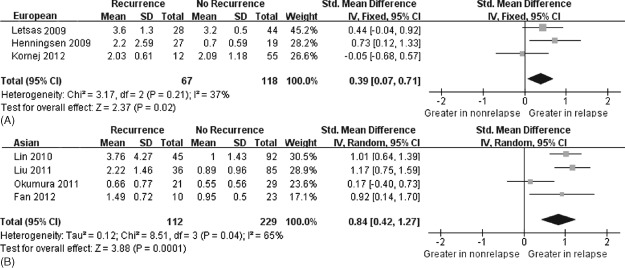
Forest plot of atrial fibrillation recurrence risk associated with increased baseline C‐reactive protein levels by ethnicity (European vs Asian). (A) Fixed‐effects model and IV method used. The result by European ethnicity. (B) Random‐effects model and IV method were used. The result by Asian ethnicity. The squares and horizontal line represent the study‐specific standardized mean difference (SMD) and 95% confidence interval (CI). The diamond represents the pooled results of SMD and 95% CI.
Publication Bias Analysis
Publication bias was assessed by Begg's funnel plot and Egger's test. The shape of the funnel plot appeared to be approximately symmetrical (recurrence vs no recurrence), and Egger's test did not show any evidence of publication bias (t = −1.43, P = 0.212 for recurrence vs no recurrence). Begg's funnel plot and Egger's test confirmed the absence of publication bias (Figure 5).
Figure 5.
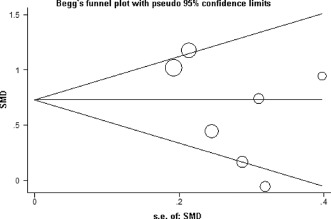
Funnel plot for elevated C‐reactive protein levels and risk of postablation atrial fibrillation recurrence. Funnel plot of all 7 eligible studies and Egger's test (P = 0.212). Abbreviations: s.e., standard error; SMD, standardized mean difference.
Discussion
The pathogenesis of AF is not exactly clear but is believed to be multifactorial. It involves triggers that initiate the arrhythmia, as well as the susceptible tissue and electrophysiological substrate that perpetuates it. The maintenance of AF is facilitated by the presence or development of abnormal tissue substrate that permits multiple re‐entrant wavelets of excitation to propagate within the atrial myocardium. AF tends to become more permanent with time, and it becomes more difficult to restore sinus rhythm when AF is present for a long time. Electrical remodeling, which develops within hours of the onset of AF, entails electrophysiological alterations including shortening of the atrial effective refractory period and action potential duration. Structural remodeling is a slower process involving myocyte degeneration, myocardial fibrosis, left atrial enlargement, and results in heterogeneity of conduction. These changes create a susceptible substrate that has been implicated in the perpetuation of AF.27
Inflammation of AF is involved in electrophysiological and structural atrial remodeling and facilitates the disease development and perpetuation.27 Atrial specimens obtained by endomyocardial biopsy showed clusters of lymphomononuclear cells compatible with a diagnosis of myocarditis in 66% of patients with lone AF.28 Activated T lymphocytes were found in left atrial endocardium, supporting the role of local inflammation as a potential trigger of AF.29 CRP, an acute‐phase protein produced in the liver,27 is a sensitive biomarker of systemic inflammation in several cardiovascular diseases.30 Elevated CRP levels are associated with increased risk of cardiovascular events.31, 32 In the study from Narducci et al,33 atrial tissue CRP was significantly more frequent in patients with paroxysmal AF than persistent AF, and there was a trend for the association between atrial tissue CRP and late recurrence of AF. The higher proportion of atrial CRP‐positive specimens in the paroxysmal AF group than in the persistent AF group may be an initial feature of myocardial damage via activation of the complement system, opsonization, chemotaxis, and activation of inflammatory cells by this acute phase protein. Furthermore, that a rise in CRP was related to left atrial size and dysfunction offered support of association between inflammation and structural remodeling.15
Many studies also focused the association between CRP and postablation AF recurrence based on the issue that the inflammatory state may influence the prognosis of catheter ablation.
In one study, for every 1 mg/dL higher, CRP level was associated with a 7‐fold increased risk of recurrent AF and a 12‐fold of permanent AF compared with controls.34 There were 4 studies included in our meta‐analysis emphasizing that increased baseline CRP had an association with AF recurrence. In the study from Lin et al,23 the cutoff of CRP value for total patients as an independent predictor was 2.92 mg/L, and another study from Liu et al22 stressed the best cutoff value was 1.41 mg/L for total patients, 1.08 mg/L for paroxysmal AF, and 1.89 mg/L for persistent AF. Available cutoff values were not obtained in the remaining 2 articles. The CRP value, as an independent predictor for AF recurrence, was higher in persistent AF. However, the exact cutoff value of CRP was inconsistent. Further analysis should be conducted.
There were some differences in the mechanism of early AF recurrence (ERAF) and late AF recurrence (LRAF). ERAF following ablation has been reported to be related to an acute inflammatory process. A longer procedural time of aggressive radiofrequency ablation could lead to massive tissue damage and profound inflammatory processes.30 Lellouche et al reported that a majority of patients (91%) with ERAF following AF ablation will suffer LRAF,35 and the reason may be that ERAF affects the remodeling of atrial anatomy after catheter ablation. In some studies,30, 36 patients with LRAF who previously suffered ERAF had an increased LAD at the time of their repeat ablation procedure. It can be presumed that ERAF may interrupt the process of postablation remodeling and result in incomplete remodeling of the atrial anatomy, which can increase the risk of LRAF.36
Catheter ablation has evolved into an effective and safe therapeutic method. A wide range of approaches has been published targeting varied putative arrhythmia mechanisms. Pulmonary vein isolation is the cornerstone of catheter ablation for patients with paroxysmal atrial fibrillation.37 The most widely used and assessed technique is that of point‐by‐point irrigated radiofrequency ablation. Three‐dimensional mapping is routinely used to accurately delineate anatomy with or without the use of an imported computed tomography scan. More recently, in an attempt to simplify the procedure, various 1‐shot technologies for circumferential isolation of the pulmonary veins have been developed.38, 39 Irrespective of technology used, the end point for antral pulmonary vein ablation is demonstration of complete conduction block into and out of the pulmonary vein.40 Because of this, we classify the ablation methods into 3 groups: pulmonary vein isolation, circumferential pulmonary vein isolation (CPVI), and CPVI plus other (Supporting Table 2). In addition, as already mentioned, metaregression according to ablation method indicated there was no statistical heterogeneity.
Catheter ablation is compelling. However, the success of ablation is often offset by the recurrence of AF.41 No meta‐analysis explored the association between the baseline CRP concentration and postablation AF recurrence, therefore, this meta‐analysis was conducted rigorously. In our meta‐analysis of 7 prospective observational studies, there was significant positive association between baseline CRP concentration and AF recurrence, and the result can be generalized to Europeans (Caucasians) and Asians.
In our analysis, the minimum duration follow‐up time is just 3 months.25 This time interval is sufficient and critical, because ERAF was defined as an AF episode during the first week after the ablation,42 and LRAF was defined as any AF episode between 3 and 6 months after the ablation (thus including a 3‐month “blanking period”).26 The follow‐up time from Fan et al was 3 months, and removal of this study25 from the meta‐analysis did not have a significant impact on the results (SMD: 0.61 units, 95% CI: 0.23‐1.00).
Heterogeneity is an important problem when interpreting the results of a meta‐analysis, and moderate heterogeneity was found in the present study. We subsequently performed sensitivity analyses. The change of heterogeneity did not make sense after excluding any article. Metaregression was then conducted, and the results indicated that the sample size was the major source of the heterogeneity. The subgroup analyses (n < 100 vs n > 100) also suggested the increased CRP levels had a significant association with postablation AF recurrence. Keeping in mind the clinical importance of sinus rhythm maintenance after catheter ablation, as well as the inconsistent published results regarding the effect of inflammation in this setting, in our meta‐analysis is of special importance.
Limitations of the Study
Although we have demonstrated that significant positive association existed between CRP concentration and AF recurrence, a few limitations and potential biases should be acknowledged. First, the cutoff value of CRP should be further conducted, which may help improve patient selection for this procedure to reduce healthcare costs and avoid exposing patients to unnecessary procedures and related complications. Additionally, there are various other inflammatory biomarkers such as interleukin (IL)‐6 and IL‐8. Examination of CRP alone is not sufficient for predicting postablation AF recurrence and direct patient selection. Various studies involving more inflammatory biomarkers should be conducted. A comprehensive scale including inflammatory biomarkers, AF history, enlarged LAD, age, and cardiac function among other characteristics can be established, which could be tested before catheter ablation can comprehensively evaluate the risk of AF recurrence. Whether undergoing catheter ablation or not, patient selection should be carefully assessed. Second, although we collected all the eligible studies, the sample size of the included studies was not large enough, which could increase the likelihood of type I and type II errors. Therefore, there was a lack of statistical power to better evaluate the association between CRP and postablation AF recurrence, especially in the subgroup analyses. However, we can see the more significant trend when studying more patients. (Figure 3). The conclusion we had come to is believed to be credible. Third, our analysis is based on observational studies and may be subject to the potential biases of such studies. Therefore, our results should be interpreted cautiously. Fourth, converting non‐normally distributed statistics (median and range) to normally distributed statistics (mean and SD) may be a cause of bias in our analysis. Fifth, although the funnel plot and Egger's test did not show any publication bias, the influence of bias in the present analysis could not be completely excluded. For example, studies with positive results are more easily published than those with negative results, and only studies published in English are included. Finally, methods of AF detection were different among the trials, and therefore some asymptomatic episodes of AF may be overlooked.
Conclusion
Our meta‐analysis provides evidence that greater baseline CRP has an association with postablation AF recurrence, which adds to the current understanding of the association between inflammation and AF recurrence. Further studies of larger, well‐designed, randomized studies with longer follow‐up periods and a variety of ethnicities should be conducted to confirm our findings. In addition, the role of other inflammatory markers such as IL‐6, IL‐8, and white blood cell count also should be examined to offer the suggestions to the theory of an association between inflammation and AF recurrence.
Supporting information
Assessment of the quality of the 7 included studies (Supplementary material)
Characteristics of the 7 Studies Included in the Meta‐Analysis (Supplementary Material )
Zhouqin Jiang, MD, and Limeng Dai, MD, contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (No. 30971228). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 2. Tsang TS, Petty GW, Barnes ME, et al. The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota: changes over three decades. J Am Coll Cardiol. 2003;42:93–100. [DOI] [PubMed] [Google Scholar]
- 3. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 4. Jais P, Cauchemez B, Macle L,et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–2505. [DOI] [PubMed] [Google Scholar]
- 5. Oral H, Pappone C, Chugh A, et al. Circumferential pulmonary‐vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–941. [DOI] [PubMed] [Google Scholar]
- 6. Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2011;57:1330–1337. [DOI] [PubMed] [Google Scholar]
- 7. Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow‐up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861. [DOI] [PubMed] [Google Scholar]
- 8. Cheema A, Vasamreddy CR, Dalal D, et al. Long‐term single procedure efficacy of catheter ablation of atrial fibrillation. J Interv Card Electrophysiol. 2006;15:145–155. [DOI] [PubMed] [Google Scholar]
- 9. Pappone C, Rosanio S, Oreto G, et al. Circumferential radiofrequency ablation of pulmonary vein ostia: a new anatomic approach for curing atrial fibrillation. Circulation. 2000;102:2619–2628. [DOI] [PubMed] [Google Scholar]
- 10. Wokhlu A, Hodge DO, Monahan KH, et al. Long‐term outcome of atrial fibrillation ablation: impact and predictors of very late recurrence. J Cardiovasc Electrophysiol. 2010;21:1071–1078. [DOI] [PubMed] [Google Scholar]
- 11. Korantzopoulos P, Kolettis T, Siogas K, et al. Atrial fibrillation and electrical remodeling: the potential role of inflammation and oxidative stress. Med Sci Monit. 2003;9:RA225–RA229. [PubMed] [Google Scholar]
- 12. Engelmann MD, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J. 2005;26:2083–2092. [DOI] [PubMed] [Google Scholar]
- 13. Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27:136–149. [DOI] [PubMed] [Google Scholar]
- 14. Liu T, Li G. Is atrial fibrillation an inflammatory disease? Med Hypotheses. 2005;64:1237–1238. [DOI] [PubMed] [Google Scholar]
- 15. Watanabe T, Takeishi Y, Hirono O, et al. C‐reactive protein elevation predicts the occurrence of atrial structural remodeling in patients with paroxysmal atrial fibrillation. Heart Vessels. 2005;20:45–49. [DOI] [PubMed] [Google Scholar]
- 16. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 17. Whitlock RP, Chan S, Devereaux PJ, et al. Clinical benefit of steroid use in patients undergoing cardiopulmonary bypass: a meta‐analysis of randomized trials. Eur Heart J. 2008;29:2592–2600. [DOI] [PubMed] [Google Scholar]
- 18. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 19. Egger M, Davey Smith G, Schneider M, et al. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Letsas KP, Weber R, Burkle G, et al. Pre‐ablative predictors of atrial fibrillation recurrence following pulmonary vein isolation: the potential role of inflammation. Europace. 2009;11:158–163. [DOI] [PubMed] [Google Scholar]
- 21. Henningsen KM, Nilsson B, Bruunsgaard H, et al. Prognostic impact of hs‐CRP and IL‐6 in patients undergoing radiofrequency catheter ablation for atrial fibrillation. Scand Cardiovasc J. 2009;43:285–291. [DOI] [PubMed] [Google Scholar]
- 22. Liu J, Fang PH, Dibs S, et al. High‐sensitivity C‐reactive protein as a predictor of atrial fibrillation recurrence after primary circumferential pulmonary vein isolation. Pacing Clin Electrophysiol. 2011;34:398–406. [DOI] [PubMed] [Google Scholar]
- 23. Lin YJ, Tsao HM, Chang SL, et al. Prognostic implications of the high‐sensitive C‐reactive protein in the catheter ablation of atrial fibrillation. Am J Cardiol. 2010;105:495–501. [DOI] [PubMed] [Google Scholar]
- 24. Okumura Y, Watanabe I, Nakai T, et al. Impact of biomarkers of inflammation and extracellular matrix turnover on the outcome of atrial fibrillation ablation: importance of matrix metalloproteinase‐2 as a predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2011;22:987–993. [DOI] [PubMed] [Google Scholar]
- 25. Fan J, Cao H, Su L, et al. NT‐proBNP, but not ANP and C‐reactive protein, is predictive of paroxysmal atrial fibrillation in patients undergoing pulmonary vein isolation. J Interv Card Electrophysiol. 2012;33:93–100. [DOI] [PubMed] [Google Scholar]
- 26. Kornej J, Reinhardt C, Kosiuk J, et al. Response of high‐sensitive C‐reactive protein to catheter ablation of atrial fibrillation and its relation with rhythm outcome. PLoS One. 2012;7:e44165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel P, Dokainish H, Tsai P, et al. Update on the association of inflammation and atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:1064–1070. [DOI] [PubMed] [Google Scholar]
- 28. Frustaci A, Chimenti C, Bellocci F, et al. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. [DOI] [PubMed] [Google Scholar]
- 29. Nakamura Y, Nakamura K, Fukushima‐Kusano K, et al. Tissue factor expression in atrial endothelia associated with nonvalvular atrial fibrillation: possible involvement in intracardiac thrombogenesis. Thromb Res. 2003;111:137–142. [DOI] [PubMed] [Google Scholar]
- 30. Chang SL, Tsao HM, Lin YJ, et al. Characteristics and significance of very early recurrence of atrial fibrillation after catheter ablation. J Cardiovasc Electrophysiol. 2011;22:1193–1198. [DOI] [PubMed] [Google Scholar]
- 31. Kaptoge S, Di Angelantonio E, Lowe G, et al. C‐reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta‐analysis. Lancet. 2010;375:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ridker PM, Rifai N, Stampfer MJ, et al. Plasma concentration of interleukin‐6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. [DOI] [PubMed] [Google Scholar]
- 33. Narducci ML, Pelargonio G, Dello Russo A, et al. Role of tissue C‐reactive protein in atrial cardiomyocytes of patients undergoing catheter ablation of atrial fibrillation: pathogenetic implications. Europace. 2011;13:1133–1140. [DOI] [PubMed] [Google Scholar]
- 34. Dernellis J, Panaretou M. Relationship between C‐reactive protein concentrations during glucocorticoid therapy and recurrent atrial fibrillation. Eur Heart J. 2004;25:1100–1107. [DOI] [PubMed] [Google Scholar]
- 35. Lellouche N, Jais P, Nault I, et al. Early recurrences after atrial fibrillation ablation: prognostic value and effect of early reablation. J Cardiovasc Electrophysiol. 2008;19:599–605. [DOI] [PubMed] [Google Scholar]
- 36. Tao H, Liu X, Dong J, et al. Predictors of very late recurrence of atrial fibrillation after circumferential pulmonary vein ablation. Clin Cardiol. 2008;31:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jais P, Haissaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. 1997;95:572–576. [DOI] [PubMed] [Google Scholar]
- 38. Neumann T, Vogt J, Schumacher B, et al. Circumferential pulmonary vein isolation with the cryoballoon technique results from a prospective 3‐center study. J Am Coll Cardiol. 2008;52:273–278. [DOI] [PubMed] [Google Scholar]
- 39. Scharf C, Boersma L, Davies W, et al. Ablation of persistent atrial fibrillation using multielectrode catheters and duty‐cycled radiofrequency energy. J Am Coll Cardiol. 2009;54:1450–1456. [DOI] [PubMed] [Google Scholar]
- 40. Lee G, Sanders P, Kalman JM. Catheter ablation of atrial arrhythmias: state of the art. Lancet. 2012;380:1509–1519. [DOI] [PubMed] [Google Scholar]
- 41. Lubitz SA, Fischer A, Fuster V. Catheter ablation for atrial fibrillation. BMJ. 2008;336:819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Husser D, Adams V, Piorkowski C, et al. Chromosome 4q25 variants and atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2010;55:747–753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Assessment of the quality of the 7 included studies (Supplementary material)
Characteristics of the 7 Studies Included in the Meta‐Analysis (Supplementary Material )


