Abstract
Background
HMG CoA (3‐hydroxy‐3‐methylglutaryl coenzyme A) reductase inhibitors, or statins, have been associated with an improvement in outcomes after coronary artery surgery for some time; however, their role in isolated valve surgery (IVS) remains undetermined.
Hypothesis
The pleiotropic effects of statins may produce similar beneficial effects on outcomes after IVS.
Methods
A systematic review of the literature was performed investigating the role of statins in bioprosthetic valve replacement.
Results
Nine observational studies (7 retrospective, 2 prospective) incorporating a total of 18 154 patients were found investigating the role of statin therapy in bioprosthetic valve replacement.
Conclusions
There is presently insufficient evidence to recommend routine statin therapy in IVS, unless concomitant hypercholesterolemia or coronary artery disease is present. A prospective study clearly defining the dose, type, and duration of therapy is now required to finally clarify whether statins alone confer a postoperative benefit in these patients.
Introduction
The beneficial role of HMG CoA (3‐hydroxy‐3‐methylglutaryl coenzyme A) reductase inhibitors (statins) in both primary and secondary prevention of cardiovascular disease has been recognized since the early 1990s, with evidence of a significant reduction in both acute coronary events and overall mortality from coronary artery disease.1, 2
Although early research focused on their lipid lowering effects, recent studies have shown more diverse pleiotropic properties, including improvement in endothelial function, inhibition of vascular smooth muscle cell proliferation, prevention of cardiac hypertrophy and atherosclerosis, and a reduction in oxidative stress and vascular inflammation.3, 4, 5 Consequently, the role of statins has been explored beyond their traditionally established indications. Cardiopulmonary bypass triggers several humoral and cellular pathways that promote inflammation, including activation of the coagulation cascade, release of proinflammatory cytokines, neutrophil adhesion, and complement and mast cell activation.6, 7 Statin pretreatment prior to on‐pump coronary artery bypass graft (CABG) surgery may significantly dampen this process, reducing cytokine release (interleukin [IL]‐6, IL‐8) and neutrophil adhesion to the vein graft endothelium.8
In the era of transcatheter valves and an ever‐aging population, bioprosthetic valve replacement is becoming increasingly common, and strategies to prevent prosthesis degeneration is of growing importance.9 Beyond their role in coronary surgery, the combination of the pleiotropic effects of statins and a reduction in hypercholesterolemia are thought to synergistically contribute to a long‐term reduction in biological prosthesis degeneration following cardiac valve replacement.10, 11 However, although a number of trials have attempted to quantify this effect, the overall mechanisms are yet to be elucidated, and no prospective randomized control trial (RCT) assessing the long‐term effects of statins on bioprosthesis degeneration exists.12
With the established view that preoperative statin therapy is protective in coronary artery surgery, this systematic literature review explores the evolving role of statins in valve surgery and evaluates the relationship between preoperative statin therapy and patient outcomes following valve replacement.
Statin Pharmacology and Pleiotropy
Statins exert their effect on lipid metabolism through the competitive inhibition of HMG‐CoA reductase, preventing the conversion of HMG‐CoA to L‐mevalonic acid, a rate‐limiting step in cholesterol biosynthesis. This results in depletion of the intercellular sterol pool and subsequent enhanced hepatic expression of low‐density lipoprotein (LDL) cholesterol receptors and an increased clearance of plasma LDL cholesterol.13, 14
However, it is now well recognized that, through a variety of different mechanisms, statins also have a number of cholesterol‐independent or pleiotropic actions, which may improve cardiovascular disease outcomes. Many of these effects result from inhibition of the downstream products of L‐mevalonic acid, which in turn act as important lipid attachments in the activation of the intracellular proteins Ras, Rho/Rho Kinase, Rac, and Rap.15, 16 Consequently, statins may inhibit vascular smooth muscle cell proliferation17 and may even lead to a reduction in cardiac hypertrophy.5, 18
Through activation of macrophage peroxisome proliferator‐activated receptors, statins also inhibit inflammatory cytokine and MMP production19 acting to stabilize atheromatous plaques (Figure 1). Furthermore, by reducing vessel wall nicotinamide adenine dinucleotide phosphate hydrogen oxidase activity, and enhancing endothelial NO production, statins improve endothelial nitric oxide synthase (eNOS) coupling, thus improving endothelial function.20 Similarly, by upregulating eNOS in platelets, statins may reduce both platelet activation and the local inflammatory response.21
Figure 1.
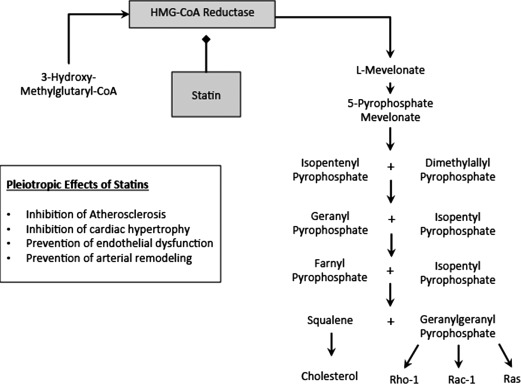
The mechanism of action and pleiotropic effects of statins. Abbreviations: HMG‐CoA, 3‐hydroxy‐3‐methylglutaryl‐coenzyme A.
Lessons From Statins in Coronary Artery Surgery
Several studies have demonstrated the beneficial effects of perioperative statins in decreasing short‐term morbidity/mortality after CABG and in reducing long‐term graft failure. Current American College of Cardiology Foundation/American Heart Association coronary revascularization guidelines reflect this, recommending statins in all patients undergoing CABG at a dose sufficient to reduce LDL cholesterol below 100 mg/dL (or 70 mg/dL in high‐risk patients) and achieve at least a 30% reduction in LDL.22
Furthermore, aside from their mortality benefits, by protecting against oxidative stress and reducing systemic inflammatory mediators such as IL‐6 and C‐reactive protein (CRP), preoperative statins have been associated with reduced atrial fibrillation (AF) after CABG23, 24, 25, 26 and may also lead to an improvement in stroke and renal function.27, 28
Guidelines for Statin Therapy in Valve Surgery
In August 2012, the European Society of Cardiology/European Association for Cardio‐Thoracic Surgery produced the most recent update on the management of valvular heart disease.29 This report underlines the lack of evidence supporting the use of statins in reducing progression of native aortic valve stenosis, and the task force recommends against the use of statins where their sole purpose is to slow disease progression. In the perioperative setting, it is recommended that statin therapy be adapted to the risk of ischemic heart disease, and no specific recommendations are given on postoperative statin use. Furthermore, although Butchart et al30 published their study in 2005, no comment was made on the effect of statins on either short‐term morbidity/mortality or long‐term bioprosthesis degeneration.
Methods
A literature search was performed using PubMed, EMBASE, and Google Scholar under the following MESH search headings: (“hydroxymethylglutaryl‐coa reductase inhibitors” OR “hydroxymethylglutaryl‐coa” AND “reductase” AND “inhibitors”OR 'hydroxymethylglutaryl‐coa reductase inhibitors' OR “statin” OR “hydroxymethylglutaryl‐coa reductase inhibitors”[Pharmacological Action]) AND valve. The Related Citations tool was used to expand the search, and all titles, abstracts, studies, and citations were retrieved and reviewed. Studies in all languages were sought. All studies assessing statin therapy in cardiac surgery were read in full, and data were extracted by 2 independent reviewers (J.C. and L.H.). Figure 2 outlines our search strategy, performed in accordance with recommendations from the Cochrane Collaboration and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines.31 The last search date was August 12, 2012.
Figure 2.
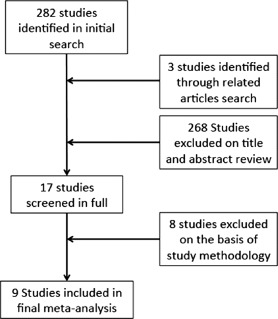
Search strategy.
Summary of Search Results
Overall Results
Our search identified 9 studies investigating the role of preoperative statins in isolated valve surgery (IVS) and valve surgery in conjunction with CABG.32, 33, 34, 35, 36, 37, 38, 39, 40 These studies reviewed a total of 18 154 patients as summarized in the Table 1.
Table 1.
Description of Included Studies
| Study (Design) | Statin, No. | No Statin, No. | Type and Dose of Statin, Median (Range) | Timing of Statin Therapy | Study Population | Outcomes | Mean Follow‐up, Y | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Clark et al., 2006 (retrospective cohort)35 | 666 | 50 | 418 Atorva, 10 mg (10–40 mg); 467 Simva, 20 mg (10–60 mg); 71 Lova, 20 mg (20–40 mg); 30 Fluva, 20 mg (20–40 mg); 58 Prava, 20 mg (10–40 mg) | Preoperative, duration NS | Elective valve ± CABG, February 1994–December 2002 | Thirty‐day mortality, MI, stroke, reoperation, composite morbidity | N/A | Lower risk‐adjusted operative mortality (OR: 0.76, CI: 0.62‐0.94) and morbidity (OR: 0.55, CI: 0.32‐0.93) with statin therapy |
| Tabata et al., 2008 (retrospective cohort)38 | 1026 | 363 | 197 Atorva, 10 mg (5–80 mg); 126 Simva, 20 mg (10–80 mg); 21 Prava, 20 mg (10–80 mg); 8 Fluva, 40 mg (20–40 mg); 7 Lova, 20 mg (20–80 mg); 4 rosuvastatin, 20 mg | Statin at time of admission | Elective IVS, January 2002–December 2005 | Thirty‐day mortality, stroke, MI | N/A | Lower operative mortality in patients receiving statin therapy (OR: 0.25, CI: 0.12‐0.54, P = 0.0004); no change in stroke (OR: 0.48, CI: 0.19‐1.22, P = 0.12) or MI (OR: 0.91, CI: 0.43, 1.91, P = 0.8025) |
| Virani et al., 2008 (retrospective cohort)40 | 570 | 255 | Atorvastatin, cerivastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, simvastatina | NS | Elective IVS, January 2001–December 2006 | Thirty‐day mortality, MACE, MI, stroke | 1.57 | No significant mortality (OR: 0.89, CI: 0.38‐1.96, P = 0.76), MACE (OR: 1.09, CI: 0.61‐1.96, P = 0.76), MI (OR: 1.28, CI: 0.37‐4.41, P = 0.70) or stroke (OR: 1.28, CI: 0.53‐3.10, P = 0.57) benefit with statin therapy |
| Fedoruk et al., 2008 (retrospective cohort)36 | 244 | 203 | NS | Statin at time of preassessment | Consecutive all IVS, July 2004–February 2006 | Thirty‐day mortality, stroke, renal failure | N/A | No significant change in adjusted mortality (OR: 2.70, CI: 0.81‐9.05, P = 0.108); lower adjusted stroke (OR: 5.82, CI: 1.01‐33.59, P = 0.049), renal failure (OR: 2.17, CI: 0.82‐5.70, P = 0.117), and MACE (OR: 2.65, CI: 1.24‐5.66, P = 0.012) with statin therapy |
| Folkeringa et al., 2011 (retrospective cohort)37 | 193 | 79 | NS | Statin ≥1 week preoperative | Consecutive all IVS, July 1996–March 2004 | Thirty‐day mortality AF | N/A | No reduction in AF (OR: 1.49, CI: 0.651‐3.403, P = 0.345) or 30‐day mortality (4% both groups, P = 0.971) with statin therapy |
| Borger et al., 2010 (prospective, observational cohort)34 | 5538 | 4216 | NS | NS | Consecutive IVS ± other, October 2001–May 2008 | MI, LCOS, neurologic injury, renal dysfunction, infection | 3.5 ± 1.9 | No difference in adjusted 30‐day mortality (OR: 0.89, CI: 0.75‐1.06], P = 0.2), long‐term survival (OR: 0.97, CI: 0.88‐1.07, P = 0.6) or combined MI/LCOS/mortality (OR: 0.85, CI: 0.5‐1.45, P = 0.6) with statin therapy |
| Angeloni et al., 2011 (retrospective cohort)33 | 1104 | 1104 | Atorvastatin, simvastatin, rosuvastatin, fluvastatina | NS | Consecutive elective IVS, May 2003–May 2009, 2 centers | Early: mortality, MI, stroke, arrhythmia; Late: mortality, MI, stroke, arrhythmia | 2.25 | Statin therapy independently associated with reduction in operative mortality (OR: 0.48, CI: 0.32‐0.89, P = 0.001), arrhythmia (OR: 0.68, CI: 0.52‐0.96, P = 0.006) and stroke (OR: 0.54, CI: 0.32‐0.92, P = 0.02); lower mortality (P = 0.04), stroke (P = 0.001), arrhythmia (P = 0.03) and MACE (P = 0.0001) at follow‐up with statin treatment; no difference in MI (P = 0.59) |
| Allou et al., 2010 (prospective, observational cohort)32 | 525 | 247 | NS | Statin for ≥2 weeks preoperative | Consecutive elective IVS, November 2005–December 2007 | Mortality, stoke, CS, AKI, sepsis; high‐risk subgroup analysis | N/A | No difference in mortality in whole patient group analysis (P = 0.6); mortality benefit with statins in high‐risk, propensity‐matched subgroup (OR: 0.41, CI: 0.17‐0.97, P = 0.043); no difference in stroke (P = 0.6), CS (P = 0.9), AKI (P = 0.2), sepsis (P = 0.9) or peak TN‐I (P = 0.2) in high‐risk patients receiving statins |
| Vaduganathan et al., 2012 (retrospective cohort)39 | 381 | 381 | NS | NS | All valve ± other, April 2004–April 2010 | Thirty‐day mortality, readmission, late mortality | 2.74 ± 1.88 | Lower 30‐day mortality (1.3% vs 4.2%, P = 0.03) and improved long‐term survival (7.3% vs 11.5%, P = 0.06) with statin therapy; No difference in total postoperative complications (P = 0.56), stroke (P = 1.0) or AF (P = 0.86) between statin and no‐statin groups |
Abbreviations: AF, atrial fibrillation; AKI, acute kidney injury; CABG, coronary artery bypass grafting; CI, 95% confidence interval; CS, cardiogenic shock; IVS, isolated valve surgery; LCOS, low cardiac output syndrome; MACE, major cardiac events; MI, myocardial infarction; N/A, not available; NS, not specified; OR, odds ratio; TN‐I, troponin‐I.
Dose not specified.
Statins in Isolated Valve Surgery
Mortality
Six studies described the effect of preoperative statin therapy on early mortality33, 36, 37, 38, 39, 40 and 3 studies assessed mortality at mid‐late follow‐up.32, 33, 39 In the largest study to date, Angeloni et al33 observed an independent association between preoperative statin therapy and lower in‐hospital and mid‐term mortality (odds ratio [OR]: 0.48, 95% confidence interval [CI]: 0.32‐0.89, P = 0.001 and 4.1% vs 6.1% at 24 months, P = 0.04), supporting Tabata et al's 2008 findings (Table 1).38 Most recently, Vaduganathan et al. were also able to show a significant reduction in 30‐day mortality with preoperative statins (1.3% vs 4.2%; P = 0.03) as well as a trend toward lower long‐term mortality (7.3% vs 11.5%, P = 0.06) at a mean follow‐up of 32.9 ± 22.5 months.39
Conversely, however, a similar number of studies report conflicting evidence. Neither the work by Virani (N = 825) nor that by Folkeringa (N = 272) was able to demonstrate an improvement in 30‐day mortality with statin therapy.37, 40 Furthermore, even though Allou et al. do highlight a potential role for statins in reducing mortality in high‐risk patients, no effect was observed in their overall patient cohort (Table 1).32
Morbidity
Three studies examined the effect of preoperative statin therapy on stroke.33, 38, 40 Neither Tabata et al. nor Virani et al. observed any relationship between stroke and statin treatment (OR: 0.48, 95% CI: 0.19‐1.22, P = 0.122 and OR: 1.28, 95% CI: 0.53‐3.10, P = 0.57, respectively). Conversely, however, Angeloni and colleagues showed statin therapy to be an independent predictor of lower early and late stroke (OR: 0.54, 95% CI: 0.32‐0.92, P = 0.02 and late 2.5% vs 5.1%, P = 0.001).33
Two studies examined the role of statins in reducing postoperative arrhythmia.33, 37 In the largest study to date, Angeloni et al33 found statin therapy to be associated with a reduction in all‐cause postoperative arrhythmia (OR: 0.76, 95% CI: 0.62‐0.73, P = 0.006), an effect also apparent at 27‐month follow‐up (25% vs 29.1%, P = 0.03). However, in their focused study of POAF, Folkeringa et al. found no significant difference between statin and non‐statin groups.37
Only 2 studies examined the effect of statins on postoperative myocardial infarction.33, 38 Neither Tabata et al. nor Angeloni et al. were able to demonstrate a relationship between statin therapy and postoperative myocardial infarction (MI) (2.2% vs 2.4% and 5.7% vs 5.1%, P = 0.59, respectively).
Three studies examined the combined effect of statins on the composite end point of combined major cardiac events (MACE).33, 38, 40 Neither Virani et al. nor Fedoruk et al. found a significant association between statin therapy and major adverse events.36, 40 However, again in their larger study, Angeloni and colleagues did demonstrate a reduction in MACE in patients receiving preoperative statins (37.2% vs 45.3%, P = 0.0001).33
Statins in Combined Valve and Coronary Artery Surgery
Two studies considered the effects of statin therapy after combined valve and CABG surgery.34, 35 Notably, however, both studies include a highly heterogeneous patient population.
Mortality
In the largest study to date, Borger et al. prospectively studied 10 061 patients, 46% of whom underwent concomitant CABG.34 Statin therapy did not lead to a significant reduction in 30‐day mortality either by univariate (7.5% vs 6.6%, P = 0.08) or multivariate analysis (OR: 0.83, 95% CI: 0.65‐1.08, P = 0.2). However, patients receiving statins did achieve improved long‐term survival (hazard ratio: 0.81; 95% CI: 0.70‐0.93, P = 0.003).
Conversely, in a similarly heterogeneous population (80% combined valve and CABG), Clark and colleagues were able to demonstrate a significantly lower 30‐day mortality with statin treatment in both adjusted and propensity‐matched groups (OR: 0.55, 95% CI: 0.32‐0.93 and OR: 0.51, 95% CI: 0.27‐0.94, respectively).35
Morbidity
After correction for preoperative comorbidities, Borger and colleagues were unable to demonstrate improvement in the composite morbidity/mortality outcome of MI, low cardiac output syndrome, and 30‐day mortality with preoperative statin therapy.34 However, using their more focused morbidity outcome of MI, stroke, reoperation, renal failure, and infection, Clark et al. observed an improvement in both risk‐adjusted (OR: 0.76, 95% CI: 0.62‐0.94) and propensity‐matched (OR: 0.71, 95% CI: 0.550‐0.92) groups.35
The Role of Statins in Bioprosthesis Degeneration
Does Hypercholesterolaemia Increase Bioprosthesis Degeneration?
In 2003, both Farivar and Cohn11 and Nollert et al42 observed a link between increased serum cholesterol and premature bioprosthesis degeneration requiring subsequent re‐operation/explant, particularly in older patients. However, in the same year, David and Ivanov were unable to reproduce this finding in either younger (≤57 years; P = 0.5) or older (>57 years; P = 0.7) patients receiving bioprosthetic aortic valve replacement (AVR).43 Similarly, later work by both Gring et al. and Le Tourneau et al. also failed to observe this relationship in their studies on AVR bioprostheses despite improved follow‐up reaching 7.3 ± 4.7 in Le Tourneau's work.44, 45
Do Statins Reduce Bioprosthesis Degeneration?
In the largest study of its kind (N = 1193), Kulik et al. failed to demonstrate any relationship between lipid‐lowering therapy and the progression of mean gradient, peak gradient, or structural valve degeneration of aortic bioprostheses at 1, 5, and 10 years.46 Similarly, statin usage did not correlate with a significant reduction in progression of mean or peak transprosthesis gradients. Conversely, however, in their smaller study (44 months follow‐up), Antonini‐Canterin et al. found less bioprosthesis degeneration progression (annual rate of increase in peak velocity of ≥0.3 m/s/y or worsening of aortic regurgitation with ≥1/3 degrees) in patients receiving statins (OR: 0.13, 95% CI: 0.03‐0.58, P = 0.002) independent of bioprosthesis type and without significant changes in lipid levels.47
Discussion
Statin therapy has long been established as a safe and effective measure in cardiovascular disease prevention. Furthermore, the multiple beneficial effects of statin therapy in CABG have led us to question whether a similar improvement in outcomes may be seen after IVS. Aside from lipid lowering, statins exert a number of beneficial pleiotropic effects, which include improvement in endothelial function, inhibition of vascular smooth muscle proliferation, and a reduction in oxidative stress. However, despite multiple biochemical studies, the clinical role of statins in IVS has not been fully established and is yet to be represented in national or international guidelines. This review, therefore, aimed to provide a systematic summary of the increasing evidence both for and against statin therapy in IVS, identify shortcomings in the current literature and propose a platform upon which future randomized studies may finally answer this difficult question.
Do Statins Have a Beneficial Effect on Mortality and Mortality After IVS?
At present, there is insufficient evidence to demonstrate a relationship between statin pretreatment and lower postoperative morbidity. Similarly, despite previously encouraging results on assessment of all cardiac procedures,27 the effect of statins on morbidity outcomes such as MACE, AF, and stroke in IVS remains conflicting.
De novo postoperative atrial fibrillation (POAF) affects 30% to 50% of patients undergoing valve surgery and is associated with increased morbidity, hospital stay, and resource utilization. It is postulated that by lowering CRP and proinflammatory cytokines such as tumor necrosis factor‐α, IL‐1, and IL‐6, as well as decreasing vascular superoxide radical formation and improving endothelial function, statins may reduce POAF. However, despite these theoretical benefits and a number of studies reporting lower AF rates with statins after CABG,23, 48, 49 results after IVS remain equivocal.33, 37 Several potential reasons for this discrepancy exist, notably due to the quality of data available. First, despite evidence for a dose‐ dependent reduction in AF,24 neither study specified the dose of drug administered and the type of statin used was highly heterogeneous. Second, the etiology of arrhythmias other than AF is often very different (particularly those of ventricular origin), and the mechanisms whereby statins reduce all‐cause arrhythmia may consequently differ from those in AF.
Also of interest is the potential benefit of statins in stroke prevention. In their 2006 study, Aboyans et al. demonstrated a protective effect of statins on stroke after CABG, thought to be mediated by a combination of lipid lowering and the pleiotropic plaque stabilizing and antiplatelet effects of statin therapy.50 However, numerous other studies have failed to show similar benefits.51, 52, 53 At present, there is insufficient evidence to demonstrate a relationship between statins and postoperative stroke reduction.
Do Statins Have a Beneficial Effect in Reducing Bioprosthesis Degeneration?
Several different questions have been raised regarding the effect of statins on bioprosthesis longevity: First, does hyperlipidemia lead to structural valve degeneration and is it an independent predictor of valve explant and reoperation? Second, if this is the case, do statins thus prevent bioprosthesis degeneration by lipid lowering? And finally, if not, do statins exert pleiotropic effects that improve bioprosthesis longevity?
Summarized in a recent review by Gilmanov et al.,54 several studies have failed to demonstrate a relationship between cholesterol, bioprosthesis calcification, and subsequent degeneration,43, 44, 45 whereas only 1 group has observed a link between cholesterol and valve calcification, explant, and rereplacement.11 Although it has also been suggested that cholesterol levels may be of greater significance in younger patients,42 this has not been supported elsewhere in the literature. It is therefore unlikely that hypercholesterolemia leads to accelerated structural valve deterioration (SVD) in vivo, and thus lipid lowering alone would not be anticipated to result in an improvement in bioprosthesis longevity.
As such, if statins were to prevent SVD, this must occur as a result of mechanisms other than lipid lowering. Such a theory has been supported by Antonini‐Canterin et al., who demonstrated a reduction in degeneration progression of all types of aortic bioprosthesis with statin treatment despite insignificant changes in lipid levels.10 This finding may be partly explained by the ability for statins to reduce serum CRP. In their 2006 work, Skowasch et al.55 demonstrated a significant increase in both valvular and serum CRP in explanted degenerative aortic valves when compared to nonstenotic control specimens. Furthermore, the increases in CRP were significantly higher in explanted bioprostheses than native stenotic valves, potentially also explaining Antonini's findings. However, the evidence for statins in reducing bioprosthesis degeneration at best remains limited, and it is plausible that these theoretical biochemical benefits may not translate into a significant improvement in clinical outcomes.
Limitations
The heterogeneity in statin type, duration, and dose as well as shortfalls in cholesterol monitoring and follow‐up, limits our ability to pool and perform statistical comparison of data available within the current literature. Furthermore, the retrospective, observational nature of these studies along with varying inclusion criteria regarding type of valve surgery, concomitant drug therapy, and numerous intraoperative variables, including type of bioprosthesis, all reduce study quality. Moreover, despite evidence from several RCTs suggesting a 3‐week period of treatment prior to CABG may be necessary to produce protective effects,8, 50 the duration of preoperative statin therapy is currently widely heterogeneous and may be too short to achieve a significant benefit.
However, even though only a randomized prospective trial is likely to finally determine any short‐ and/or long‐term benefits of statins in IVS, there are several ethical considerations when designing such a trial. Stopping preexisting statin therapy may have deleterious rebound effects, and a nonrandomized prospective trial will carry inherent bias.56 As such, a well‐designed, multicenter, prospective, cohort study may be the only way to answer this question, performing multiple subgroup analyses by statin type and dosage as well as controlling for intraoperative variables including position and type of bioprosthesis. Furthermore, such a study would also benefit from an assessment of the duration of preoperative statin use, in order to ascertain the most favorable time window to attain an improvement in short‐ and long‐term outcomes.
Should All Patients Receive Statin Therapy Anyway?
Although questions remain regarding the quality of available evidence, the potential morbidity and mortality benefits of statins have led to increasing support for their administration to all patients undergoing IVS.57 However, statins may not always be tolerated, with complications including myalgia, transaminase elevation, and rhabdomyolysis.58 Of particular note is their under‐recognized anticoagulant effect, potentially increasing perioperative bleeding through the same isoprenylation pathways responsible for their pleiotropy.59 Although largely rare, these complications highlight the need for some caution when prescribing routine statin therapy in patients without concomitant hypercholesterolemia or coronary artery disease.
Conclusion
Statins have long been associated with improved outcomes after CABG; however, their role after IVS remains uncertain. Our findings underline the lack of evidence to support an improvement in either mortality or morbidity outcomes after IVS. However, insufficient prospective data and heterogeneity in statin type, dosage, and duration of therapy limits the validity of pooled data analysis and prevents a quantitative interpretation of these results. Nonetheless, at present we conclude that statins alone are unlikely to significantly improve clinical outcomes or delay structural valve degeneration after IVS. However, the optimal dose, type, and duration of therapy remains undetermined and warrants further investigation.
Thanos Athanasiou proposed the original concept for the study and supervised this research. Jacob Chacko and Leanne Harling performed the literature search, and wrote and edited the manuscript. Data analysis was performed by Leanne Harling and Hutan Ashrafian. Leanne Harling, Hutan Ashrafian, and Thanos Athanasiou together edited and finalized the manuscript. All authors have read and approved the manuscript prior to submission.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 2. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long‐Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339:1349–1357. [DOI] [PubMed] [Google Scholar]
- 3. Palinski W. New evidence for beneficial effects of statins unrelated to lipid lowering. Arterioscler Thromb Vasc Biol. 2001;21:3–5. [DOI] [PubMed] [Google Scholar]
- 4. Takemoto M, Liao JK. Pleiotropic effects of 3‐hydroxy‐3‐methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–1719. [DOI] [PubMed] [Google Scholar]
- 5. Zhou Q, Liao JK. Statins and cardiovascular diseases: from cholesterol lowering to pleiotropy. Curr Pharm Des. 2009;15:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:S715–S720. [DOI] [PubMed] [Google Scholar]
- 7. Bruins P, te Velthuis H, Yazdanbakhsh AP, et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C‐reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–3548. [DOI] [PubMed] [Google Scholar]
- 8. Chello M, Patti G, Candura D, et al. Effects of atorvastatin on systemic inflammatory response after coronary bypass surgery. Crit Care Med. 2006;34:660–667. [DOI] [PubMed] [Google Scholar]
- 9. Hoffmann G, Lutter G, Cremer J. Durability of bioprosthetic cardiac valves. Dtsch Arztebl Int. 2008;105:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Antonini‐Canterin F, Zuppiroli A, Popescu BA, et al. Effect of statins on the progression of bioprosthetic aortic valve degeneration. Am J Cardiol. 2003;92:1479–1482. [DOI] [PubMed] [Google Scholar]
- 11. Farivar RS, Cohn LH. Hypercholesterolemia is a risk factor for bioprosthetic valve calcification and explantation. J Thorac Cardiovasc Surg. 2003;126:969–975. [DOI] [PubMed] [Google Scholar]
- 12. Antonini‐Canterin F, Popescu BA, Zuppiroli A, et al. Are statins effective in preventing bioprosthetic aortic valve failure? A need for a prospective, randomized trial. Ital Heart J. 2004;5:85–88. [PubMed] [Google Scholar]
- 13. Brown MS, Goldstein JL. A receptor‐mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. [DOI] [PubMed] [Google Scholar]
- 14. Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG‐CoA reductase. Science. 2001;292:1160–1164. [DOI] [PubMed] [Google Scholar]
- 15. Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. [DOI] [PubMed] [Google Scholar]
- 16. Van Aelst L, D'Souza‐Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. [DOI] [PubMed] [Google Scholar]
- 17. Chandrasekar B, Mummidi S, Mahimainathan L, et al. Interleukin‐18‐induced human coronary artery smooth muscle cell migration is dependent on NF‐kappaB‐ and AP‐1‐mediated matrix metalloproteinase‐9 expression and is inhibited by atorvastatin. J Biol Chem. 2006;281:15099–15109. [DOI] [PubMed] [Google Scholar]
- 18. Nakagami H, Jensen KS, Liao JK. A novel pleiotropic effect of statins: prevention of cardiac hypertrophy by cholesterol‐independent mechanisms. Ann Med. 2003;35:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yano M, Matsumura T, Senokuchi T, et al. Statins activate peroxisome proliferator‐activated receptor gamma through extracellular signal‐regulated kinase 1/2 and p38 mitogen‐activated protein kinase‐dependent cyclooxygenase‐2 expression in macrophages. Circ Res. 2007;100:1442–1451. [DOI] [PubMed] [Google Scholar]
- 20. Antoniades C, Bakogiannis C, Leeson P, et al. Rapid, direct effects of statin treatment on arterial redox state and nitric oxide bioavailability in human atherosclerosis via tetrahydrobiopterin‐mediated endothelial nitric oxide synthase coupling. Circulation. 2011;124:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laufs U, Gertz K, Huang P, et al. Atorvastatin upregulates type III nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke. 2000;31:2442–2449. [DOI] [PubMed] [Google Scholar]
- 22. Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:2610–2642. [DOI] [PubMed] [Google Scholar]
- 23. Karimi A, Bidhendi LM, Rezvanfard M, et al. The effect of a high dose of atorvastatin on the occurrence of atrial fibrillation after coronary artery bypass grafting. Ann Thorac Surg. 2012;94:8–14. [DOI] [PubMed] [Google Scholar]
- 24. Kourliouros A, De Souza A, Roberts N, et al. Dose‐related effect of statins on atrial fibrillation after cardiac surgery. Ann Thorac Surg. 2008;85:1515–1520. [DOI] [PubMed] [Google Scholar]
- 25. Kourliouros A, Roberts N, Jahangiri M. Statins with equivalent lipid‐lowering capacity exhibit differential effects on atrial fibrillation after cardiac surgery. J Thorac Cardiovasc Surg. 2008;136:1100–1101; author reply 1101. [DOI] [PubMed] [Google Scholar]
- 26. Patti G, Chello M, Candura D, et al. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA‐3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation. 2006;114:1455–1461. [DOI] [PubMed] [Google Scholar]
- 27. Liakopoulos OJ, Kuhn EW, Slottosch I, et al. Preoperative statin therapy for patients undergoing cardiac surgery. Cochrane Database Syst Rev. 2012;4:CD008493. [DOI] [PubMed] [Google Scholar]
- 28. Mithani S, Kuskowski M, Slinin Y, et al. Dose‐dependent effect of statins on the incidence of acute kidney injury after cardiac surgery. Ann Thorac Surg. 2011;91:520–525. [DOI] [PubMed] [Google Scholar]
- 29. The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS) ; Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 30.Butchart EG, Gohlke‐Barwolf C, Antunes MJ, et al. Working Groups on Valvular Heart Disease, Thrombosis, and Cardiac Rehabilitation and Exercise Physiology, European Society of Cardiology. Recommendations for the management of patients after heart valve surgery. Eur Heart J. 2005;26:2463–2471 [DOI] [PubMed] [Google Scholar]
- 31. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Allou N, Augustin P, Dufour G, et al. Preoperative statin treatment is associated with reduced postoperative mortality after isolated cardiac valve surgery in high‐risk patients. J Cardiothorac Vasc Anesth. 2010;24:921–926. [DOI] [PubMed] [Google Scholar]
- 33. Angeloni E, Melina G, Benedetto U, et al. Statins improve outcome in isolated heart valve operations: a propensity score analysis of 3,217 patients. Ann Thorac Surg. 2011;92:68–73. [DOI] [PubMed] [Google Scholar]
- 34. Borger MA, Seeburger J, Walther T, et al. Effect of preoperative statin therapy on patients undergoing isolated and combined valvular heart surgery. Ann Thorac Surg. 2010;89:773–779; discussion 779–780. [DOI] [PubMed] [Google Scholar]
- 35. Clark LL, Ikonomidis JS, Crawford FA Jr, et al. Preoperative statin treatment is associated with reduced postoperative mortality and morbidity in patients undergoing cardiac surgery: an 8‐year retrospective cohort study. J Thorac Cardiovasc Surg. 2006;131:679–685. [DOI] [PubMed] [Google Scholar]
- 36. Fedoruk LM, Wang H, Conaway MR, et al. Statin therapy improves outcomes after valvular heart surgery. Ann Thorac Surg. 2008;85:1521–1525; discussion 5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Folkeringa RJ, Tieleman RG, Maessen JG, et al. Statins Do not reduce atrial fibrillation after cardiac valvular surgery: a single centre observational study. Neth Heart J. 2011;19:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tabata M, Khalpey Z, Cohn LH, et al. Effect of preoperative statins in patients without coronary artery disease who undergo cardiac surgery. J Thorac Cardiovasc Surg. 2008;136:1510–1513. [DOI] [PubMed] [Google Scholar]
- 39. Vaduganathan M, Stone NJ, Andrei AC, et al. Midterm benefits of preoperative statin therapy in patients undergoing isolated valve surgery. Ann Thorac Surg. 2012;93:1881–1887. [DOI] [PubMed] [Google Scholar]
- 40. Virani SS, Nambi V, Lee VV, et al. Does preoperative statin therapy improve outcomes in patients undergoing isolated cardiac valve surgery? Am J Cardiol. 2008;102:1235–1239. [DOI] [PubMed] [Google Scholar]
- 41. Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e652–e735. [DOI] [PubMed] [Google Scholar]
- 42. Nollert G, Miksch J, Kreuzer E, et al. Risk factors for atherosclerosis and the degeneration of pericardial valves after aortic valve replacement. J Thorac Cardiovasc Surg. 2003;126:965–968. [DOI] [PubMed] [Google Scholar]
- 43. David TE, Ivanov J. Is degenerative calcification of the native aortic valve similar to calcification of bioprosthetic heart valves? J Thorac Cardiovasc Surg. 2003;126:939–941. [DOI] [PubMed] [Google Scholar]
- 44. Le Tourneau T, Marechaux S, Vincentelli A, et al. Cardiovascular risk factors as predictors of early and late survival after bioprosthetic valve replacement for aortic stenosis. J Heart Valve Dis. 2007;16:483–488. [PubMed] [Google Scholar]
- 45. Gring CN, Houghtaling P, Novaro GM, et al. Preoperative cholesterol levels do not predict explant for structural valve deterioration in patients undergoing bioprosthetic aortic valve replacement. J Heart Valve Dis. 2006;15:261–268. [PubMed] [Google Scholar]
- 46. Kulik A, Masters RG, Bedard P, et al. Postoperative lipid‐lowering therapy and bioprosthesis structural valve deterioration: justification for a randomised trial? Eur J Cardiothorac Surg. 2010;37:139–144. [DOI] [PubMed] [Google Scholar]
- 47. Antonini‐Canterin F, Zuppiroli A, Baldessin F, et al. Is there a role of statins in the prevention of aortic biological prostheses degeneration. Cardiovasc Ultrasound. 2006;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Song YB, On YK, Kim JH, et al. The effects of atorvastatin on the occurrence of postoperative atrial fibrillation after off‐pump coronary artery bypass grafting surgery. Am Heart J. 2008;156:373 e9–e16. [DOI] [PubMed] [Google Scholar]
- 49. Sakamoto H, Watanabe Y, Satou M. Do preoperative statins reduce atrial fibrillation after coronary artery bypass grafting? Ann Thorac Cardiovasc Surg. 2011;17:376–382. [DOI] [PubMed] [Google Scholar]
- 50. Aboyans V, Labrousse L, Lacroix P, et al. Predictive factors of stroke in patients undergoing coronary bypass grafting: statins are protective. Eur J Cardiothorac Surg. 2006;30:300–304. [DOI] [PubMed] [Google Scholar]
- 51. Pan W, Pintar T, Anton J, et al. Statins are associated with a reduced incidence of perioperative mortality after coronary artery bypass graft surgery. Circulation. 2004;110:II45–II49. [DOI] [PubMed] [Google Scholar]
- 52. Koenig MA, Grega MA, Bailey MM, et al. Statin use and neurologic morbidity after coronary artery bypass grafting: A cohort study. Neurology. 2009;73:2099–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Subramaniam K, Koch CG, Bashour A, et al. Preoperative statin intake and morbid events after isolated coronary artery bypass grafting. J Clin Anesth. 2008;20:4–11. [DOI] [PubMed] [Google Scholar]
- 54. Gilmanov D, Bevilacqua S, Mazzone A, et al. Do statins slow the process of calcification of aortic tissue valves? Interact Cardiovasc Thorac Surg. 2010;11:297–301. [DOI] [PubMed] [Google Scholar]
- 55. Skowasch D, Schrempf S, Preusse CJ, Likungu JA, Welz A, Luderitz B, et al. Tissue resident C reactive protein in degenerative aortic valves: correlation with serum C reactive protein concentrations and modification by statins. Heart. 2006;92:495–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pineda A, Cubeddu LX. Statin rebound or withdrawal syndrome: does it exist? Curr Atheroscler Rep. 2011;13:23–30. [DOI] [PubMed] [Google Scholar]
- 57. Paraskevas KI, Mikhailidis DP. Statins may not prevent structural valve degeneration of aortic bioprosthetic valves, but should probably be prescribed to patients undergoing heart valve surgery nonetheless. Interact Cardiovasc Thorac Surg. 2010;11:302. [DOI] [PubMed] [Google Scholar]
- 58. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C–60C. [DOI] [PubMed] [Google Scholar]
- 59. Hauer‐Jensen M, Fort C, Mehta JL, et al. Influence of statins on postoperative wound complications after inguinal or ventral herniorrhaphy. Hernia. 2006;10:48–52. [DOI] [PubMed] [Google Scholar]


