Abstract
Background
Atrial fibrillation is associated with inflammation and oxidative stress.
Hypothesis
Carvedilol and N‐acetyl cysteine (NAC) combination decreases inflammation, oxidative stress, and postoperative atrial fibrillation (POAF) rates more than metoprolol or carvedilol.
Methods
Preoperative and postoperative total oxidative stress (TOS), total antioxidant capacity (TAC), and white blood cells (WBC) were measured in metoprolol, carvedilol, or carvedilol plus NAC groups, and association with POAF was evaluated.
Results
Preoperative TAC, TOS, and WBC levels were similar among the groups. Postoperative TAC levels were lower in the metoprolol group compared with the carvedilol group (1.0 vs 1.4) or the carvedilol plus NAC group (1.0 vs 1.9) and were also lower in the carvedilol group compared with the carvedilol plus NAC group (all P < 0.0001). Postoperative TOS levels were higher in the metoprolol group as compared with the carvedilol (29.6 vs 24.2; P < 0.0001) or the carvedilol plus NAC groups (P < 0.0001), and were also higher in the carvedilol group as compared with the carvedilol plus NAC group (24.2 vs 19.3; P < 0.0001). Postoperative WBC counts were lower in the carvedilol plus NAC group compared with the metoprolol group (12.9 vs 14.8; P = 0.004), were similar between the carvedilol and the metoprolol groups (13 vs 14.8) and between the carvedilol plus NAC group and the carvedilol group (both P > 0.05). Postoperative TAC, TOS, and WBC were associated with POAF.
Conclusions
Carvedilol plus NAC reduced oxidative stress and inflammation compared with metoprolol and decreased oxidative stress compared with carvedilol. Postoperative TAC, TOS, and WBC were associated with POAF.
Introduction
Cardiopulmonary bypass may cause oxidative stress and inflammation1 processes that have been shown to be associated with atrial fibrillation.2, 3, 4, 5 Carvedilol is a nonselective β‐blocker and N‐acetyl cysteine (NAC) is a mucolytic agent. These agents have anti‐inflammatory and antioxidant properties,6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 and they decreased postoperative atrial fibrillation (POAF) rates in previous studies.2, 17, 18, 19, 20, 21, 22 In a randomized study, we have shown that carvedilol plus NAC decreased POAF incidence compared with metoprolol or carvedilol.23 However, it is not known whether the effect of carvedilol and NAC is associated with changes in the levels of oxidative markers or whether there is a correlation between these markers and the development of POAF. Therefore, this study was designed to compare the effects of metoprolol, a β‐blocker without antioxidant and anti‐inflammatory action, vs carvedilol or carvedilol plus NAC on the markers of oxidative status and inflammation, and to evaluate the relationship between these markers and POAF. This is a prespecified substudy of our randomized study.23
Methods
This was a single‐center, prospective, double‐blind, randomized study.23 The overall study population included 311 patients undergoing coronary artery bypass graft (CABG) or combined CABG and valve surgery. Patients undergoing their first cardiothoracic surgery without contraindications to β‐blockers or NAC were included. Exclusion criteria were hyperthyroidism, age <18 years, prior cardiac surgery, class III or IV heart failure, previous atrial fibrillation, left atrial diameter >55 mm, left ventricular ejection fraction <0.25, sepsis, heart rate <60 bpm, systolic blood pressure <90 mm Hg, inflammatory disease, and being already on antiarrhythmic or NAC treatment. According to these criteria, 36 patients were excluded due to previous atrial fibrillation (n = 12), heart rate <60 bpm (n = 10), previous NAC use (n = 10), and hyperthyroidism (n = 4). Measurement of preoperative and postoperative serum oxidative status and inflammation markers were not available in 16 patients. Therefore, 259 patients were included for this substudy.
In brief, patients were randomized to metoprolol succinate plus saline (n = 103), carvedilol plus saline (n = 104), and carvedilol plus NAC (n = 104). Metoprolol and carvedilol were started at doses of 50 mg once daily and 6.25 mg twice daily, respectively. The doses were up‐titrated to maximum tolerated doses. The target doses for metoprolol and carvedilol were 200 mg once daily and 25 mg twice daily, respectively. N‐acetyl cysteine (Asist, Husnu Arsan, Turkey) was administered intravenously at a dose of 50 mg/kg for 1 hour before surgery and at the same dose for 48 hours after the procedure. In the metoprolol and the carvedilol groups, normal saline solution was infused as placebo. The infusion rate and duration were similar for saline and NAC. Informed consent was obtained from each patient, and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee. The detailed information about the study design is given elsewhere.23 Similar operative techniques were used in all of the patients.
End Points
The end points were to compare the effects of treatment groups on the change in serum preoperative to postoperative markers of oxidative status and inflammation and to evaluate the relationship between these markers and POAF. Therefore, serum total oxidative stress (TOS), total antioxidant capacity (TAC) levels, and white blood cell (WBC) counts were measured preoperatively and at postoperative 48th hour. Decreased TAC and increased TOS were used as markers of oxidative stress, and WBCs were used as markers of inflammation. TAC and TOS levels were determined with new methods using a spectrophotometric kit (Rel Assay Diagnostics, Gaziantep, Turkey) and were assayed in an autoanalyzer (Olympus AU2700; Olympus, Tokyo, Japan). The results of TAC and TOS were expressed as mmol Trolox equivalent/L and mmol H2O2 equivalent/L, respectively.24, 25
Rhythm Follow‐up
The rhythms were followed by continuous electrocardiogram monitoring during intensive care unit stay and by all‐day Holter during the rest of hospitalization. A 12‐lead electrocardiogram was recorded every morning routinely and whenever the patients had symptoms suggestive of dysrhythmia. Atrial fibrillation was defined as an irregular rhythm with the absence of discrete P waves in the 12‐lead electrocardiogram. An atrial fibrillation episode lasting 5 minutes during hospitalization was defined as POAF.23
Statistical Analysis
Categorical variables were expressed as frequency (%) and compared with the χ2 test. A Kolmogorov‐Smirnov test was used to test the distribution of numeric variables, and those with normal distribution were expressed as mean ± standard deviation and were compared with the Student t test or analysis of variance as appropriate. Scheffe correction test was used for post hoc pairwise comparison of variables showing normal distribution (postoperative heart rates). On the other hand, those without normal distribution were expressed as median (minimum–maximum) and were compared with the Mann–Whitney U test or Kruskal‐Wallis test as appropriate. Preoperative to postoperative differences in paired observations in each group were compared with the Wilcoxon rank test (Table 1, Figure 1). A 2‐sided P value <0.05 was considered significant. SPSS version 11.0 (SPSS, Inc., Chicago, IL) was used for the analysis. Predictors of POAF were determined by logistic regression analysis. The strength of association between variables and the occurrence of POAF were represented by odds ratios and their accompanying 95% confidence intervals. Factors with P < 0.10 with univariate regression were entered in the multivariate model. Preoperative and postoperative TAC, TOS, and WBC counts; age; left‐atrial diameter; ejection fraction; hypertension; diabetes mellitus; bypass duration; the carvedilol plus NAC group vs the metoprolol group; the carvedilol plus NAC group vs the carvedilol group; the carvedilol group vs the metoprolol group; prerandomization, preoperative, and postoperative heart rates, postoperative spironolactone treatment; and CABG plus valve surgery or CABG had P < 0.10 with univariate comparison in terms of the end point of POAF.
Table 1.
Preoperative, Postoperative, and Procedural Characteristics
| Metoprolol (n = 87) | Carvedilol (n = 87) | Carvedilol + NAC (n = 85) | Carv + NAC vs Met, P Value | Carv + NAC vs Carv, P Value | Carv vs Met, P Value | |
|---|---|---|---|---|---|---|
| Preoperative characteristics | ||||||
| Age, y | 63 ± 10.5 | 63 ± 9.4 | 62 ± 9.2 | 0.83 | 0.98 | 0.90 |
| Male gender (%) | 67 (77) | 63 (72.4) | 65 (76.5) | 1.00 | 0.6 | 0.6 |
| Heart failure (%) | 12 (13.8) | 16 (18.4) | 14 (16.5) | 0.67 | 0.84 | 0.54 |
| Hypertension, (%) | 54 (62.1) | 55 (63.2) | 53 (62.4) | 1.00 | 1.00 | 1.00 |
| Diabetes mellitus, (%) | 35 (40.2) | 37 (42.5) | 35 (41.2) | 1.00 | 0.87 | 0.87 |
| COPD, (%) | 6 (6.9) | 12 (13.8) | 9 (10.6) | 0.42 | 0.64 | 0.21 |
| Clinical presentation | ||||||
| Stable angina pectoris (%) | 40 (46) | 41 (47.1) | 31 (36.5) | 1.00 | 0.17 | 1.00 |
| Unstable angina pectoris/ myocardial infarction (%) | 47 (54) | 46 (52.9) | 54 (63.5) | |||
| Body mass index, kg/m2 | 26 ± 3.9 | 27 ± 3.9 | 26 ± 4.2 | 0.99 | 0.79 | 0.73 |
| Ejection fraction, % | 50 ± 10.3 | 49 ± 12.3 | 49 ± 11.9 | 0.85 | 0.94 | 0.66 |
| Left atrial diameters, mm | 40.4 ± 4 | 40.7 ± 3.8 | 40.9 ± 4.8 | 0.76 | 0.93 | 0.93 |
| Preoperative systolic BP | 119 ± 13 | 120 ± 12 | 118 ± 13 | 0.88 | 0.74 | 0.96 |
| Preoperative diastolic BP | 73 ± 9 | 75 ± 9 | 73 ± 10 | 0.98 | 0.36 | 0.45 |
| Prerandomization heart rate | 73 ± 7 | 71 ± 5 | 72 ± 7 | 0.37 | 0.73 | 0.10 |
| Preoperative heart rate | 70 ± 7 | 69 ± 5 | 69 ± 6 | 0.43 | 0.97 | 0.56 |
| Preoperative medications | ||||||
| β‐Blocker (before randomization) | 78 (89.7) | 74 (85.1) | 67 (78.8) | 0.06 | 0.32 | 0.49 |
| ACEI and/or ARB | 35 (40.2) | 35 (40.2) | 38 (44.7) | 0.64 | 0.64 | 1.00 |
| Spironolactone | 7 (8) | 13 (14.9) | 14 (16.5) | 1.00 | 0.83 | 0.23 |
| Statin | 20 (23) | 32 (36.8) | 24 (28.2) | 0.48 | 0.25 | 0.07 |
| Procedural and postoperative characteristics | ||||||
| Procedure (%) | ||||||
| CABG | 80 (92) | 79 (90.8) | 81 (95.3) | 0.53 | 0.37 | 1.00 |
| CABG + MVR | 4 (4.6) | 4 (4.6) | 3 (3.5) | |||
| CABG + AVR | 3 (3.4) | 4 (4.6) | 1 (1.2) | |||
| Beating heart surgery (%) | 26 (29.9) | 34 (39.1) | 23 (27.1) | 0.73 | 0.10 | 0.26 |
| Cross‐clamp duration, min | 58 ± 21 | 65 ± 27 | 59 ± 23 | 0.98 | 0.33 | 0.24 |
| Bypass duration, min | 109 ± 39 | 109 ± 35 | 104 ± 24 | 0.70 | 0.61 | 0.98 |
| Postoperative systolic BP (48th hour) | 112 ± 17 | 114 ± 14 | 112 ± 13 | 0.97 | 0.54 | 0.67 |
| Postoperative diastolic BP (48th hour) | 64 ± 10 | 63 ± 11 | 61 ± 9 | 0.20 | 0.31 | 0.96 |
| Postoperative heart rate | 93 ± 5 | 87 ± 2 | 86 ± 10 | 0.001 | 0.96 | 0.02 |
| Postoperative medications (%) | ||||||
| ACEI and/or ARB | 11 (12.6) | 10 (11.5) | 10 (11.8) | 1.00 | 1.00 | 0.81 |
| Spironolactone | 3 (3.4) | 3 (3.4) | 4 (4.7) | 1.00 | 0.71 | 1.00 |
| Statin | 16 (18.4) | 12 (13.8) | 12 (14.1) | 0.53 | 1.00 | 0.41 |
| β‐Blocker | 77 (88.5) | 82 (94.3) | 81 (95.5) | 0.32 | 0.72 | 0.56 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; AVR, aortic valve replacement; BP, blood pressure; CABG, coronary artery bypass graft; Carv, carvedilol; COPD, chronic obstructive pulmonary disease; Met, metoprolol; MVR, mitral valve replacement; NAC, N‐acetyl cysteine.
Data are presented as mean ± standard deviation or number (%) of patients.
Figure 1.
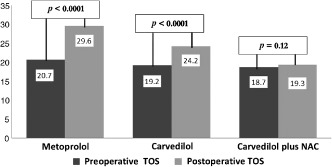
Preoperative to postoperative paired differences in the total oxidative stress (TOS) levels by intervention groups. Abbreviations: NAC, N‐acetyl cysteine; TOS, total oxidative stress.
Results
Patients
A total 259 patients (mean age, 63 ± 10 years; 231 male) constituted the study population. Postoperative heart rate was higher in the metoprolol group compared with the carvedilol group (P = 0.02) or the carvedilol plus NAC group (P = 0.001), but was similar between the carvedilol and the carvedilol plus NAC groups (P = 0.9). There were no statistically significant differences with respect to other preoperative, postoperative, and procedural characteristics (all P values >0.05; Table 1).
Oxidative Status and Inflammation
Preoperative TAC and TOS levels and WBC counts were similar among the 3 groups (all P values >0.05) (Table 2). Compared with preoperative levels, postoperative TAC levels decreased in the metoprolol and the carvedilol groups, but increased in the carvedilol‐plus NAC group (all P values <0.0001). Compared with preoperative levels, postoperative TOS levels increased in the metoprolol (P < 0.0001) and the carvedilol groups (P < 0.0001), but no significant change occurred in the carvedilol plus NAC group (P = 0.12). Postoperative WBC counts increased significantly as compared with preoperative levels in all 3 groups (all P values < 0.0001; Figure 1). Postoperative TAC levels were lower in the metoprolol group compared with the carvedilol group (P < 0.0001) or the carvedilol plus NAC group (P < 0.0001) and were also lower in the carvedilol group compared with the carvedilol plus NAC group (P < 0.0001). Postoperative TOS levels were higher in the metoprolol group as compared with the carvedilol group (P < 0.0001) or the carvedilol plus NAC group (P < 0.0001), and were also higher in the carvedilol group as compared with the carvedilol plus NAC group (P < 0.0001). Postoperative WBC counts were lower in the carvedilol plus NAC group compared with the metoprolol group (P = 0.004), and were similar between the carvedilol and the metoprolol groups and between the carvedilol plus NAC group and the carvedilol group (both P values > 0.05).
Table 2.
Levels of the Markers of Oxidative Status and Inflammation
| Metoprolol, n = 87 | Carvedilol, n = 87 | Carvedilol + NAC, n = 85 | Carv + NAC vs Met, P Value | Carv + NAC vs Carv, P Value | Carv vs Met, P Value | |
|---|---|---|---|---|---|---|
| Marker | ||||||
| Preoperative TAC | 1.4 (0.7‐2.9) | 1.6 (0.7‐3.0) | 1.6 (0.7‐2.9) | 0.08 | 0.89 | 0.12 |
| Preoperative TOS | 20.7 (4.8‐39.3) | 19.2 (4.9‐38.8) | 18.7 (3.0‐65.0) | 0.1 | 0.81 | 0.11 |
| Preoperative WBC count | 8.5 (3.6‐14.5) | 8.5 (4.9‐12.3) | 8.5 (4.2‐15.7) | 0.4 | 0.47 | 0.11 |
| Postoperative TAC | 1.0 (0.2‐2.04) | 1.4 (0.6‐3.2) | 1.9 (0.9‐3.9) | <0.0001 | <0.0001 | <0.0001 |
| Postoperative TOS | 29.6 (5.3‐76.9) | 24.2 (2.2‐41.9) | 19.3 (4.0‐41.0) | <0.0001 | <0.0001 | <0.0001 |
| Postoperative WBC count | 14.8 (5.7‐22.7) | 13.0 (6.5‐28) | 12.9 (6.4‐19.6) | 0.004 | 0.28 | 0.09 |
| Group | Preoperative TAC | Postoperative TAC | P Value | |||
| Metoprolol | 1.4 (0.7‐2.9) | 1.0 (0.2‐2.0) | <0.0001 | |||
| Carvedilol | 1.6 (0.7‐3.0) | 1.4 (0.6‐3.2) | <0.0001 | |||
| Carvedilol + NAC | 1.6 (0.7‐2.9) | 1.9 (0.9‐3.9) | <0.0001 | |||
| Group | Preoperative TOS | Postoperative TOS | P Value | |||
| Metoprolol | 20.7 (4.8‐39.3) | 29.6 (5.3‐76.9) | <0.0001 | |||
| Carvedilol | 19.2 (4.9‐38.8) | 24.2 (2.2‐41.9) | <0.0001 | |||
| Carvedilol + NAC | 18.7 (3.0‐65.0) | 19.3 (4.0‐41.0) | 0.12 | |||
| Group | Preoperative WBC Count | Postoperative WBC Count | P Value | |||
| Metoprolol | 8.0 (3.6‐14.5) | 14.8 (5.7‐22.7) | <0.0001 | |||
| Carvedilol | 8.5 (4.9‐12.3) | 13.0 (6.5‐28.0) | <0.0001 | |||
| Carvedilol + NAC | 8.5 (4.2‐15.7) | 12.9 (6.4‐19.6) | <0.0001 | |||
| Marker | Patients Without POAF, n = 197 | Patients With POAF, n = 62 | P Value | |||
| Preoperative TAC | 1.6 (0.7‐3.0) | 1.4 (0.7‐2.9) | 0.02 | |||
| Preoperative TOS | 18 (3.0‐65.0) | 22.0 (5.7‐38.8) | <0.0001 | |||
| Preoperative WBC count | 8.4 (4.2‐15.7) | 7.9 (3.6‐14.5) | 0.08 | |||
| Postoperative TAC | 1.5 (0.3‐3.9) | 1.0 (0.2‐2.1) | <0.0001 | |||
| Postoperative TOS | 21.4 (2.2‐55.9) | 32.1 (6.3‐76.9) | <0.0001 | |||
| Postoperative WBC count | 12.5 (5.7‐28.0) | 17.1 (10.2‐22.7) | <0.0001 | |||
Abbreviations: Carv, carvedilol; H2O2, hydrogen peroxide; Met, metoprolol; NAC, N‐acetyl cysteine; POAF, postoperative atrial fibrillation; TAC, total antioxidant capacity; TOS, total oxidative stress; WBC, white blood cell.
The results of TAC TOS levels and WBC counts were expressed as mmol Trolox equivalent/L, mmol H2O2 equivalent/L and × 1000/mm3, respectively. Data presented as median (maximum‐minimum).
Treatment Groups, POAF, and Markers of Oxidative Stress and Inflammation
Preoperative TOS levels were higher, and preoperative TAC levels were lower in patients with POAF as compared with those without POAF (both P values < 0.05). There was no significant difference with respect to preoperative WBC counts in patients with POAF as compared with those without POAF (P = 0.08). Postoperative TAC and TOS levels and WBC counts were higher in patients with POAF as compared with those without POAF (all P values < 0.0001).
Carvedilol plus NAC reduced the POAF rate as compared with carvedilol (10.6% vs 24.1%; P = 0.02) or metoprolol (10.6% vs 36.8%; P < 0.0001). There was a trend for lower POAF rates in the carvedilol group compared with the metoprolol group (P = 0.09; Figure 2). Postoperative TAC and TOS levels and WBC counts were independent predictors of POAF in multivariate regression analysis (all the multivariate predictors of POAF are given in Table 3).
Figure 2.
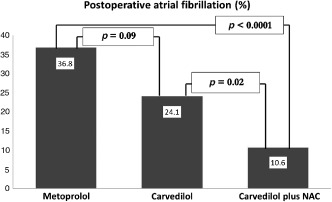
The incidence of postoperative atrial fibrillation according to intervention groups. Abbreviations: NAC, N‐acetyl cysteine.
Table 3.
Multivariate Predictors of Postoperative Atrial Fibrillation
| Predictors | Odds Ratio | Confidence Interval | P Value |
|---|---|---|---|
| Postoperative WBC count | 1.94 | 1.47‐2.55 | <0.0001 |
| Postoperative TAC | 0.18 | 0.04‐0.69 | 0.01 |
| Postoperative TOS | 1.24 | 1.13‐1.36 | <0.0001 |
| Carvedilol plus NAC vs carvedilol | 0.16 | 0.05‐0.51 | 0.002 |
| Carvedilol plus NAC vs metoprolol | 0.18 | 0.03‐0.92 | 0.04 |
| Hypertension | 10.93 | 2.41‐49.45 | 0.002 |
| Diabetes mellitus | 3.87 | 1.20‐12.47 | 0.02 |
| Left atrial diameter | 1.30 | 1.13‐1.50 | <0.0001 |
| Bypass duration | 1.01 | 1.00‐1.03 | 0.01 |
| Prerandomization heart rate | 1.22 | 1.10‐1.34 | <0.0001 |
| Preoperative heart rate | 1.24 | 1.11‐1.39 | <0.0001 |
Abbreviations: NAC, N‐acetyl cysteine; TAC, total antioxidant capacity; TOS, total oxidative stress; WBC, white blood cell.
Discussion
The present study shows that carvedilol plus NAC reduced oxidative stress and inflammation as compared with metoprolol and reduced oxidative stress as compared with carvedilol. Similarly, the combination decreased POAF rates as compared with carvedilol or metoprolol. On the other hand, better antioxidant but similar anti‐inflammatory actions and a trend for lower POAF rates were found in the carvedilol group as compared with the metoprolol group. Postoperative TAC and TOS levels and WBC counts were independent predictors of POAF. To the best of our knowledge, no previous study compared the antioxidant and anti‐inflammatory actions of metoprolol vs carvedilol or carvedilol plus NAC using the present markers and evaluated their association with POAF.
NAC has been shown to reduce oxidative and inflammatory response8, 9, 10, 11 and ischemia/reperfusion injury after myocardial infarction12 and after cardiac surgery.13 Sucu et al8 evaluated myeloperoxidase, malondialdehyde, interleukin‐6, α1‐acid glycoprotein, and C‐reactive protein, and found that NAC decreased oxidoinflammatory response during surgery. Koramaz et al9 compared cold‐blood cardioplegia enriched with NAC and cold‐blood cardioplegia alone, and found that postoperative troponin I levels were lower and malondialdehyde levels were higher in the control group compared with the NAC group. Vento et al have shown that myocardial glutathione content is better preserved, myeloperoxidase activity is lower, and leukocytes sequestered in the coronary circulation are lower in patients receiving NAC than in the control group.10 In the study of Tossios et al,11 left ventricular biopsy specimens collected before and at the end of cardiopulmonary bypass were subjected to immunocytochemical staining against 8‐iso‐prostaglandin F2‐α as an indicator for reactive oxygen species‐mediated lipid peroxidation and nitrotyrosine as a marker for peroxynitrite‐mediated tissue injury. They found that the preoperative to postoperative change in ventricular cardiomyocyte staining for both 8‐iso‐prostaglandin F2‐α and nitrotyrosine differed significantly in the NAC group compared with the placebo group.11
Carvedilol has been shown to have anti‐inflammatory actions.6, 7, 14, 15, 16 Arumanayagam et al showed that it has significant antioxidant effects as compared with metoprolol.6 Similarly, it decreased interleukin‐6 and tumor necrosis factor‐α levels and increased tumor necrosis factor‐α levels in patients with ischemic and nonischemic cardiomyopathy, respectively.14, 15 Alfieri et al showed that asymmetric dimethylarginine and interleukin‐18 levels decreased with carvedilol in heart failure.16
Bypass surgery may induce oxidative stress and inflammation, and these processes may be associated with complications after cardiac surgery including POAF.4, 26, 27 Upregulated reactive oxygen species genes,28 injured atrial myofibrils,29 and increased reactive oxygen species and ratios of oxidized to reduced glutathione and cysteine30 have been found in patients with atrial fibrillation. However, urinary F2‐isoprostanes did not increase in these patients.31 Leukocytes are inflammatory cells that may regulate oxidative stress by releasing reactive oxygen species.32 They have been shown to be increased after cardiac surgery and may predict POAF.33, 34, 35 In a very recent study, Rodrigo et al. randomized the patients undergoing cardiac surgery to n‐3 polyunsaturated fatty acids, vitamin C, and vitamin E or placebo, and found that POAF rate and postoperative biomarkers of inflammation and oxidative stress were lower in the intervention group as compared with the placebo group.36
Carvedilol has been shown to be effective in preventing POAF.20, 21, 22 However, conflicting results have been obtained with respect to the effects of NAC on POAF.2, 17, 18, 19, 23, 37 Postoperative TAC and TOS levels and WBC counts were independent predictors of POAF, suggesting that any treatment methods that effectively prevent postoperative oxidative stress and inflammation may markedly decrease POAF rates. Therefore, we speculated that either longer administration and/or a higher dose of carvedilol and NAC or the addition of other agents are needed to obtain better results.
Limitations
This was a substudy. Patients without POAF had lower values of the markers of inflammation and oxidative stress. In the present study, new methods using a spectrophotometric kit have been used to measure oxidative capacity and total oxidative stress levels. There is a lack of data regarding specific oxidative stress and inflammatory markers, and this is the main deficiency of the present study. To confirm our data, it would be better if we had measured levels of other oxidative stress markers routinely used, such as malondialdehyde, nitrotyrosine, plasma lipid hydrogen peroxide levels, or paraoxonase and arylesterase activities. Also, instead of WBC count, it would be more reliable to use more sensitive markers of inflammation such as C‐reactive protein. There was a trend of lower preoperative β‐blocker use in the carvedilol plus NAC group as compared with the metoprolol group (P = 0.06). Therefore, if β‐blocker use was higher in the carvedilol plus NAC group, the beneficial effects of carvediol plus NAC would be higher. To explore whether the effect of carvedilol plus NAC is due to the NAC alone or combination, a control group would have been interesting. Also, a metoprolol plus NAC group could be interesting to determine whether carvedilol is necessary for the antiarrhythmic effect. However, those groups are lacking.
Conclusion
NAC plus carvedilol reduced oxidative stress and inflammation compared with metoprolol and decreased oxidative stress compared with carvedilol. Carvedilol showed better antioxidant effects than metoprolol, but their anti‐inflammatory effects were similar. Postoperative TAC and TOS levels and WBC counts were independent predictors of POAF in multivariate regression analysis.
Acknowledgments
The authors thank Daiichi‐Sankyo Co. for providing the kits for determining TAC and TOS levels.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Elahi MM, Flatman S, Matata BM. Tracing the origins of postoperative atrial fibrillation: the concept of oxidative stress‐mediated myocardial injury phenomenon. Eur J Cardiovasc Prev Rehabil. 2008;15:735–741. [DOI] [PubMed] [Google Scholar]
- 2. Baker WL, Anglade MW, Baker EL, et al. Use of N‐acetyl cysteine to reduce post‐cardiothoracic surgery complications: a metaanalysis. Eur J Cardiothorac Surg. 2009;35:521–527. [DOI] [PubMed] [Google Scholar]
- 3. Kumagai K, Nakashima H, Saku K. The HMG‐CoA reductase inhibitor atorvastatin prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Cardiovasc Res. 2004;62:105–111. [DOI] [PubMed] [Google Scholar]
- 4. Carnes CA, Chung MK, Nakayama T, et al. Ascorbate attenuates atrial pacing‐induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:e32–e38. [DOI] [PubMed] [Google Scholar]
- 5. Koroglu S, Tuncer C, Acar G, et al. Relation of inflammatory and oxidative markers to the occurrence and recurrence of persistent atrial fibrillation. Turk Kardiyol Dern Ars. 2012;40:499–504. [DOI] [PubMed] [Google Scholar]
- 6. Arumanayagam M, Chan S, Tong S, et al. Antioxidant properties of carvedilol and metoprolol in heart failure: a double‐blind randomized controlled trial. J Cardiovasc Pharmacol. 2001;37:48–54. [DOI] [PubMed] [Google Scholar]
- 7. El‐Sheriff N, Turitto G. Electrophysiologic effects of carvedilol: is carvedilol an antiarrhythmic agent? Pacing Clin Electrophysiol. 2005;28:985–990. [DOI] [PubMed] [Google Scholar]
- 8. Sucu N, Cinel I, Unlu A, et al. N‐Acetylcysteine for preventing pump induced oxidoinflammatory response during cardiopulmonary bypass. Surg Today. 2004;34:237–242. [DOI] [PubMed] [Google Scholar]
- 9. Koramaz I, Pulathan Z, Usta S, et al. Cardioprotective effect of cold blood cardioplegia enriched with N‐acetylcysteine during coronary artery bypass grafting. Ann Thorac Surg. 2006;81:613–618. [DOI] [PubMed] [Google Scholar]
- 10. Vento AE, Nemlander A, Aittomaki J, et al. N‐Acetylcysteine as an additive to crystalloid cardioplegia increased oxidative stress capacity in CABG patients. Scand Cardiovasc J. 2003;37:349–355. [DOI] [PubMed] [Google Scholar]
- 11. Tossios P, Bloch W, Huebner A, et al. N‐Acetylcysteine prevents reactive oxygen species‐mediated myocardial stress in patients undergoing cardiac surgery: results of a randomized, double‐blind, placebo‐controlled clinical trial. J Thorac Cardiovasc Surg. 2003;126:1513–1520. [DOI] [PubMed] [Google Scholar]
- 12. Forman MB, Puett DW, Cates CU, et al. Glutathione redox pathway and reperfusion injury. Effect of N‐acetylcysteine on infarct size and ventricular function. Circulation. 1988;78:202–213. [DOI] [PubMed] [Google Scholar]
- 13. Orhan G, Yapici N, Yuksel M, et al. Effects of N‐acetylcysteine on myocardial ischemia‐reperfusion injury in bypass surgery. Heart Vessels. 2006;21:42–47. [DOI] [PubMed] [Google Scholar]
- 14. Kurum T, Tatli E, Yuksel M. Effects of carvedilol on plasma levels of pro‐inflammatory cytokines in patients with ischemic and nonischemic dilated cardiomyopathy. Tex Heart Inst J. 2007;34:52–59. [PMC free article] [PubMed] [Google Scholar]
- 15. Tatli E, Kurum T. A controlled study of the effects of carvedilol on clinical events, left ventricular function and proinflammatory cytokines levels in patients with dilated cardiomyopathy. Can J Cardiol. 2005;21:344–348. [PubMed] [Google Scholar]
- 16. Alfieri AB, Briceno L, Fragasso G, et al. Differential long‐term effects of carvedilol on proinflammatory and antiinflammatory cytokines, asymmetric dimethylarginine, and left ventricular function in patients with heart failure. J Cardiovasc Pharmacol. 2008;52:49–54. [DOI] [PubMed] [Google Scholar]
- 17. Gu WJ, Wu ZJ, Wang PF, et al. N‐Acetyl cysteine supplementation for the prevention of atrial fibrillation after cardiac surgery: a meta‐analysis of eight randomized controlled trials. BMC Cardiovasc Disord. 2012;12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang G, Bainbridge D, Martin J, et al. N‐acetyl cysteine in cardiac surgery: do the benefits outweigh the risks? A meta‐analytic reappraisal. J Cardiothorac Vasc Anesth. 2011;25:268–275. [DOI] [PubMed] [Google Scholar]
- 19. Ozaydin M, Peker O, Erdogan D, et al. N‐acetyl cysteine for the prevention of postoperative atrial fibrillation: a prospective, randomized, placebo‐controlled pilot study. Eur Heart J. 2008;29:625–631. [DOI] [PubMed] [Google Scholar]
- 20. Haghjoo M, Saravi M, Hashemi MJ, et al. Optimal beta‐blocker for prevention of atrial fibrillation after on‐pump coronary artery bypass graft surgery: carvedilol versus metoprolol. Heart Rhythm. 2007;4:1170–1174. [DOI] [PubMed] [Google Scholar]
- 21. Acikel S, Bozbas H, Gultekin B, et al. Comparison of the efficacy of metoprolol and carvedilol for preventing atrial fibrillation after coronary bypass surgery. Int J Cardiol. 2008;126:108–113. [DOI] [PubMed] [Google Scholar]
- 22. Merritt JC, Niebauer M, Tarakji K, et al. Comparison of effectiveness of carvedilol versus metoprolol or atenolol for atrial fibrillation appearing after coronary artery bypass grafting or cardiac valve operation. Am J Cardiol. 2003;92:735–736. [DOI] [PubMed] [Google Scholar]
- 23. Ozaydin M, Icli A, Yucel H, et al. Metoprolol versus carvedilol or carvedilol plus N‐acetyl cysteine on postoperative atrial fibrillation: a randomized, double‐blind, placebo‐controlled study. Eur Heart J. 2013;34:597–604. [DOI] [PubMed] [Google Scholar]
- 24. Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. [DOI] [PubMed] [Google Scholar]
- 25. Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. [DOI] [PubMed] [Google Scholar]
- 26. Korantzopoulos P, Kolettis TM, Galaris D, et al. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol. 2007;115:135–143. [DOI] [PubMed] [Google Scholar]
- 27. Patti G, Chello M, Candura D, et al. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA‐3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation. 2006;114:1455–1461. [DOI] [PubMed] [Google Scholar]
- 28. Kim YH, Lim DS, Lee JH, et al. Gene expression profiling of oxidative stress on atrial fibrillation in humans. Exp Mol Med. 2003;35:336–349. [DOI] [PubMed] [Google Scholar]
- 29. Mihm MJ, Yu F, Carnes CA, et al. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–180. [DOI] [PubMed] [Google Scholar]
- 30. Neuman RB, Bloom HL, Shukrullah I, et al. Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem. 2007;53:1652–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li J, Solus J, Chen Q, et al. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farah R, Shurtz‐Swirski R, Bolotin Y, et al. Oxidative stress and inflammation due to peripheral polymorphonuclear leukocytes after coronary angiography vs percutaneous coronary intervention. Minerva Cardioangiol. 2008;56:189–195. [PubMed] [Google Scholar]
- 33. Lamm G, Auer J, Weber T, et al. Postoperative white blood cell count predicts atrial fibrillation after cardiac surgery. J Cardiothorac Vasc Anesth. 2006;20:51–56. [DOI] [PubMed] [Google Scholar]
- 34. Sabol F, Jakubova M, Mitro P, et al. Is there a relationship between inflammatory markers, oxidative stress and postoperative atrial fibrillation [in Slovak]? Vnitr Lek. 2012;58:730–734. [PubMed] [Google Scholar]
- 35. Abdelhadi RH, Gurm HS, Van Wagoner DR, et al. Relation of an exaggerated rise in white blood cells after coronary bypass or cardiac valve surgery to development of atrial fibrillation postoperatively. Am J Cardiol. 2004;93:1176–1178. [DOI] [PubMed] [Google Scholar]
- 36. Rodrigo R, Korantzopoulos P, Cereceda M, et al. A randomized controlled trial to prevent post‐operative atrial fibrillation by antioxidant reinforcement. J Am Coll Cardiol. 2013;62:1457–1465. [DOI] [PubMed] [Google Scholar]
- 37. El‐Hamamsy I, Stevens LM, Carrier M, et al. Effect of intravenous N‐acetylcysteine on outcomes after coronary artery bypass surgery: a randomized, double‐blind, placebo‐controlled clinical trial. J Thorac Cardiovasc Surg. 2007;133:7–12. [DOI] [PubMed] [Google Scholar]


