Abstract
Background:
In patients with chronic heart failure, physical evaluation and clinical judgment may be inadequate for prognostic stratification.
Hypothesis:
Information obtained with simple bedside tests would be helpful in patient management.
Methods:
We report on 142 outpatients with systolic heart failure seen at our heart failure unit from 2007 to 2010 (ages 69.4 ± 8.9 years; ejection fraction [EF] 30.6 ± 6.1%; 43% with implanted defibrillators and/or resynchronization devices). At their first visit, we assessed levels of brain natriuretic peptide (BNP) (pg/mL), evaluated transthoracic conductance (TFC) (1/kΩ) by transthoracic bioimpedance, and performed echocardiography.
Results:
Four‐year mortality was 21.2%. At multivariate analysis, surviving and deceased subjects did not differ regarding New York Heart Association, age, gender, heart failure etiology, or EF at index visit. Patients who died had higher BNP and TFC (BNP = 884 ± 119 pg/mL vs 334 ± 110 pg/mL; TFC = 50 ± 8/kΩ vs 37 ± 7/kΩ, both P < 0.001]. Patients with BNP < 450 pg/mL and TFC < 40/kΩ had a 2.1% 4‐year mortality, compared to 46.5% mortality of patients having BNP ≥ 450 pg/mL and TFC ≥ 40/kΩ. BNP ≥ 450 pg/mL and TFC ≥ 40/kΩ showed high sensitivity (91%) and specificity (88%)in identifying patients who died at follow‐up.
Conclusions:
The combined use of BNP and impedance cardiography during the first assessment of a patient in a heart failure unit identified those carrying a worse medium‐term prognosis. This approach could help the subsequent management of patients, allowing better clinical and therapeutic strategies.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
In patients with chronic heart failure, symptoms and signs of congestion affect quality of life and survival1, 2 and are targets for therapy, because their identification and treatment may prevent hospitalizations, slow the progression of the disease, and possibly affect prognosis.3, 4, 5 Available data support the concept that congestion results in hospitalizations more than low cardiac output.6 However, physical signs often overlook pulmonary congestion,7 and physicians do not treat heart failure aggressively enough. Data from the Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure (COMPASS) trial8 indicate that ambulatory filling pressures are far higher than clinically suspected, and they begin to increase over 3 weeks before heart failure events. Thus, the challenge is to intensify not only acute management (with results that may be disappointing as demonstrated by the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness [ESCAPE] trial9) but to improve outpatients surveillance to allow early intervention and reduce rehospitalizations.10
Natriuretic peptides are secreted from the cardiac chambers during hemodynamic stress and increased wall tension, as a result of increased volume and/or pressure overload; they have been proven useful in the diagnosis of acute heart failure.11, 12, 13 Moreover, recent data14, 15, 16, 17, 18, 19 and a meta‐analysis20 suggest that its use in outpatient management may reduce all‐cause mortality.
Another tool for detecting pulmonary congestion21, 22, 23, 24 and predict heart failure readmissions25, 26, 27 may be the assessment of thoracic conductance by intra‐ or transthoracic impedance cardiography. It has been proposed that the combined use of brain natriuretic peptide (BNP) levels and transthoracic conductance values may be superior to either data alone in detecting abnormal filling pressures and signs of congestion, both in stable patients and in the acute setting.23, 24, 28
In this report, we present our experience on a nonselected population of outpatients, referred to our congestive heart failure unit, in whom we obtained BNP levels and thoracic conductance values at the entry visit. The aim of the study was to assess if the combined information could identify high‐risk patients and to find target values of BNP and transthoracic bioimpedance that might be used for therapy adjustments.
Methods
Between January 2007 and June 2010, 205 consecutive patients with systolic heart failure seen for the first time at our congestive heart failure unit were screened. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected by the approval by our ethical committee. Informed consent was obtained from each patient.
The majority of patients (125, 61%) had a recent hospitalization, and 80 patients (39%) were referred for advice by their general practitioners for worsening clinical status. The only exclusion criteria for the study were the impossibility to obtain simultaneously a meaningful BNP measurement, a reliable impedance cardiography recording, and echocardiographic examination of diastolic function and mitral regurgitation. Therefore, we excluded patients whose creatinine clearance was below 20 mL/min, patients whose body mas index was below 20 and above 40, and patients with severe chronic obstructive pulmonary disease (COPD), pleural or pericardial effusion, prosthetic mitral valves, and atrial fibrillation. Overall, 63 patients (31%) could not be included, 20 with isolated severe renal failure, 14 because of a prosthetic mitral valve, and 29 because of a combination of causes.
When they were first seen in our center at the index visit, patients underwent clinical examination, electrocardiography (ECG), echocardiography, BNP dosage, and transthoracic bioimpedance. Table 1 shows the clinical characteristics of the population. All patients had systolic heart failure, mainly of ischemic origin (76%), and 43% already had an implantable cardioverter‐defibrillator (ICD) or cardiac resynchronization (CRT or CRT‐D) device. On the other hand, medical treatment was not optimized, because for some of them the diagnosis of heart failure was recent.
Table 1.
Patients' General Characteristics
| Number of patients | 142 |
|---|---|
| Age, y | 69.4 ± 8.9 |
| Gender (males/females) | 94/48 |
| Ischemic/nonischemic cardiomyopathy | 76%/24% |
| NYHA class I | 19% |
| NYHA class II | 33% |
| NYHA class III | 40% |
| NYHA class IV | 8% |
| SBP/DBP (mm Hg) | 114 ± 11/70 ± 9 |
| HR (beats/min) | 71 ± 10 |
| Treatment | |
| ACE inhibitors or ARB | 95% |
| Aldosterone antagonists | 35% |
| β‐Blockers | 71% |
| Diuretics | 80% |
| Nitrates | 45% |
| Digoxin | 6% |
| CRT/CRT + ICD | 43% |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin‐receptor blocker; CRT, cardiac resynchronization therapy (CRT); DPB, diastolic blood pressure; HR, heart rate; ICD, implantable cardioverter‐defibrillator; NYHA, New York Heart Association; SBP, systolic blood pressure.
Transthoracic Bioimpedance
Transthoracic bioimpedance was performed by the staff nurse soon after the ECG using commercial equipment (Niccomo; Medis, Ilmenau, Germany). Four couples of silver‐silver chloride sensors were placed, 2 at the base of the neck under each ear and 2 on either side of the chest in the midaxillary line at the level of the xiphoid. A cable with 8 bioimpedance lead wires was then attached to the sensor sites. An integrated oscillometric blood pressure cuff was connected to the patient's arm. The recording was performed for 10 minutes, and the average transthoracic bioimpedance status report was stored for analysis. Variables evaluated in this study have been described previously.24, 28 For the present study dealing with pulmonary congestion, we took into account only thoracic conductance (the inverse of impedance measurement as representative of total fluid volume in the chest [thoracic fluid content TFC = 1/Z0*1000 = 1/kΩ]25).
Neuropeptides
Plasma BNP levels were assessed by a point‐of care system based on a fluorescence immunoassay (Triage BNP Test; Biosite Inc., San Diego, CA18).
Transthoracic Echocardiography
Echocardiography, including Doppler and tissue Doppler imaging (TDI) analysis, was performed immediately before the clinical examination, according to recommendations of the American Society of Echocardiography.29 Left ventricular ejection fraction and estimated systolic pulmonary artery pressure were calculated routinely. Pulsed Doppler was used to record transmitral and pulmonary venous flow in the apical 4‐chamber view. Tissue Doppler velocities were acquired at the septal and lateral annular sites. Mitral inflow measurements included peak early (E) and peak late (A) velocities, E/A ratio, and deceleration time of E velocity. For pulmonary venous flow, measurements included peak systolic, diastolic, atrial reversal (Ar) velocities, systolic filling fraction, and duration of Ar. The early diastolic (E′) velocity by TDI at the septal and lateral annular sites was measured; the E/E′ ratio was calculated from the average of septal and lateral E′, because this approach has been shown to yield optimum accuracy in patients with regional wall motion abnormalities.30, 31 We present here both E/E′ values and a multiparametric evaluation of diastolic function (estimated with filling pressures) on a 4‐point scale, from normal (grade 1) to restrictive (grade 4). The severity of mitral regurgitation was graded semiquantitatively on a 4‐point scale, from minimal (grade 1) to severe (grade 4), using color‐flow Doppler images of the apical 4‐chamber view.32, 33
Follow‐up
When starting this study, we decided to use BNP levels as an index of congestion and adjust the treatment according to data available at that time.14, 15 Patients were then referred to their primary care physician, but were seen at the heart failure unit every 6 months for control and for further treatment adjustment and clinical tests if needed. The end point considered was all‐cause mortality recorded at each visit. The average follow‐up was 38 months (range, 12–52 months), and we considered mortality at 1 and 4 years.
Statistical Analysis
Descriptive variables are summarized as mean ± 1 standard deviation. Correlation among variables was determined through linear regression with least squares approach; differences between patient groups were compared using the unpaired t test. Variables resulting significantly different in determining survival at a preliminary univariate analysis were subsequently analyzed using a multivariate stepwise regression analysis (Origin version 7.0; MicroCal, Loma Linda, CA). Cutoff values of variables that differed significantly at multivariate analyses were used to build receiver operating characteristic (ROC) curves for prediction of mortality at 1 and 4 years (MedCalc version 11.6.1; Broekstraat, Mariakerke, Belgium). Kaplan‐Meier survival curves were built by grouping patients in accordance to the values of BNP and TFC at the index visit that resulted best predictors of mortality. A P < 0.05 was used to define statistical significance.
Results
Table 2 describes the population in terms of echocardiographic, humoral, and impedance cardiography data at their index visit. At baseline, confirming previous evidence obtained in a more selected population,28 a significant correlation was present between BNP values, TFC, and E/E′ (taken as a numerical index of restrictive filling pattern). Not surprisingly, also patients with 3rd and 4th degree mitral regurgitation showed higher BNP levels and TFC values (data not shown).
Table 2.
Echocardiographic, Humoral, and Impedance Cardiography Data at the First Visit in All Patients (n = 142)
| Echocardiography | |
|---|---|
| Left ventricular EF (%) | 30.6 ± 6.1 |
| E/E′ | 13.6 ± 6.6 |
| Pulmonary pressure (mm Hg) | 40.1 ± 12.9 |
| Left ventricular end‐diastolic volume (mL) | 188.9 ± 44.7 |
| Restrictive flow pattern | 30 (20.4 %) |
| FMR grade 3 and 4 | 28 (19.9%) |
| Renal function—blood tests | |
| eGFR (mL/min) | 55.6 ± 6.5 |
| Hemoglobin (g/dL) | 13.1 ± 2.9 |
| BNP (pg/mL) | 420.5 ± 346.2 |
| Impedance cardiography | |
| TFC (1/kΩ) | 39.2 ± 8.4 |
Abbreviations: BNP, brain natriuretic peptide; E, peak early; E′, early diastolic velocity; EF, ejection fraction; eGFR, estimated glomerular filtration rate; FMR, functional mitral regurgitation; TFC, thoracic fluid content.
Short‐ and Long‐term Mortality
Global mortality in the whole population of 142 patients was 4.9% at 1 year (7 deaths), and 21.2% at 4 years (30 deaths). Table 3 shows that at univariate analysis, both in the short‐term and in the long‐term follow‐up, deceased patients had lower hemoglobin levels, worse renal function, higher pulmonary pressure, worse diastolic function, more severe functional mitral regurgitation, and higher BNP levels and TFC values. However, when multivariate analysis was performed, the only independent predictors of mortality in the population study at 1 and 4 years (P < 0.05) were the degree of mitral regurgitation, BNP levels, and TFC values.
Table 3.
Echocardiographic, Humoral, and Impedance Cardiography Data at the First Visit According to Short‐ and Long‐term Outcome
| 1‐Year Follow‐up | 4‐Year Follow‐up | Analysis | ||||
|---|---|---|---|---|---|---|
| Alive | Deceased | Alive | Deceased | Univariate | Multivariate | |
| Number of patients | 135 | 7 | 112 | 30 | ||
| Age (y) | 67.9 ± 9.6 | 73.0 ± 6.9 | 69.1 ± 9.0 | 72.1 ± 6.7 | ||
| NYHA class | 2.50 ± 0.52 | 2.70 ± 0.75 | 2.49 ± 0.53 | 2.74 ± 0.65 | ||
| Echocardiography | ||||||
| Left ventricular EF (%) | 30.9 ± 6.2 | 28.6 ± 6.7 | 31.0 ± 5.9 | 28.6 ± 6.4 | ||
| E/E′ | 13.3 ± 6.7 | 19.6 ± 6.6 | 12.7 ± 6.3 | 17.9 ± 5.9 | P < 0.05 | |
| Pulmonary pressure (mm Hg) | 40.1 ± 12.1 | 49.4 ± 14.0 | 37.2 ± 11.7 | 46.5 ± 13.7 | P < 0.05 | |
| Left ventricular end‐diastolic volume (mL) | 186.8 ± 44.1 | 182.9 ± 65.6 | 186.8 ± 44.1 | 182.9 ± 65.6 | ||
| Restrictive flow pattern | 26 (19.3%) | 4 (57.1%) | 16 (14.3%) | 14 (46.6%) | P < 0.05 | |
| FMR grade 3 and 4 | 23 (17.7%) | 5 (71.4%) | 13 (11.6%) | 15 (50.0%) | P < 0.05 | P < 0.05 |
| Renal function—blood tests | ||||||
| eGFR (mL/min) | 58.3 ± 5.5 | 53.9 ± 7.7 | 58.1 ± 6.3 | 52.5 ± 9.4 | P < 0.05 | |
| Hemoglobin (g/dL) | 13.8 ± 2.1 | 12.9 ± 2.3 | 13.7 ± 1.9 | 12.3 ± 2.2 | P < 0.05 | |
| BNP (pg/mL) | 349.5 ± 234.1 | 923.5 ± 397.3 | 324.2 ± 211.0 | 837.4 ± 368.7 | P < 0.05 | P < 0.05 |
| Impedance cardiography | ||||||
| TFC (1/kΩ) | 38.0 ± 7.5 | 50.7 ± 8.2 | 37.2 ± 7.1 | 49.6 ± 7.4 | P < 0.05 | P < 0.05 |
Abbreviations: BNP, brain natriuretic peptide; E, peak early; E′, early diastolic velocity; EF, ejection fraction; FMR, functional mitral regurgitation; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; TFC, thoracic fluid content.
Predictive Value of BNP, TFC, and Mitral Regurgitation
From the analysis of ROC curves, the association of BNP ≥450 pg/mL and TFC ≥40/kΩ at the index visit was found to be the strongest indicator of patient death at 1‐year follow‐up, with sensitivity 89%, specificity 86%, positive predictive value 88%, and negative predictive value 92% (Figure 1). This combination retained its powerful predictive power also for mortality at 4 years. The addition of moderate and severe mitral regurgitation to this model and its association with BNP ≥450 pg/mL or TFC ≥40/kΩ did not add further statistical significance; therefore, we did not use it for the subsequent Kaplan‐Meyer analysis.
Figure 1.
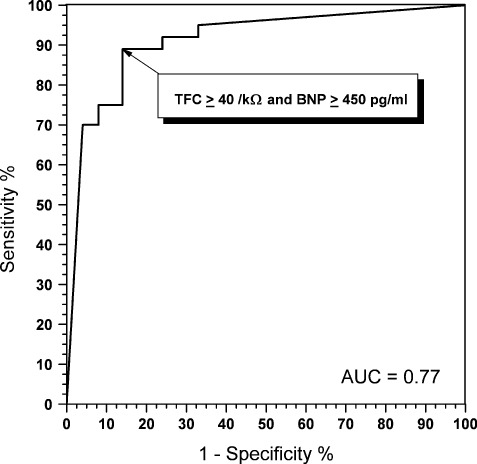
Receiver operating characteristic curve showing the predictive value for 1‐year mortality of the association of brain natriuretic peptide (BNP) ≥450 pg/mL and thoracic fluid content (TFC) ≥40/kΩ. Abbreviations: AUC, area under the curve.
Survival curves (Kaplan‐Meier analysis, Figure 2) were built keeping in mind 4 subgroups of patients, classified as follows by the threshold values of BNP of 450 pg/mL and TFC of 40/kΩ; group 1, BNP <450 pg/mL and TFC ≤40/kΩ; group 2, BNP ≥450 pg/mL and TFC <40/kΩ; group 3, BNP <450 pg/mL and TFC ≥40/kΩ; and group 4, BNP ≥450 pg/mL and TFC ≥40/kΩ. The worst prognosis, when compared to each of the other groups of patients, was found in group 4 (18.9% 1‐year mortality, 46.5% 4‐year mortality, P < 0.001). Patients presenting with only 1 abnormal value (either BNP or TFC) had a similar survival probability (P = 0.21), which was intermediate between the 2 extreme groups (P < 0.001). Of note, no deaths were observed at 1 year in the group with BNP <450 pg/mL and TFC <40/kΩ (group 1), and 4‐year mortality in this group was 2.1%.
Figure 2.
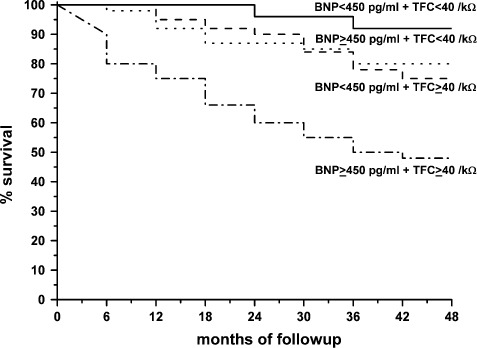
Kaplan‐Meier survival curves in patients divided into 4 groups according to brain natriuretic peptide (BNP) and thoracic fluid content (TFC) values at index visit. Group 1 = BNP < 450 pg/mL and TFC ≤ 40/ kΩ; group 2 = BNP ≥ 450 pg/mL and TFC < 40/kΩ; group 3 = BNP < 450 pg/mL and TFC ≥ 40/kΩ; group 4 = BNP ≥ 450 pg/mL and TFC ≥ 40/kΩ. Comparisons were as follow: groups 2 and 3 vs group 1, P = 0.0028; group 2 and 3 vs group 4, P = 0.0136; group 1 vs group 4, P < 0.0001.
Discussion
We started this investigation because due to the increasing number of patients with chronic heart failure in need of periodic clinical evaluation, the adequacy of such evaluation must be improved to optimize the overall management of their disease.1 Clinical evaluation alone, as offered by standard outpatient visits may not identify early signs of hemodynamic congestion, and symptoms are often not specific.7 Thus, an operator‐independent, easily accessible, inexpensive assessment of the hemodynamic status would be helpful in confirming or modifying the clinical impression.
Echocardiographic examination such as the examination we performed for research purposes may provide a comprehensive noninvasive evaluation of hemodynamic status. However, echocardiography requires time, expensive equipment, and skilled operators; at least in our country this test is unattainable during a routine outpatient visit. Interestingly, in our unselected population, diastolic flow pattern and E/E′, despite their relationship with BNP levels and TFC values and their predictive value at univariate analysis, were not related to mortality. When the number of observations is small, multivariate analysis may sometimes overlook differences identified by larger studies. However, it must be noted that many patients of this study had large spherical left ventricles and carried ventricular resynchronization devices; thus, mitral annulus motion could have been reduced independently from an increase in filling pressure.34 Moreover, a qualitative assessment of the severity of mitral regurgitation predicted a poor outcome without additive predictive power of BNP levels and TFC values.
Determination of BNP levels may represent a valuable tool in the outpatient clinic. Its use for differential diagnosis of acute dyspnea is already documented.12, 13 In recent years, BNP‐ or N‐terminal proBNP (NT‐proBNP)‐guided therapy in chronic heart failure outpatients have been compared with usual care in a number of studies and in 1 meta‐analysis,14, 15, 16, 17, 18, 19, 20 the composite end points being mortality and hospitalizations. Most studies14, 15, 16, 17, 18 showed reduction in the composite end point, sometimes only in younger patients.17, 18 The improvement in prognosis was usually attributed to the achievement of better therapy titration.20 One study did not show favorable results.35 It remains to be defined whether any difference exists between the use of NT‐proBNP (more stable over time and possibly accumulating with more severe renal impairment), and BNP (whose level are less dependent on renal clearance and thus may better show impending congestion). As stated in the Methods section, treatment for all patients in our center was BNP‐guided, so that our purpose was merely a search for a prognostic cutoff value of BNP in the outpatient setting.
Transthoracic bioimpedance does not have the same widespread use of natriuretic peptides, albeit being a noninvasive, rather inexpensive and simple technique that does not require interpretative skills. The working hypothesis behind the use of transthoracic bioimpedance, despite some controversial evidence36 is that when comparing surface and invasive measurements simultaneously, a significant correlation is found between thoracic conductance and pulmonary wedge pressure.22, 24 In fact, thoracic conductance has proven useful in detecting high‐risk heart failure patients25 and in identifying severe diastolic dysfunction.28 Moreover, the continuous monitoring of intrathoracic impedance with an internal system included in most of the newest ventricular resynchronization devices (CRT or CRT‐D) may show impedance changes before the appearance of symptoms and signs of heart failure linked to congestion.26, 27
The interest in the combined use of BNP determination and impedance cardiography for prognostic stratification of patients is growing. In subjects undergoing a screening echocardiogram (of whom only 25% had heart failure), BNP levels and systolic time ratio index at impedance cardiography (a marker of inotropism) predicted subsequent heart failure events, whereas TFC did not.37 This is not surprising. In a population where most patients are unaffected at the index visit, the first occurrence of heart failure would depend on reduced inotropism, whereas late complications in longer‐lasting disease, such as in our study patients, would be related to congestion.1, 2, 3 In patients discharged after an episode of acute heart failure, TFC and BNP were univariate predictors of the outcome, but in multivariate Cox regression analysis, only BNP was independently associated with prognosis.38 In this study, about 25% of patients had heart failure with preserved systolic function, a population not considered in the present report.
In our study, BNP level determination and thoracic conductance evaluation performed at the entry visit identified patients who, despite being subsequently treated by the same medical team following international guidelines, had higher mortality at follow‐up. In this population, not taking into account the information obtained by echocardiography, which as described above had minor additive prognostic relevance, we could classify patients into classes of increasing mortality risk. In retrospect, patients in these classes of risk could have been handled differently. Low‐risk patients could have been managed by their general practitioner with the help of a telemonitoring and /or telecare system and with yearly referrals to the heart failure unit, whereas high‐risk patients should have been followed almost exclusively by a specialized heart failure team. Due to the wide availability of a local telecare system, our current disease management approach is shifting toward this model, which increased the number of patients that can be followed by our unit.
Limitations of the Study
We only analyzed patients with systolic dysfunction, therefore our results cannot be extended to patients with preserved systolic function who may have different risk profile.39 We chose to exclude patients with preserved systolic function from data analysis because they were very few. In earlier years (2007 and 2008), possibly due to a referral bias, they were not being sent to the heart failure unit, and only after 2009 did their numbers increase to about 20% of all patients. Moreover, about one‐fourth of eligible patients were not studied because of the impossibility of simultaneously obtaining a meaningful BNP measurement, a reliable impedance cardiography recording, and echocardiographic examination of diastolic function and mitral regurgitation (see Methods). In so doing, we excluded patients with more severe prognoses, such as patients with advanced renal failure, obesity, or severe COPD, which would explain the low mortality in the study population. In fact, in the excluded patients, at least 1 of the 3 methods of evaluation could have been applied (BNP assessment, impedance cardiography, echocardiography). The availability of a multiparametric approach to patients with heart failure in search of signs of congestion is therefore a key issue. To this end, a further help might come from the use of pulmonary echography for the detection of pulmonary tails, a method that has been thus far used in the acute setting in the differential diagnosis of acute dyspnea.40
Conclusion
In the setting of an outpatient heart failure unit, the combined use of clinical evaluation, transthoracic bioimpedance, and BNP assessment, even without the help of echocardiography, hinted to the presence of thoracic congestion and allowed a clear prognostic stratification. The reliability of this preliminary stratification for the subsequent disease management of the increasing population of chronic heart failure patients should be tested in multicenter prospective studies.
Acknowledgements
The authors are grateful to Ada Spiezia, RN, and to Cosetta Corapi, RN, for their help in performing the study and their invaluable care of the patients both in the outpatient heart failure unit and in the TeleCare and telemonitoring systems.
References
- 1. Metra M, Ponikowski P, Dickstein K, et al; Heart Failure Association of the European Society of Cardiology. Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2007;9:684–694. [DOI] [PubMed] [Google Scholar]
- 2. Damman K, Voors AA, Hillege HL, et al; CIBIS‐2 Investigators and Committees. Congestion in chronic systolic heart failure is related to renal dysfunction and increased mortality. Eur J Heart Fail. 2010;12:974–982. [DOI] [PubMed] [Google Scholar]
- 3. Gheorghiade M, Shin DD, Thomas TO, et al. Congestion is an important diagnostic and therapeutic target in heart failure. Rev Cardiovasc Med. 2006;7(suppl 1):S12–S24. [PubMed] [Google Scholar]
- 4. The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J. 2008;29:2388–2442. [DOI] [PubMed] [Google Scholar]
- 5. Lucas C, Johnson W, Hamilton MA, et al. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am Heart J. 2000;140:840–847. [DOI] [PubMed] [Google Scholar]
- 6. Guglin M. Key role of congestion in natural history of heart failure. Int J Gen Med. 2011;4:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884–888. [PubMed] [Google Scholar]
- 8. Bourge RC, Abraham WT, Adamson PB, et al; COMPASS‐HF Study Group. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS‐HF study. J Am Coll Cardiol. 2008;51:1073–1079. [DOI] [PubMed] [Google Scholar]
- 9. Drazner MH, Hellkamp AS, Leier CV, et al. Value of clinician assessment of hemodynamics in advanced heart failure: the ESCAPE trial. Circ Heart Fail. 2008;1:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stevenson LW; ESCAPE and COMPASS trials. Theodore E. Woodward Award: coming in out of the rain. Relieving congestion in heart failure. Trans Am Clin Climatol Assoc. 2009;120:177–187. [PMC free article] [PubMed] [Google Scholar]
- 11. Yu CM, Sanderson JE. Plasma brain natriuretic peptide—an independent predictor of cardiovascular mortality in acute heart failure. Eur J Heart Fail. 1999;1:59–65. [DOI] [PubMed] [Google Scholar]
- 12. Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B‐type natriuretic peptide (BNP) in the diagnosis of heart failure in an urgent care setting. J Am Coll Cardiol. 2001;37:379–385. [DOI] [PubMed] [Google Scholar]
- 13. Maisel A, Mueller C, Adams K Jr, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824–839. [DOI] [PubMed] [Google Scholar]
- 14. Troughton RW, Frampton CM, Yandle TG, et al. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N‐BNP) concentrations. Lancet. 2000;355:1126–1130. [DOI] [PubMed] [Google Scholar]
- 15. Monica R Shah. STARBRITE: a randomized pilot trial of BNP‐guided therapy in patients with advanced heart failure. Circulation. 2006;114:528–533. [Google Scholar]
- 16. Jourdain P, Jondeau G, Funck F, et al. Plasma brain natriuretic peptide‐guided therapy to improve outcome in heart failure: the STARS‐BNP multicenter study. J Am Coll Cardiol. 2007;49:1733–1739. [DOI] [PubMed] [Google Scholar]
- 17. Lainchbury JG, Troughton RW, Strangman KM, et al. N‐terminal pro‐B‐type natriuretic peptide‐guided treatment for chronic heart failure: results from the BATTLESCARRED (NT‐proBNP‐Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol. 2009;55:53–60. [DOI] [PubMed] [Google Scholar]
- 18. Pfisterer M, Buser P, Rickli H, et al; TIME‐CHF Investigators. BNP‐guided vs symptom‐guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME‐CHF) randomized trial. JAMA. 2009;301:383–392. [DOI] [PubMed] [Google Scholar]
- 19. Januzzi JL Jr, Rehman SU, Mohammed AA, et al. Use of amino‐terminal pro‐B‐type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol. 2011;58:1881–1889. [DOI] [PubMed] [Google Scholar]
- 20. Porapakkham P, Porapakkham P, Zimmet H, et al. B‐type natriuretic peptide‐guided heart failure therapy: a meta‐analysis. Arch Intern Med. 2010;170:507–514. [DOI] [PubMed] [Google Scholar]
- 21. Rosenberg P, Yancy CW. Noninvasive assessment of hemodynamics: an emphasis on bioimpedance cardiography. Curr Opin Cardiol. 2000;15:151–155. [DOI] [PubMed] [Google Scholar]
- 22. Greenberg BH, Hermann DD, Pranulis MF, et al. Reproducibility of impedance cardiography hemodynamic measures in clinical stable heart failure patients. Congest Heart Fail. 2000;6:74–80. [DOI] [PubMed] [Google Scholar]
- 23. Barcarse E, Kazanegra R, Chen A, et al. Combination of B‐type natriuretic peptide levels and non‐invasive hemodynamic parameters in diagnosing congestive heart failure in the emergency department. Congest Heart Fail. 2004;10:171–176. [DOI] [PubMed] [Google Scholar]
- 24. Malfatto G, Blengino S, Perego GB, et al. Transthoracic impedance accurately estimates pulmonary wedge pressure in patients with decompensated chronic heart failure. Congest Heart Fail. 2012;18:25–31. [DOI] [PubMed] [Google Scholar]
- 25. Packer M, Abraham WT, Mehra MR, et al; Prospective Evaluation and Identification of Cardiac Decompensation by ICG Test (PREDICT) Study Investigators and Coordinators. Utility of impedance cardiography for the identification of short term risk of clinical decompensation in stable patients with chronic heart failure. J Am Coll Cardiol. 2006;47:2245–2252. [DOI] [PubMed] [Google Scholar]
- 26. Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841–848. [DOI] [PubMed] [Google Scholar]
- 27. Catanzariti D, Lunati M, Landolina M, et al; Italian Clinical Service Optivol‐CRT Group. Monitoring intrathoracic impedance with an implantable defibrillator reduces hospitalizations in patients with heart failure. Pacing Clin Electrophysiol. 2009;32:363–370. [DOI] [PubMed] [Google Scholar]
- 28. Malfatto G, Branzi G, Giglio A, et al. Transthoracic bioimpedance and brain natriuretic peptide levels accurately indicate additional diastolic dysfunction in patients with chronic advanced systolic heart failure. Eur J Heart Fail. 2010;12:928–935. [DOI] [PubMed] [Google Scholar]
- 29. Gerhard‐Herman M, Gardin JM, Jaff M, et al; American Society of Echocardiography and the Society of Vascular Medicine and Biology. Guidelines for noninvasive vascular laboratory testing. J Am Soc Echocardiogr. 2006;19:955–972. [DOI] [PubMed] [Google Scholar]
- 30. Bruch C, Klem I, Breithardt G, et al. Diagnostic usefulness and prognostic implications of the mitral E/E' ratio in patients with heart failure and severe secondary mitral regurgitation. Am J Cardiol. 2007;100:860–865. [DOI] [PubMed] [Google Scholar]
- 31. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 32. Enriquez‐Sarano M, Tribouilloy C. Quantitation of mitral regurgitation: rationale, approach, and interpretation in clinical practice. Heart. 2002;88:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomas JD. Doppler echocardiographic assessment of valvar regurgitation. Heart. 2002;88:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mullens W, Borowski AG, Curtin RJ, et al. Tissue Doppler imaging in the estimation of intracardiac filling pressure in decompensated patients with advanced systolic heart failure. Circulation. 2009;119:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eurlings LW, van Pol PE, Kok WE, et al. Management of chronic heart failure guided by individual N‐terminal pro‐B‐type natriuretic peptide targets: results of the PRIMA (Can PRo‐brain‐natriuretic peptide guided therapy of chronic heart failure IMprove heart fAilure morbidity and mortality?) study. J Am Coll Cardiol. 2010;56:2090–2100. [DOI] [PubMed] [Google Scholar]
- 36. Kamath SA, Drazner MH, Tasissa G, et al. Correlation of impedance cardiography with invasive hemodynamic measurements in patients with advanced heart failure: the BioImpedance CardioGraphy (BIG) substudy of the Evaluation Study of congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) Trial. Am Heart J. 2009;158:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Castellanos LR, Bhalla V, Isakson S, et al. B‐Type natriuretic peptide and impedance cardiography at the time of routine echocardiography predict subsequent heart failure events. J Cardiac Fail. 2009;15:41–47. [DOI] [PubMed] [Google Scholar]
- 38. Pimenta J, Paulo C, Mascarenhas J, et al. BNP at discharge in acute heart failure patients: is it all about volemia? A study using impedance cardiography to assess fluid and hemodynamic status. Int J Cardiol. 2010;145:209–214. [DOI] [PubMed] [Google Scholar]
- 39. Inglis SC, Clark RA, McAlister FA, et al. Structured telephone support or telemonitoring programmes for patients with chronic heart failure. Cochrane Database Syst Rev. 2010;(8):CD007228. [DOI] [PubMed] [Google Scholar]
- 40. Picano E, Frassi F, Agricola E, et al. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19:356–363. [DOI] [PubMed] [Google Scholar]


