Abstract
Background
Improvement in the left ventricular ejection fraction (LVEF) may occur in patients with dilated cardiomyopathy (DCM).
Hypothesis
There are different implications of persistent versus transient improvement in LVEF among DCM patients receiving contemporary therapy.
Methods
We studied 188 patients with nonischemic DCM. Persistent improvement in LVEF (PIEF) was defined as LVEF increase by at least 10% compared to baseline, and found in 2 separate echo‐Doppler exams performed at least 12 months apart. Increased LVEF in echo 2, which was not sustained in echo 3, was defined as transient improvement in LVEF (TIEF).
Results
Over an average follow‐up of 6.8 years, PIEF occurred in 61 (33%) patients, predicting a better long‐term outcome (P < 0.001) in a combined end‐point comprising death, heart transplantation, or the need for a ventricular assist device. The TIEF group had an intermediate course and were closer to nonimprovers (P = 0.003 vs PIEF). Multivariate logistic regression identified the following independent predictors of PIEF: shorter disease duration, pregnancy‐associated disease, left ventricular hypertrophy, and baseline LVEF ≤25%. A score to predict PIEF assigned 1 point to each of the following: disease duration <3 years and no familial cardiomyopathy; pregnancy‐associated presentation; basal LVEF ≤25%; and left ventricular wall thickness ≥12. A score of ≥3 was present in 44% of the patients, reliably predicting PIEF in 91% (P = 0.01).
Conclusions
Persistent improvement in LVEF is associated with improved long‐term prognosis. Baseline clinical parameters can be used to identify patients likely to demonstrate PIEF, thereby allowing tailored management in this population.
Introduction
The term cardiac remodeling was originally used to describe changes in the cardiac morphology occurring after myocardial infarction, as well as those occurring in dilated cardiomyopathy (DCM). The pathological process is triggered by physiological mechanisms compensating for cardiovascular overload or dysfunction, but then progresses independently of the original cause of myocardial injury. Reverse remodeling (RR) is a concept that refers to the functional and structural rehabilitation of the heart.1 This fascinating phenomenon gained publicity with description of heart recovery following revascularization, timely valve surgery, cardiac resynchronization, or implantation of an assist device. RR may also occur with heart failure therapies and, occasionally, spontaneously.
The IMAC (Intervention in Myocarditis and Acute Cardiomyopathy) study in patients with recent‐onset DCM showed that 70% of recipients of optimal heart failure therapy improved their left ventricular ejection fraction (LVEF) by at least 10%. The low rates of death (4%) and heart transplantation (5%) over a mean of 2.2 years imply that contemporary heart failure therapy revolutionized the natural history of DCM.2, 3 In our previous study, RR (defined as 10% LVEF increase combined with a 10% decrease in the left ventricular dimension) occurred in 26% of DCM patients after an average follow‐up of 32 months.4
Fluctuations in LVEF are well recognized in DCM patients. Although several studies investigated the RR phenomenon in DCM, its long‐term persistence, once achieved, was rarely addressed.5 In the study by Choi and coworkers, 10% of the group who underwent RR had recurrence of heart failure symptoms combined with a decrement in their ejection fraction on a long‐term follow‐up.3 The purpose of the current study was to examine the predictors and the prognostic implications of sustained, as compared to transient, LVEF improvement in DCM.
Methods
Study Population
The investigational part of the study conforms to the principles outlined in the Declaration of Helsinki and was approved by the institutional review board. A cohort of consecutive patients with DCM was evaluated between July 1, 2004 and July 1, 2008 in the Heart Failure/Cardiomyopathy Clinic of Sheba Hospital4 and followed since then. Significant coronary disease was ruled out by angiography or radionuclide scan. Patients suspected to have an acute myocardial injury, such as myocarditis, hypertensive crisis, sepsis, or stress‐induced cardiomyopathy, were reevaluated and excluded if myocardial function recovered within 3 months.
Data Collection
Patients were treated according to the contemporary heart failure guidelines6 and were followed until May 31, 2012. Serial echo‐Doppler studies were performed every 6 to 24 months or as clinically indicated. We encouraged performing on‐site studies; however, echo reports by outpatient clinics were accepted. LVEF was determined by eyeballing or (when necessary) by Simpson's method. Epidemiologic and clinical data including the approximate duration of symptoms, the principal complaint on presentation, New York Heart Association (NYHA) functional class and baseline electrocardiogram (ECG) were collected. We documented comorbidities and potential causes of secondary cardiomyopathy such as hypertension, diabetes, obesity, chemotherapy exposure, sustained tachyarrhythmia, and association with pregnancy.
Definitions and Outcome Measures
The echo‐Doppler study used for DCM diagnosis was defined as echo 1. Echo 2 had to be separated by at least 6 months from echo 1. Improved LVEF was defined as an increase of at least 10% in echo 2 units relative to baseline. A time interval between echo 2 and echo 3 had to be at least 12 months. An increase in LVEF by at least 10% units in both echo 2 and echo 3 compared to echo 1 was defined as persistent improvement in LVEF (PIEF). An increase in echo 2 that was not sustained in echo 3 was defined as transient improvement in LVEF (TIEF). Familial cardiomyopathy was defined according to the consensus document.7 When several affected members of a family were available, only the proband was included in the database. Coexistent coronary artery disease was defined as stenosis that did not involve a section of a major coronary artery and when the myocardial dysfunction could not be attributed to scar tissue according to a radionuclide perfusion scan. Left ventricular hypertrophy (LVH) on ECG was defined by the voltage criteria of Sokolow‐Lyon.8 LVH by echo was defined as maximal left ventricular wall thickness (LVWT) ≥12 mm.
Therapies and interventions were recorded at the time of echo 2. The dose of β‐blockers, angiotensin‐converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) were also presented by a fraction of the maximal recommended dose in each category. Mineralocorticoid antagonism and device therapy were defined by a binary variable.
Outcome measures were obtained from the patient's chart and ascertained by phone, if required. They included NYHA functional class recorded at the end of follow‐up and the combined end point of death and heart transplantation or implanting an assist device.
Statistical Analysis
The objectives of the study were to define the clinical predictors of PIEF and its impact on the outcome measures. PIEF, TIEF and no improvement in LVEF (NIEF) were set as categorical variables. Baseline clinical and heart failure therapy features, and echocardiographic and ECG characteristics were compared using analysis of variance or χ2 for categorical variables and t test for continuous measures (SAS version 9; SAS Institute, Inc., Cary, NC).
Identification of Factors Associated With PIEF
We included the potential binary risk factors (Tables 1 and 2) for the PIEF model. Variables were made binary by the use of cut points to derive a simple scoring method. Univariate relationships between candidate covariates and PIEF were assessed as defined above. The covariates with values of P < 0.20 were further evaluated by carrying out a best‐subset regression analysis, examining the models created from all possible combinations of predictor variables, and using a penalty of 3.84 on the likelihood ratio of x 2 value for any additional factor included. Model selection was repeated after unselected factors were dismissed, 1 at a time, to minimize the effects of missing data. Survival for the composite outcome after echo 2 by the 3 groups was analyzed by the Kaplan‐Meier method, and the statistical difference between groups was assessed by the log‐rank test.
Table 1.
Baseline Clinical Features and Heart Failure Therapies
| NIEF | TIEF | PIEF | P Value | |
|---|---|---|---|---|
| No. of patients | 101 (55%) | 21 (11%) | 61 (33%) | |
| Age, y | 57 ± 17 | 65 ± 16 | 58 ± 16 | 0.117 |
| Disease duration, y | 4.9 ± 6 | 4.6 ± 5.7 | 2.1 ± 3.9 | 0.007 |
| Male sex | 68 (67%) | 14 (67%) | 33 (54%) | 0.223 |
| BMI, kg/m2 | 27 ± 6 | 27 ± 6 | 26 ± 5 | 0.661 |
| Familial CMP | 27 (27%) | 5 (24%) | 5 (8%) | 0.016 |
| Hypertension | 36 (36%) | 10 (48%) | 27 (44%) | 0.413 |
| Diabetes | 26 (26%) | 4 (19%) | 12 (20%) | 0.607 |
| Chemotherapy | 4 (4%) | 3 (14%) | 11 (18%) | 0.011 |
| Substance abuse (alcohol/narcotics) | 8 (8%) | 0 (0%) | 3 (5%) | 0.287 |
| Pregnancy | 5 (5%) | 2 (10%) | 12 (20%) | 0.012 |
| Renal failure | 19 (19%) | 9 (43%) | 12 (20%) | 0.046 |
| Pulmonary disease | 13 (13%) | 1 (5%) | 4 (7%) | 0.301 |
| Possible TICM | 10 (10%) | 4 (19%) | 11 (18%) | 0.257 |
| Conduction disease | 14 (14%) | 1 (5%) | 4 (7%) | 0.225 |
| Heart rate, bpm | 77 ± 13 | 77 ± 14 | 83 ± 18 | 0.060 |
| Systolic BP, mm Hg | 120 ± 20 | 129 ± 24 | 126 ± 30 | 0.255 |
| Mineralocorticoid antagonist | 51 (50.5%) | 9 (43%) | 28 (46%) | 0.748 |
| ACEI | 62 (61%) | 12 (57%) | 38 (62%) | 0.915 |
| ARB | 28 (28%) | 5 (24%) | 16 (26%) | 0.928 |
| ACEI or ARB (% of maximal dose) | 50 ± 36 | 45 ± 37 | 54 ± 37 | 0.646 |
| β‐Blocker | 81 (80%) | 16 (79%) | 55 (90%) | 0.176 |
| β‐Blocker (% of maximal dose) | 58 ± 29 | 75 ± 34 | 70 ± 31 | 0.027 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CMP, cardiomyopathy; LVEF, left ventricular ejection fraction; NIEF, no improvement in LVEF; PIEF, persistent improvement in LVEF; TICM, tachycardia‐induced cardiomyopathy; TIEF, transient improvement in LVEF.
The data show number and percent of patients unless otherwise indicated.
Table 2.
Comparison of ECG and Echo‐Doppler Parameters
| NIEF | TIEF | PIEF | P Value | |
|---|---|---|---|---|
| ECG parameters | ||||
| Sinus rhythm | 84 (87.5%) | 14 (74%) | 52 (93%) | 0.088 |
| QRS width, msec | 116 ± 31 | 111 ± 31 | 107 ± 34 | 0.304 |
| Normal or LVH ECG (by voltage criteria) | 10 (10%) | 4 (21%) | 16 (28%) | 0.017 |
| Echo 1 parameters | ||||
| LVEF, % | 30 ± 9 | 27 ± 7 | 25 ± 7 | 0.002 |
| LVEDD, mm | 61 ± 8 | 59 ± 6 | 58.5 ± 8 | 0.225 |
| LVH, LVWT ≥12 mm | 20 (20%) | 2 (10%) | 23 (39%) | 0.007 |
| LAD, mm | 44 ± 8 | 43 ± 5 | 42. ± 7 | 0.486 |
| LVESD, mm | 48 ± 9 | 46.5 ± 8 | 47 ± 10 | 0.582 |
| Normal diastolic filling | 18 (25%) | 3 (23%) | 4 (10%) | 0.163 |
| Severe diastolic dysfunctiona | 31 (46%) | 6 (50%) | 28 (68%) | 0.080 |
| Estimated PAP, mm Hg | 38 ± 13 | 37 ± 8 | 38 ± 10 | 0.886 |
| Normal RV function | 77 (79%) | 16 (80%) | 44 (76%) | 0.898 |
| Echo 2 | ||||
| Time interval between echo 1 and echo 2, mo | 33 ± 24 | 31 ± 21 | 33 ± 28 | 0.941 |
| LVEF, % | 27 ± 10 | 45 ± 10 | 50 ± 10 | <0.001 |
| LVEDD, mm | 61 ± 9 | 53 ± 8 | 52 ± 7 | <0.001 |
| Echo 3 | ||||
| Time interval between echo 2 and echo 3, mo | NA | 38 ± 14 | 35 ± 14 | 0.471 |
| LVEF, % | NA | 29 ± 8 | 49 ± 11 | <0.001 |
| LVEDD, mm | NA | 56 ± 8 | 51 ± 6 | 0.007 |
Abbreviations: ECG, electrocardiograph; LAD, left atrial dimension; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; LVH, left ventricular hypertrophy; LVWT, left ventricular wall thickness; NA, nonapplicable; NIEF, no improvement in LVEF; PAP, pulmonary artery pressure; PIEF, persistent improvement in LVEF; RV, right ventricle; TIEF, transient improvement in LVEF.
The data show number and percentage of patients unless otherwise indicated.
Severe diastolic dysfunction was defined as diastolic filling level 2 and 3.
PIEF Response Score
After selection of binary covariates, each was assigned a unit value based on its regression coefficient in the multivariate regression model. A response score was constructed in each patient by adding the assigned numeric values of the factors identified in each patient, and the study population was categorized into approximate quintiles based on the distribution of the score and the likelihood of PIEF.
Results
One hundred eighty‐eight DCM patients were evaluated and followed in our heart failure/cardiomyopathy clinic between July 1, 2004 and July 1, 2008. Improved LVEF was found in 87 (46%) increasing from 26 ± 7 to 48 ± 10% (P < 0.001 vs no significant change in the others).4
Between 2008 and 2012 we studied the natural history of LVEF improvement in these DCM patients. Echo 3 was available in 183 (97%) patients and followed echo 2 by an average of 36 ± 12 months. There were no significant differences in the time intervals between the echo‐Doppler exams among the groups (Table 2).
PIEF was observed in 61 patients (70% of those who improved between echo 1 to echo 2, constituting 33% of the entire cohort). Figure 1 shows that PIEF is associated with a markedly improved long‐term survival regarding the end point of death or heart transplantation or ventricular assisted device (VAD) (P < 0.001). At a 5‐year follow‐up after echo 2 (defining the improvement in ejection fraction) the cumulative probability of the composite outcome measure of all‐cause mortality/heart transplantation/VAD was only 5% among patients who demonstrated PIEF, compared to 29% and 31% among those who had TIEF or NIEF, respectively (P < 0.001).
Figure 1.
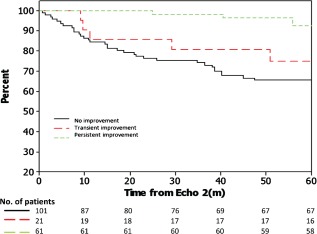
Five‐year survival plot (starting from the date of echo 2) using the Kaplan‐Meier method. Survival includes freedom from death, heart transplantation, or implantation of a ventricular assist device (P < 0.001).
PIEF was also associated with a significant improvement in the NYHA functional class. Figure 2 compares the NYHA functional class at the end of follow‐up in the survivors comparing PIEF with all other patients.
Figure 2.
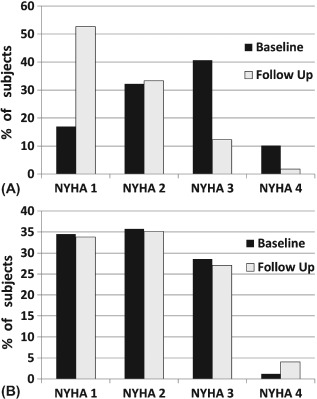
New York Heart Association (NYHA) functional class on baseline and at the end of follow‐up among those who survived. (A) An improvement occurred in the persistent improvement in LVEF (PIEF) group (P = 0.001). (B) There was no change in the no‐PIEF group.
Factors Associated With PIEF
The baseline characteristics, clinical features, and therapies in the 3 groups are presented in Table 1. Patients with pregnancy or chemotherapy‐associated cardiomyopathy had a higher frequency of having PIEF, whereas either long‐standing disease or family history of DCM had a worse outcome. The groups did not differ in their baseline medical therapy. Yet, the dose of β‐adrenergic blockers (adjusted to the maximal recommended dose) was significantly higher in PIEF and TIEF groups. The medical therapy did not differ between the groups in the course of the study. There was no difference in the biventricular pacing, whereas there was a trend for less implantable cardioverter defibrillators (ICDs) implanted in the PIEF group during the course of the study (see Supporting Table 1 in the online version of this article).
Echo‐Doppler and ECG parameters related to improvement of LVEF and its persistence are shown in Table 2. Among baseline echocardiographic measurements, greater LVWT and lower baseline LVEF, but not ventricular dimensions, or valve regurgitation were associated with LVEF recovery. Normal ECG or voltage criteria for LVH also showed a trend for a positive effect. We therefore defined a combined electrocardiographic parameter of normal or LVH ECG.
Parameters that showed a significant univariate association with outcome measures were studied by multivariate logistic regression. Disease duration, pregnancy‐associated disease, LVH by echo, and LVEF ≤25% at baseline emerged as independent predictors of PIEF (see Supporting Table 2 in the online version of this article).
PIEF Score
To establish a model to predict left ventricular recovery using the baseline clinical features, we developed a score based on the results of multivariate analysis. Because score covariates had similar relative values of regression coefficients, each variable was assigned 1 point: disease duration shorter than 3 years and no familial cardiomyopathy, pregnancy‐associated presentation, basal LVEF ≤25%, and LVWT ≥12 mm. Figure 3 demonstrates the remarkable relationship between the score and the propensity to undergo PIEF. The prevalence of PIEF ranged between 3% among patients with a score of 0 and 100% in those with score of 4. Most of the patients in the PIEF group (64%) had a score of 3 or 4. Logistic regression analysis showed a graded relation between the score and the likelihood to achieve PIEF. Notably, patients with a score of 4 were >17‐fold more likely to undergo PIEF than those with a score of 0. Furthermore, when the score was dichotomized at the median, 44% had a score of ≥3, and were 4.6 were more likely to undergo PIEF than those with a lower score, thereby predicting PIEF in 91% of patients (see Supporting Table 3 in the online version of this article) (P = 0.01). The C statistic for the propensity risk score was 0.82.
Figure 3.
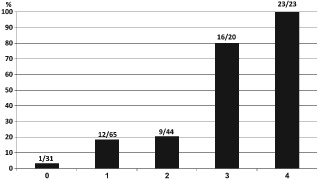
The likelihood of persistent improvement in left ventricular ejection fraction (PIEF) according to the propensity score. The score was calculated by adding 1 point for each of the following parameters: no history of familial disease and duration <3 years; pregnancy‐associated presentation; left ventricular wall thickness ≥12 mm; baseline left ventricular ejection fraction ≤25%. The probability of PIEF is strongly related to the score (see Supporting Table 3 in the online version of this article).
Discussion
The molecular pathways underlying reverse remodeling remain to be elucidated but may include mechanical unloading, reversal of abnormalities in calcium handling, mobilization of cardiac stem cells, and normalization of ultrastructure rearrangement within the cardiomyocytes.1 RR may be defined by changes in LVEF and/or the left ventricular dimension.9 In the current study, we followed these changes over an average period of approximately 5 years. PIEF occurred in 33% of the study population and was associated with a significant improvement in the NYHA functional class and long‐term prognosis (Figures 1 and 2). TIEF occurred in 11% and had a similar clinical prognosis to those who did not improve (NIEF).
We have previously reported that 26% of DCM patients qualified as reverse remodeling.4 In the long‐term, persistent decrease in the left ventricular end diastolic dimension was detected in only 18% of the cohort (data not shown). This subgroup was a part of, and had similar characteristics to, the PIEF population. We hereby chose to elaborate on the implications of improved LVEF alone, because this parameter was applicable to a larger portion of the DCM population.
In the IMAC study, which followed patients with recent onset DCM, smaller ventricular dimension on presentation was the strongest predictor of LVEF recovery.2 In contrast, many of our patients had an established disease. Disease duration prior to diagnosing DCM appears to be a major factor in determining reversibility. Early diagnosis implies timely evaluation of precipitating and aggravating factors as well as early institution of evidence‐based therapies. Familial cardiomyopathy is an example of an indolent disease, precluding accurate definition of the time of onset.10, 11 The etiology of cardiomyopathy may also affect the response to therapy. A recent study compared the response to treatment of DCM between men and women with or without peripartum cardiomyopathy. At 4 years, the most pronounced improvement in LVEF was in the peripartum group, followed by other women. Males had a worse prognosis.12 Chemotherapy‐induced cardiomyopathy is historically associated with poor prognosis. In our study, a considerable portion of these patients underwent PIEF. Most had a late‐onset variant (ie, presenting more than 1 year after exposure), which is often precipitated by another comorbidity such as hypertension.13 Recent reports suggest that early diagnosis and contemporary therapies may change the natural history of chemotherapy‐induced cardiomyopathy.14, 15, 16 Collectively, these findings suggest that recent‐onset DCM, which is associated with distinct insults, might have a better prognosis when diagnosed early and properly treated.
In contrast to other studies associating low ejection fraction with poor prognosis,17 we found that lower LVEF at presentation is an independent predictor of PIEF. We believe that a recent‐onset disease (characterized by subacute presentation) allows for a greater opportunity to intervene and respond to the modern heart failure therapy (Table 2).
Left ventricular hypertrophy by either echo‐Doppler or ECG appeared to be another predictor of improvement (Table 2). A normal ECG, which is an uncommon finding in DCM (11% of the entire cohort), shows a similar association with the outcome.18
Evidence‐based medical therapies such as β adrenergic blockers, ACE inhibitors, ARBs, and aldosterone antagonists improve cardiac function when given separately or together.19, 20, 21 Cardiac resynchronization therapy (CRT) improved LVEF and diminished the end systolic and diastolic dimensions independently of, and in synergy with, pharmacological therapy.22 Our study was not designed to study the effect of pharmacological therapies or CRT, because all subjects were treated according to the same heart failure guidelines pertinent at the time of the study.6, 23 Most of the patients had a narrow QRS complex (Table 2). We did find that a higher dose of β‐blockers, but not RAAS inhibitors, was related to improvement of LVEF.24 The long‐standing effect of an ICD on cardiac remodeling remains to be investigated. Notwithstanding its survival benefit, detrimental ICD effects were reported due to inappropriate shocks, right ventricular pacing, and other complications. There have also been notes of caution regarding ICD and heart failure exacerbation.25 We believe that ICD implantation should be postponed if possible, because some patients with low LVEF may eventually not need an ICD if observed for a reasonable time interval to allow LVEF recovery.26, 27
Limitations
This observational, single‐center, clinical study lacks an echocardiographic core lab and a stringent time schedule. The study cohort was nonhomogenous, reflecting large variability in etiology, disease duration, and previous therapy so characteristic of nonselected DCM populations. We often had difficulty defining the disease duration and found a statistical overlap between this parameter and having familial cardiomyopathy. Important predictors such as brain natriuretic peptide and magnetic resonance imaging,28 as well as genetic analysis, were not available in the majority of patients, and thus could not be analyzed.
Conclusion
Not withstanding these reservations, we developed a propensity score (presented in Figure 3 and Supporting Table 3) to identify patients likely to undergo PIEF using simple and easily available baseline clinical characteristics. This score allows identifying patients with a better prognosis who may benefit from observation on optimal medical therapy. If validated, such a score might guide clinical decision‐making and may help reduce the mounting costs of medical care in heart failure patients.
Supporting information
TableS1. Device therapies and medications at baseline and follow‐up
TableS2. Multivariate predictors of PIEF
TableS3. Relationship between the propensity score and the likelihood of PIEF
Acknowledgments
The authors are grateful to Elaine Finkelstein for secretarial assistance.
This work was supported in part by Israel Science Foundation grant 763/10.
This work was performed by Ido Blechman in partial fulfillment of the M.D. thesis requirements of the Sackler Faculty of Medicine, Tel Aviv University.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Pieske B. Reverse remodeling in heart failure—fact or fiction? Eur Heart J. 2004;6(suppl D):D66–D78. [Google Scholar]
- 2. McNamara DM, Starling RC, Cooper LT, et al; IMAC Investigators . Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)‐2 study; J Am Coll Cardiol. 2011;58:1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi JO, Kim EY, Lee GY, et al. Predictors of left ventricular reverse remodeling and subsequent outcome in nonischemic dilated cardiomyopathy. Circ J. 2013;77:462–469. [DOI] [PubMed] [Google Scholar]
- 4. Arad M, Nussbaum T, Blechman I, et al. Prevalence and clinical predictors of reverse remodeling in patients with dilated cardiomyopathy. Isr Med Assoc J. 2014;16:405–411. [PubMed] [Google Scholar]
- 5. Park JS, Kim JW, Seo KW, et al. Recurrence of left ventricular dysfunction in patients with restored idiopathic dilated cardiomyopathy. Clin Cardiol. 2014;37:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dickstein K, Cohen‐Solal A, Filippatos G, et al; ESC Committee for Practice Guidelines (CPG) . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29:2388–2442. [DOI] [PubMed] [Google Scholar]
- 7. Mestroni L, Maisch B, Mckenna WJ, et al. Guidelines for the study of familial dilated cardiomyopathies. Eur Heart J. 1999;20:93–102. [DOI] [PubMed] [Google Scholar]
- 8. Surawicz B, Knilans TK. Chou's Electrocardiography in Clinical Practice. 5th ed Philadelphia, PA: Saunders; 2001. [Google Scholar]
- 9. Merlo M, Pyxaras SA, Pinamonti B, et al. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol. 2011;57:1468–1476. [DOI] [PubMed] [Google Scholar]
- 10. Grunig E, Benz A, Mereles D, et al. Prognostic value of serial cardiac assessment and familial screening in patients with dilated cardiomyopathy. Eur J Heart Fail. 2003;5:55–62. [DOI] [PubMed] [Google Scholar]
- 11. Pasotti M, Klersy C, Pilotto A, et al. Long‐term outcome and risk stratification in dilated cardiolaminopathies. J Am Coll Cardiol. 2008;52:1250–1260. [DOI] [PubMed] [Google Scholar]
- 12. Cooper LT, Mather PJ, Alexis JD, et al; IMAC2 Investigators . Myocardial recovery in peripartum cardiomyopathy: prospective comparison with recent onset cardiomyopathy in men and nonperipartum women. J Card Fail. 2012;18:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Curigliano G, Cardinale D, Suter T, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Ann Oncol. 2012;23(suppl 7):vii155–vii66. [DOI] [PubMed] [Google Scholar]
- 14. Shakir DK, Rasul KI. Chemotherapy induced cardiomyopathy: pathogenesis, monitoring and management. J Clin Med Res. 2009;1:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wells QS, Lenihan DJ. Reversibility of left ventricular dysfunction resulting from chemotherapy: can this be expected? Prog Cardiovasc Dis. 2010;53:140–148. [DOI] [PubMed] [Google Scholar]
- 16. Cardinale D, Colombo A, Lamantia G, et al. Anthracycline‐induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–220. [DOI] [PubMed] [Google Scholar]
- 17. Hoshikawa E, Matsumura Y, Kubo T, et al. Effect of left ventricular reverse remodeling on long‐term prognosis after therapy with angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers and β blockers in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2011;107:1065–1070. [DOI] [PubMed] [Google Scholar]
- 18. Iida K, el Sersi M, Fujieda K, et al. Pathophysiologic significance of left ventricular hypertrophy in dilated cardiomyopathy. Clin Cardiol. 1996;19:704–708. [DOI] [PubMed] [Google Scholar]
- 19. Doughty RN, Whalley GA, Gamble G, et al. Left ventricular remodeling with carvedilol in patients with congestive heart failure due to ischemic heart disease. Australia‐New Zealand Heart Failure Research Collborative Group. J Am Coll Cardiol. 1997;29:1060–1066. [DOI] [PubMed] [Google Scholar]
- 20. Chan AK, Sanderson JE, Wang T, et al. Aldosterone receptor antagonism induces reverse remodeling when added to angiotensin receptor blockade in chronic heart failure. J Am Coll Cardiol. 2007;50:591–596. [DOI] [PubMed] [Google Scholar]
- 21. Remme WJ, Riegger G, Hildebrandt P, et al. The benefits of early combination treatment of carvedilol and ACE‐inhibitors in mild heart failure and left ventricular systolic dysfunction: the Carvedilol and ACE‐inhibitor Remodeling Mild Heart Failure Evaluation Trial (CARMEN). Cardiovasc Drugs Ther. 2004;18:57–66. [DOI] [PubMed] [Google Scholar]
- 22. St John Sutton MG, Plappert T, Abraham WT, et al; Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Study Group . Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107:1985–1990. [DOI] [PubMed] [Google Scholar]
- 23. Hunt SA, Abraham WT, Chin MH, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American College of Chest Physicians; International Society for Heart and Lung Transplantation; Heart Rhythm Society . ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the HeartRhythm Society. Circulation. 2005;112:e154–e235. [DOI] [PubMed] [Google Scholar]
- 24. Frigerio M, Roubina E. Drugs for left ventricular remodeling in heart failure. Am J Cardiol. 2005;96:10–18. [DOI] [PubMed] [Google Scholar]
- 25. Goldenberg I, Moss AJ, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial (MADIT) II Investigators . Causes and consequences of heart failure after prophylactic implantation of a defibrillator in the multicenter automatic defibrillator implantation trial II. Circulation. 2006;113:2810–2817. [DOI] [PubMed] [Google Scholar]
- 26. Yancy CW, Jessup M, Bozkurt B, et al; ACCF/AHATask Force Members . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 27. Kini V, Soufi MK, Deo R, et al. Appropriateness of primary prevention implantable cardioverter defibrillators at time of generator replacement: are indications still met? J Am Coll Cardiol. 2014;63:2388–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kubanek M, Sramko M, Maluskova J, et al. Novel predictors of left ventricular reverse remodeling in individuals with recent‐onset dilated cardiomyopathy. J Am Coll Cardiol. 2013;61:54–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TableS1. Device therapies and medications at baseline and follow‐up
TableS2. Multivariate predictors of PIEF
TableS3. Relationship between the propensity score and the likelihood of PIEF


