Abstract
Background
In patients with left‐sided native‐valve infective endocarditis (IE), the risk of embolism is increased with a vegetation size ≥10 mm. For this reason—according to guidelines—surgery may be considered in patients with isolated large vegetations (without any other indication for surgery). However, the value of surgery in this patient subset has never been systematically studied.
Hypothesis
We hypothesized that surgery may be superior to medical therapy in terms of clinical outcome in the aforementioned patients.
Methods
All patients who presented at our institution between January 2000 and July 2012 with isolated left‐sided native‐valve IE and large vegetations (≥10 mm) were examined. Patients with conventional indications for surgical treatment were excluded. Follow‐up for clinical events was performed according to predefined definitions.
Results
A total of 71 patients with left‐sided native‐valve IE and isolated large vegetations qualified for inclusion into the study cohort. Mean vegetation length was 17 ± 5 mm. A total of 59 patients underwent surgery after a mean of 5 ± 6 days following the initiation of antibiotic treatment. Mean follow‐up duration was 6.0 ± 2.9 years. Surgical compared to purely medical treatment was associated with a significant increase in long‐term all‐cause mortality (P = 0.03 by log‐rank test, unadjusted analysis). Upon multivariable Cox regression analysis, surgical treatment, affection of mitral valve, blood cultures positive for Staphylococcus aureus, and increasing age showed trends as independent predictors of long‐term mortality.
Conclusions
Surgical treatment in patients with left‐sided native‐valve IE and isolated large vegetations without any other indication for surgery seems to be associated with excess mortality. A randomized controlled trial of surgery vs conservative treatment in this subset of patients is justified.
Introduction
Infective endocarditis (IE) is a life‐threatening disease with mortality rates of approximately 30% at 1 year.1, 2 About half of the patients with native‐valve IE are treated surgically.3 According to current guidelines, several constellations are considered a class I indication for surgery in left‐sided native‐valve IE: heart failure due to progressive destruction of affected valves and surrounding structures, locally uncontrolled infection (ie, abscess, pseudoaneurysm, fistula, or enlarging vegetations), persistent fever and positive blood cultures despite appropriate antibiotic therapy, infection caused by fungi or multiresistant organisms, and 1 or more embolic episode in the presence of large vegetations (>10 mm) despite appropriate antibiotic therapy.4, 5 The evidence in favor of a surgical approach is less clear in patients with large vegetations without embolic events. It is well known that the risk of embolism is increased with a vegetation size ≥10 mm and is particularly high for very large (≥15 mm) and mobile vegetations.2, 6 For this reason, surgery may be considered in isolated very large vegetations >15 mm according to the European Society of Cardiology guidelines and mobile vegetations in excess of 10 mm according to the American Heart Association guidelines to prevent systemic embolism.4, 5 However, the class of recommendation is weak (class IIb) and is mainly based on expert consensus (level of evidence C). To date, the value of surgery to prevent embolism in the subset of patients with large vegetations as the only reason for surgery has never been systematically studied.
We present characteristics and clinical outcome of a cohort of patients with left‐sided native‐valve IE and isolated large vegetations without any other indication for surgical treatment.
Methods
Study Population
The cohort included all patients who presented at the Department of Internal Medicine/Cardiology, Heart Center, University of Leipzig between January 2000 and July 2012 with isolated left‐sided native‐valve IE and large vegetations. All patients had to meet criteria for definite IE according to modified Duke criteria.7 Large vegetation was defined as ≥10 mm maximal extent. Patients with conventional indications for surgical treatment were excluded: presence of large vegetations (>10 mm) and 1 or more embolic episodes despite appropriate antibiotic therapy, aortic or mitral IE with severe acute regurgitation or valve obstruction (independent of clinical signs of heart failure or echocardiographic signs of poor hemodynamic tolerance), locally uncontrolled infection (abscess, false aneurysm, fistula, enlarging vegetation), persistent fever and positive blood cultures >7 to 10 days despite appropriate antibiotic therapy, and infection caused by fungi or multiresistant organisms. A flowchart of the patient selection process is shown in Figure 1. Data were extracted from medical records by 1 of the authors (A.F.) using a standard form and cross‐checked by a second investigator (S.D.), with any disagreements resolved by consensus. Clinical events were confirmed by direct contact with the treating hospital, general practitioner, and/or relatives using a standard questionnaire.
Figure 1.
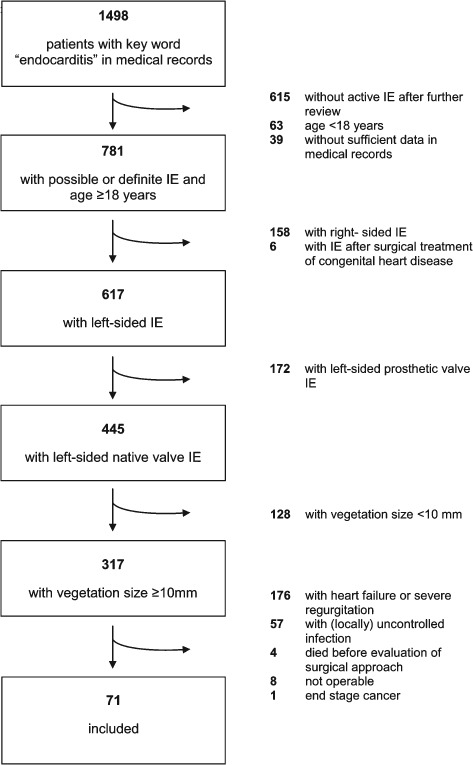
Flowchart of patient selection process. Abbreviations: IE, infective endocarditis.
Echocardiography and Computed Tomography Scanning
Transesophageal echocardiography was routinely performed on admission. Echocardiographic assessment included vegetation length and mobility as well as valvular regurgitation. Length was measured in multiple planes, and maximal length was used for the analysis. Grading of valvular regurgitation was performed according to published guidelines at the respective time points. Whole‐body computed tomography (CT) scanning was systematically performed upon admission except when contraindicated.
Definition of Clinical Events
Death was categorized into (1) definite or presumed cardiovascular and (2) noncardiovascular according to medical records and information provided by treating physicians and relatives.8 Cardiovascular death was considered present unless an unequivocal noncardiovascular cause could be established. This included any death of unknown cause. Diagnosis of clinically silent embolic events was based on imaging studies (CT scanning, magnetic resonance imaging, or sonography). Symptomatic stroke was defined as any new focal neurological deficit of central origin lasting ≥24 hours that resulted in irreversible brain damage or body impairment in association with signs of ischemia or hemorrhage in a cranial CT or magnetic resonance scan. Surgery‐related bleeding was classified as type 4 according to the Bleeding Academic Research Consortium (BARC) definitions.9 In its original context, BARC type 4 bleeding denotes bleeding related to bypass surgery; however, for the present analysis, all bleeding related to surgery was classified as BARC type 4.
Statistics
Categorical variables are expressed as number and percentage of patients. Continuous data are reported as means and standard deviation. Differences between groups (ie, surgery vs conservative treatment), were assessed by the χ2 test for categorical variables and by the Student t test for continuous data with normal distribution; otherwise, the nonparametric Wilcoxon rank‐sum test was used. A multivariable Cox regression model was applied to assess predictors of long‐term mortality. Variables assessed for inclusion in the model were age, gender, body mass index, diabetes mellitus, embolization before antibiotic therapy, symptom onset to antibiotic therapy, number of vegetations, maximum length of vegetation, mobile vegetation, affection of mitral valve, blood culture positive for Staphylococcus aureus, previous heart surgery, and surgical treatment. To reduce the number of predictor variables in light of the limited number of clinical events, only variables presenting with a P value <0.10 on univariable analysis qualified for entering the multivariable model. All qualifying variables were entered simultaneously in the multivariable model. A 2‐sided P value of <0.05 was considered statistically significant. Statistical analyses were performed with SPSS 17.0 (SPSS Inc., Chicago, IL).
Results
A total of 71 patients with left‐sided native‐valve IE and isolated large vegetations qualified for inclusion into the study cohort. Baseline characteristics are shown in Table 1. The mean age was 66 years, and the majority of patients had a heart condition predisposing for IE. As defined by the exclusion criteria, all patients were hemodynamically stable. However, in 22% of patients, neurological vigilance was compromised. Microorgansims most frequently found in blood cultures were S aureus, Viridans streptococci, and Enterococcus species (Table 1). Mean vegetation length was 17 ± 5 mm, with most patients displaying some degree of mobility. Mild or moderate regurgitation was present in the majority of patients. More than half of the patients experienced systemic embolization before the initiation of antibiotic therapy (Table 2). The time from the onset of the first IE‐related symptoms to the initiation of antibiotic treatment was almost 4 weeks. A total of 59 patients underwent surgery after a mean of 5 ± 6 days following the initiation of antibiotic treatment. Of these, 73% underwent biological valve replacement.
Table 1.
Characteristics of Study Cohort
| Overall Cohort | Patients Treated Medically | Patients Treated Surgically | P | |
|---|---|---|---|---|
| Baseline patient features | ||||
| Age, y | 66.2 ± 13.2 | 63.4 ± 14.8 | 66.7 ± 12.9 | 0.42 |
| Male sex | 45/71 (63%) | 8/12 (67%) | 37/59 (63%) | 0.80 |
| Hypertension | 48/71 (68%) | 8/12 (67%) | 40/59 (68%) | 0.94 |
| Diabetes mellitus | 26/71 (37%) | 3/12 (25%) | 23/59 (39%) | 0.36 |
| Diabetes subtype | 0.11 | |||
| Diet only | 7/26 (27%) | 0/7 | 7/7 (100%) | |
| Oral medication | 4/26 (15%) | 2/4 (50%) | 2/4 (50%) | |
| Insulin requiring | 15/26 (58%) | 1/15 (7%) | 14/15 (93%) | |
| Body mass index, kg/m2 | 27.1 ± 6.2 | 27.2 ± 7.4 | 27.1 ± 5.9 | 0.96 |
| Coronary artery disease | 21/71 (30%) | 1/12 (8%) | 20/59 (34%) | 0.08 |
| Previous endocarditis | 1/71 (1%) | 0/12 | 1/59 (2%) | 0.65 |
| History of fever | 50/71 (70%) | 9/12 (75%) | 41/59 (70%) | 0.70 |
| Predisposing heart condition | 53/71 (75%) | 8/12 (67%) | 47/59 (80%) | 0.33 |
| Injection drug use | 2/71 (3%) | 1/12 (8%) | 1/59 (2%) | 0.21 |
| Previous heart surgery | 5/71 (7%) | 0/12 | 5/59 (9%) | 0.30 |
| Logistic EuroSCOREa | 16.9 ± 12.4 | 11.4 ± 7.0 | 18.1 ± 13.0 | 0.02 |
| Glomerular filtration rate (mL/min/1.73 m2)b | 82 ± 46 | 114 ± 48 | 76 ± 43 | 0.01 |
| Hemoglobin, mmol/L | 6.6 ± 1.2 | 6.7 ± 0.8 | 6.5 ± 1.3 | 0.65 |
| White blood cell count, Gpt/L | 11.4 ± 6.3 | 7.1 ± 2.7 | 12.2 ± 6.5 | 0.009 |
| C‐reactive protein, mg/L | 84 ± 87 | 30 ± 17 | 99 ± 92 | <0.001 |
| Medication on admission | ||||
| Antiplatelet agent | 14/71 (20%) | 3/12 (25%) | 11/59 (19%) | 0.61 |
| Oral anticoagulant | 2/71 (3%) | 0/12 | 2/59 (3%) | 0.52 |
| Statin | 11/71 (16%) | 2/12 (17%) | 9/59 (15%) | 0.90 |
| β‐Blocker | 21/71 (30%) | 5/12 (42%) | 16/59 (27%) | 0.31 |
| Inhibitor of renin angiotensin system | 32/71 (45%) | 6/12 (50%) | 26/59 (44%) | 0.71 |
| Mental status on admission | 0.12 | |||
| Alert | 55/71 (78%) | 12/12 (100%) | 43/59 (73%) | |
| Lethargic, somnolent or disoriented | 13/71 (18%) | 0 | 13/59 (22%) | |
| Comatose | 3/71 (4%) | 0 | 3/59 (5%) | |
| Chronic illnessc | 44/71 (62%) | 5/12 (42%) | 39/59 (66%) | 0.11 |
| Charlson Comorbidity Indexd | 1.4 ± 1.3 | 1.0 ± 1.4 | 1.4 ± 1.3 | 0.32 |
| Symptom onset to first medical contact, d | 14 ± 35 | 9 ± 10 | 15 ± 38 | 0.56 |
| Transfer from another medical facility | 66/71 (93%) | 10/12 (83%) | 56/59 (95%) | 0.15 |
| Microorganisms and antibiotic therapy | ||||
| Microorganisms in blood cultures | 0.19 | |||
| Staphylococcus aureus | 19/69 (28%) | 1/12 (8%) | 18/57 (32%) | |
| Sensitive | 17/69 (25%) | 0 | 17/57 (30%) | |
| Methicillin‐resistant | 2/69 (3%) | 1/12 (8%) | 1/57 (2%) | |
| Viridans streptococci | 11/69 (16%) | 4/12 (33%) | 7/57 (12%) | |
| Enterococcus species | 11/69 (16%) | 4/12 (33%) | 7/57 (12%) | |
| Streptococcus bovis | 8/69 (11%) | 1/12 (8%) | 7/57 (12%) | |
| Coagulase‐negative staphylococci | 5/69 (7%) | 1/12 (8%) | 4/57 (7%) | |
| Mixed infection | 2/69 (3%) | 0 | 2/57 (4%) | |
| Other | 7/69 (10%) | 1/12 (8%) | 6/57 (11%) | |
| Blood culture negative | 6/69 (9%) | 0 | 6/57 (11%) | |
| Symptom onset to initiation of antibiotic therapy, d | 25 ± 44 | 22 ± 16 | 26 ± 48 | 0.76 |
| Echocardiography and computed tomography | ||||
| Affected valve | 0.34 | |||
| Mitral | 43/71 (60%) | 8/12 (67%) | 35/59 (59%) | |
| Aortic | 19/71 (27%) | 4/12 (33%) | 15/59 (25%) | |
| Both | 9/71 (13%) | 0/12 | 9/59 (15%) | |
| No. of vegetations | 1.4 ± 0.6 | 1.6 ± 0.7 | 1.4 ± 0.5 | 0.27 |
| Maximum length of vegetation(s), mm | 17 ± 5 | 14 ± 4 | 18 ± 6 | 0.03 |
| Mobile vegetation | 64/68 (94%) | 11/12 (92%) | 53/56 (95%) | 0.69 |
| Valvular regurgitation | 0.80 | |||
| None | 4/71 (6%) | 1/12 (8%) | 3/56 (5%) | |
| Mild | 32/67 (48%) | 6/12 (50%) | 26/56 (44%) | |
| Moderate | 35/67 (52%) | 5/12 (42%) | 30/56 (51%) | |
| Left ventricular ejection fraction, % | 62 ± 8 | 65 ± 8 | 61 ± 8 | 0.17 |
| Computed tomography on admission | 66/71 (93%) | 8/12 (67%) | 58/59 (98%) | <0.001 |
| Surgery | ||||
| Type of surgery | ||||
| Valve replacement (biological) | NA | NA | 43/59 (73%) | NA |
| Valve replacement (mechanical) | NA | NA | 9/59 (15%) | NA |
| Reconstruction | NA | NA | 5/59 (8%) | NA |
| Both biological and mechanical valve replacement | NA | NA | 1/59 (2%) | NA |
| Both valve replacement and reconstruction | NA | NA | 1/59 (2%) | NA |
| Time between symptom onset and surgery, d | NA | NA | 31 ± 48 | NA |
| Time between first medical contact and surgery, d | NA | NA | 16 ± 31 | NA |
Abbreviations: Gpt, giga (109) particles; NA, not applicable.
Continuous data are presented as means and standard deviations.
The logistic EuroSCORE (European System for Cardiac Operative Risk Evaluation) is a scoring system used to predict mortality from cardiac surgery with increasing values indicating a higher risk.17
Estimated according to modification of diet in renal disease study.18
Defined as the presence of chronic comorbidities such as diabetes mellitus, cancer, immunosuppression, hemodialysis, chronic obstructive pulmonary disease, and cirrhosis.
The Charlson Comorbidity Index is a scoring system to predict mortality with increasing values indicating a higher risk.19
Table 2.
Clinical Events
| Overall Cohort | Patients Treated Medically | Patients Treated Surgically | P | |
|---|---|---|---|---|
| Pre‐ and intrahospital events | ||||
| Any systemic embolization before initiation of antibiotic therapy | 41/66 (62%) | 5/8 (63%) | 36/58 (62%) | 0.98 |
| Cerebral | 23/64 (36%) | 5/8 (63%) | 18/56 (23%) | 0.09 |
| Silent | 7/64 (11%) | 2/8 (25%) | 5/56 (9%) | 0.17 |
| Symptomatic (stroke) | 16/64 (25%) | 3/8 (38%) | 13/56 (23%) | 0.38 |
| Splenic | 29/63 (46%) | 1/7 (14%) | 28/56 (50%) | 0.07 |
| Renal | 15/63 (24%) | 1/7 (14%) | 14/56 (25%) | 0.53 |
| Any clinically symptomatic systemic embolization after initiation of antibiotic therapy | 5/71 (7%) | 0/12 (0%) | 5/59 (9%) | 0.30 |
| Cerebral symptomatic (stroke) | 3/71 (4%) | 0 | 3/59 (5%) | 0.43 |
| Splenic | 2/71 (3%) | 0 | 2/59 (3%) | 0.52 |
| Renal | 1/71 (1%) | 0 | 1/59 (2%) | 0.65 |
| Death | 26/71 (37%) | 1/12 (8%) | 25/59 (42%) | 0.03 |
| Cardiovascular death | 13/71 (18%) | 0/12 | 13/59 (22%) | 0.07 |
| Renal failure requiring replacement therapy | 18/69 (26%) | 0 | 18/57 (32%) | 0.02 |
| BARC type 4 bleeding | NA | NA | 6/57 (11%) | NA |
| Events at long‐term follow‐up | ||||
| Death | 37/71 (52%) | 2/12 (17%) | 35/59 (59%) | 0.007 |
| Cardiovascular death | 19/71 (28%) | 0/12 | 19/59 (32%) | 0.02 |
| Recurrent endocarditis | 8/64 (13%) | 2/12 (17%) | 6/52 (12%) | 0.63 |
Abbreviations: BARC, Bleeding Academic Research Consortium; NA, not applicable.
Intrahospital and Long‐term Clinical Events
Clinical events are displayed in Table 2. A total of 26 patients (37%) died during hospitalization. In‐hospital death occurred significantly more often in patients undergoing surgical treatment compared to patients managed conservatively (unadjusted analysis, P = 0.03). After a mean follow‐up of 6.0 ± 2.9 years, overall mortality was 52%. In unadjusted analysis, the difference in short‐term mortality between surgery and medical therapy alone remained statistically significant at long‐term follow‐up (P = 0.03 by log‐rank test). Among the 45 patients who survived the initial hospital stay, long‐term mortality was not significantly different between patients who had been treated medically and surgically (medical therapy 1/11 [9%], surgical treatment 10/34 [29%], P = 0.73 by long‐rank test).
Predictors of Long‐term Mortality
Upon multivariable Cox regression analysis, several characteristics showed trends as independent predictors of long‐term mortality, albeit only on the verge of statistical significance: surgical treatment, affection of mitral valve, blood cultures positive for S aureus, and increasing age (Table 3). The hazard associated with surgery was particularly high (hazard ratio: 3.9, 95% CI: 0.9‐16.6; Table 3 and Figure 2).
Table 3.
Prediction of Long‐term Mortality on Multivariable Cox Regression Analysisa
| Variable | Coefficient B | Hazard Ratio (95% CI) | P |
|---|---|---|---|
| Surgical treatment | 1.4 | 3.9 (0.9‐16.7) | 0.06 |
| Female gender | 0.7 | 2.0 (0.8‐4.7) | 0.12 |
| Blood cultures positive for Staphylococcus aureus | 0.6 | 1.8 (0.9‐3.7) | 0.09 |
| Age | 0.3 | 1.03 (1.00‐1.07) | 0.08 |
| Diabetes | 0.4 | 1.4 (0.7‐2.9) | 0.34 |
| Affection of mitral valve | 0.9 | 2.6 (0.9‐7.5) | 0.09 |
Abbreviations: CI, confidence interval.
All variables presenting with a P value <0.10 on univariable analysis are displayed.
Figure 2.
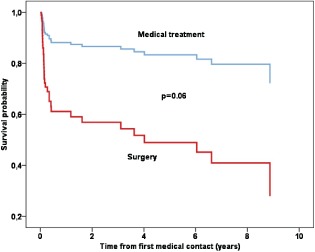
Survival plot from multivariate Cox regression analysis estimating the cumulative probability of long‐term death according to medically and surgically managed patients.
Discussion
The present study is the first to report on clinical outcome exclusively in patients with left‐sided native‐valve IE and isolated large vegetations without any other indication for surgery. There is considerable uncertainty with regard to the optimal management in this specific group of patients. The major findings are: (1) both intrahospital and long‐term mortality are high in this subset of patients; and (2) as compared to a pure antimicrobial approach, surgical treatment seems to be associated with an increase in the risk of death mainly attributed to significant intrahospital mortality.
Evidence for surgical treatment (indications and timing) in IE is characterized by a distinct lack of randomized data. In fact, only recently has the first small randomized controlled trial been published (pertaining to the optimal timing of surgical intervention in patients with left‐sided native‐valve IE with a clear indication for surgery due to both severe valve dysfunction and large vegetations—a distinctly different cohort from the cohort described in the present report).10 Surgery is currently recommended in specific situations in left‐sided native‐valve IE such as destruction of affected valves and surrounding structures with or without clinical signs of heart failure, locally uncontrolled infection, nonresponse to appropriate antibiotic therapy, difficult‐to‐treat microorganisms, and in patients with large vegetations (>10 mm) following embolic episodes after initiation of appropriate antibiotic therapy.4, 5 These recommendations are derived from nonrandomized studies or based on expert opinion (levels of evidence B and C).5
An area of particular uncertainty pertains to patients with isolated large vegetations without embolic events after the start of appropriate antimicrobial therapy. Current guidelines state that surgery may be considered in these patients.4, 5 However, it is acknowledged that this recommendation is mainly based on expert consensus, and the decision to operate must be carefully individualized considering the risks of a surgical approach. The published literature is of little help in the decision‐making process in favor or against a surgical approach in these patients. To date, all previous registries on the value of surgical therapy in left‐sided native‐valve IE mainly reported on patients with conventional surgical indications, and if specifically mentioned at all, only a few patients with isolated large vegetations were included. For example, the Euro Heart Survey reported that only 4 out of 62 surgical patients with native‐valve IE were treated surgically on the basis of vegetation size alone.3
The rationale for a surgical intervention in the subset of patients with left‐sided native‐valve IE and isolated large vegetations is the prevention of systemic embolism.5 Systemic embolism is frequent in these patients and occurred in more than 60% of patients in the present cohort. The brain and spleen were the organs most frequently affected. It is well known that the risk of embolism is increased with a vegetation size ≥10 mm and is particularly high for very large (>15 mm) and mobile vegetations.2, 6 Of note, in the present analysis, vegetation length was not predictive of short‐ or long‐term mortality, likely due to the fact that the study exclusively included patients with large vegetations. Progression from large to very large vegetation size seems to be of only minor importance for outcome. Most embolic events occur before the diagnosis of IE is made, and the risk falls considerably once antimicrobial therapy is initiated.2, 6, 11, 12 This aspect has important implications for surgical intervention, because the decision to operate or not must usually be made after the start of antibiotic therapy. Although surgery seems to be effective in reducing the risk of embolism, the risks of surgery have to be balanced against this benefit.10 In the present cohort, intrahospital mortality was 42% among surgical patients. On the other hand, the overall rate of clinically symptomatic embolic events after the initiation of antibiotic therapy was only 6% (with all events occurring among surgical patients). Therefore, the risks of surgery outweighed any possible benefit in the prevention of embolism. The difference in outcome between medical and surgical management was mainly driven by high intrahospital mortality among surgical patients. The observed mortality rate is higher than in previous registries of patients with IE. This is most likely explained by the fact that patients were on average over 10 years older than in previous reports.3, 13, 14, 15 It is unclear whether the risk‐benefit ratio would shift toward surgical therapy in younger patients.
Limitations
The major limitation of the present analysis is its observational cohort study character, which leaves the possibility of scientific bias. This is especially relevant with regard to the choice of a surgical or conservative approach by the attending physicians. The exact reasons why 1 treatment strategy was favored over the other could not be determined due to the design. However, it is possible that clinical judgment (possibly incorporating patient factors not adequately reflected in baseline characteristics and the multivariable regression model of the present study) led the attending physicians to send patients preferentially for surgery deemed at too high a risk for a conservative treatment course. This might have introduced a selection bias where higher‐risk patients underwent surgery, whereas in seemingly suitable low‐risk patients a purely antimicrobial approach was attempted. Our institution is a tertiary care referral center with a large cardiac surgery unit, which might have led to over‐representation of patients considered to be candidates for surgery. The present cohort might therefore not reflect the overall population of patients with left‐sided native‐valve IE and large vegetations.
Survivor treatment selection bias is another potential source of error whereby patients who live longer might be more likely to undergo surgery than those who die early during hospitalization and are therefore no longer available for the choice between medical therapy and surgical intervention. In principle, this could introduce a bias in favor of surgical treatment.16 However, in the context of the present analysis, only 1 patient in the medical management died during the initial hospital stay 34 days after admission. Survivor treatment selection bias can therefore be excluded.
Most patients underwent routine whole body CT on admission. However, in the subsequent course of disease, repeat CT was only performed if there were clinical signs of suspected new embolism (ie, new neurological deficit). Therefore, although the rate of embolism on admission is highly accurate, silent embolism at later stages may have been underdiagnosed.
Finally, it must be reemphasized that the present cohort differs significantly from all previously published surgical registries in that only patients with left‐sided native‐valve IE with isolated large vegetations were included. The results should therefore not be extrapolated to other indications of surgery in IE where the benefits of surgery over medical therapy are well established.
Conclusion
Surgical treatment in patients with left‐sided native‐valve IE and isolated large vegetations without any other indication for surgery seems to be associated with excess mortality. We therefore believe a randomized controlled trial of surgery vs conservative treatment in this subset of patients is justified.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Cabell CH, Jollis JG, Peterson GE, et al. Changing patient characteristics and the effect on mortality in endocarditis. Arch Intern Med. 2002;162:90–94. [DOI] [PubMed] [Google Scholar]
- 2. Thuny F, Di Salvo G, Belliard O, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation. 2005;112:69–75. [DOI] [PubMed] [Google Scholar]
- 3. Tornos P, Iung B, Permanyer‐Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart. 2005;91:571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–e231. [DOI] [PubMed] [Google Scholar]
- 5. Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009;30:2369–2413. [DOI] [PubMed] [Google Scholar]
- 6. Vilacosta I, Graupner C, San Roman JA, et al. Risk of embolization after institution of antibiotic therapy for infective endocarditis. J Am Coll Cardiol. 2002;39:1489–1495. [DOI] [PubMed] [Google Scholar]
- 7. Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. [DOI] [PubMed] [Google Scholar]
- 8. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 9. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 10. Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med. 2012;366:2466–2473. [DOI] [PubMed] [Google Scholar]
- 11. Dickerman SA, Abrutyn E, Barsic B, et al. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: an analysis from the ICE Prospective Cohort Study (ICE‐PCS). Am Heart J. 2007;154:1086–1094. [DOI] [PubMed] [Google Scholar]
- 12. Fabri J Jr, Issa VS, Pomerantzeff PM, et al. Time‐related distribution, risk factors and prognostic influence of embolism in patients with left‐sided infective endocarditis. Int J Cardiol. 2006;110:334–339. [DOI] [PubMed] [Google Scholar]
- 13. Lalani T, Cabell CH, Benjamin DK, et al. Analysis of the impact of early surgery on in‐hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment‐selection bias. Circulation. 2010;121:1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tleyjeh IM, Ghomrawi HM, Steckelberg JM, et al. The impact of valve surgery on 6‐month mortality in left‐sided infective endocarditis. Circulation. 2007;115:1721–1728. [DOI] [PubMed] [Google Scholar]
- 15. Vikram HR, Buenconsejo J, Hasbun R, et al. Impact of valve surgery on 6‐month mortality in adults with complicated, left‐sided native valve endocarditis: a propensity analysis. JAMA. 2003;290:3207–3214. [DOI] [PubMed] [Google Scholar]
- 16. Sy RW, Bannon PG, Bayfield MS, et al. Survivor treatment selection bias and outcomes research: a case study of surgery in infective endocarditis. Circ Cardiovasc Qual Outcomes. 2009;2:469–474. [DOI] [PubMed] [Google Scholar]
- 17. Roques F, Michel P, Goldstone AR, Nashef SA. The logistic EuroSCORE. Eur Heart J 2003;24:881–882. [DOI] [PubMed] [Google Scholar]
- 18. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1–S266. [PubMed] [Google Scholar]
- 19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]


