Abstract
Background
Hypercholesterolemia is a strong risk factor for myocardial infarction (MI). There is scarce information regarding lipoprotein levels among patients with MI in Latin America as well as about the association of very early statin therapy during the course of acute MI.
Hypothesis
Very early statin prescription might be associated with a reduction on in‐hospital mortality in MI patients with nearly normal lipid levels.
Methods
Prospective registry database analysis of MI patients admitted between 2001 and 2007 at a single university hospital from which demographics, treatments, clinical variables, and mortality were assessed. Patients naïve to statin therapy were divided in 2 groups, according to whether they received (group A) or did not receive (group B) statins during the first 24 hours after admission.
Results
In the 1465 patients analyzed, mean plasma levels of total cholesterol, low‐density lipoprotein cholesterol, and high‐density lipoprotein cholesterol (HDL‐C) were 197, 117, and 44 mg/dL, respectively, and 41.8% had HDL‐C ≤40 mg/dL. Among statin naïve patients (n = 1272), 67% were classified in group A and 33% in group B. Overall in‐hospital mortality was 4.1%: 1.8% in group A and 8.5% in group B. In the multivariate analysis, including propensity score for statin prescription, the odds ratio for in‐hospital mortality for group A was 0.971 (95% confidence interval: 0.944‐0.999, P = 0.04).
Conclusions
In the Chilean registry of MI patients, low HDL‐C was the main lipid disturbance. Very early statin use after MI appears to be associated with a borderline significant and independent reduction of in‐hospital mortality.
Introduction
High total cholesterol (total‐C) and low‐density lipoprotein cholesterol (LDL‐C) levels are strong risk factors for myocardial infarction (MI).1 Use of statins is a mainstay of therapy for lowering cholesterol levels and has been associated with significant reductions in mortality in patients with coronary artery disease (CAD).2, 3 Accordingly, clinical guidelines support the achievement of strict goals for LDL‐C, high‐density lipoprotein cholesterol (HDL‐C), and non–HDL‐C levels for primary and secondary prevention of CAD.4 However, approximately two‐thirds of patients presenting with MI have LDL‐C levels <130 mg/dL, a cutoff within current primary prevention guideline‐recommended targets for LDL‐C.5 In addition to reducing LDL‐C, statins exert beneficial pleiotropic properties on endothelial function, platelet mediated aggregation, and inflammation, effects that have been associated with reduced reperfusion injury and infarct size.6, 7 These pleiotropic effects might confer additional benefit during the early hours following MI.
In the present study, we analyzed the lipid profiles of patients admitted with MI, their relationships with statin prescription, and the impact of very early administration of statins following MI (ie, within the first 24 hours) on in‐hospital mortality.
Methods
We analyzed the database of patients admitted with acute MI to the coronary care unit (CCU) at a university tertiary center between January 2001 and December 2007. Each admitted patient with the diagnosis of MI was prospectively registered in a specialized observational clinical record form designed by the working group of the Chilean MI Registry (GEMI), which has been previously described.8
Patients with MI fulfilled at least 2 of the following diagnostic criteria: (1) typical chest pain; (2) ST‐segment deviation > 1 mm in 2 or more consecutive electrocardiographic leads; and (3) characteristic elevation of creatine kinase and/or troponin I. Thus, both patients with ST‐segment elevation myocardial infarction (STEMI) as well as non‐STEMI were included in the analysis. Data entry into the registry was performed by a trained and skilled nurse and reviewed by a staff cardiologist from the CCU.
Data Collection
Information from 1465 MI patients was analyzed, including demographics, coronary risk factors, prior cardiovascular history, previous and in‐hospital treatments, clinical variables at presentation (eg, heart rate, systolic arterial pressure, Killip class, time from onset of chest pain to treatment), basic laboratory workup, electrocardiography, location and type of MI, adverse events during hospitalization (eg, arrhythmias, heart failure, reinfarction, mechanical complications), and in‐hospital mortality. This analysis included all patients who had full information with respect to statin use, lipid profile measured at admission, and in‐hospital mortality.
All lipid profiles were measured by the same laboratory and with the same enzymatic method (Hitachi). HDL‐C was calculated using the Friedewald formula. Because of the observational nature of this study, patients received a variety of statins, including atorvastatin, simvastatin, rosuvastatin, and pravastatin.
For the purposes of analysis, patients who were not previously receiving statins were divided into 2 groups: (group A) very early administration of statins (defined as initiation within the first 24 hours after MI) and (group B) no very early administration.
Data Analysis
Statistical analysis was made using Stata 8.0 software (StataCorp, College Station, TX). Descriptive statistics were generated for baseline demographics and clinical characteristics across patient groups. Fisher exact test, Student t test, and univariate and multivariate logistic regression were used. Unadjusted odds ratios (ORs) and 95% confidence intervals (CI) for in‐hospital mortality according to statin use groups (A and B) and type of MI (STEMI and non‐STEMI) were determined. A 2‐tailed P value ≤0.05 was considered statistically significant.
To control for confounding variables associated with in‐hospital mortality, a logistic regression analysis was performed. In the first block, data on demographics, prior cardiovascular history, duration of symptoms upon admission, and year of infarction were entered. The second block included clinical variables on admission: heart rate, systolic arterial pressure, Killip class, MI type and location, and lipid profile. Medical and interventional treatments received during the first 24 hours after MI were added to the third block. Blocks were entered cumulatively until the complete model was produced.
To minimize any bias due to the prescription of statins during the first 24 hours, a propensity score was generated.9 This method indicates the propensity of having the same baseline characteristics for each patient. The probability of receiving statins was assessed by a multivariate regression analysis that included all variables previously described. This propensity score was added to the analysis and led to the final model.
Results
We analyzed 1465 patients in this study, 46% of whom had STEMI. Demographic and clinical characteristics of patients according to gender are shown in Table 1. Mean plasma levels of total‐C, LDL‐C, and HDL‐C were 197 mg/dL, 117 mg/dL, and 44 mg/dL, respectively. Women showed significantly higher total‐C levels, mainly driven by higher HDL‐C. The histograms in Figure 1 show the distribution of LDL‐C and HDL‐C for the whole population. Interestingly, 62.3% of patients had LDL‐C levels within acceptable limits according to current guidelines4 (ie, LDL‐C <130 mg/dL), and few patients (14.9%) had high LDL‐C (>160 mg/dL). The majority of patients (89.2%) had HDL‐C levels <60 mg/dL, and a significant proportion (41.8%) had very low HDL‐C levels (≤40 mg/dL). Therefore, most patients recorded in our registry had normal range LDL‐C and markedly low HDL‐C levels.
Table 1.
Demographic, Cardiovascular, and Clinical Characteristics of Patients With Myocardial Infarction on Admission, According to Gender
| Total, N = 1465 (100) | Males, n = 1075 (73.3) | Females, n = 390 (26.7) | P Value | |
|---|---|---|---|---|
| Age, y | 64 (13) | 61 (11) | 70 (14) | <0.001 |
| STEMI, % | 45.3 | 44 | 49 | 0.044 |
| Hypertension, % | 58.9 | 57 | 64.1 | 0.007 |
| Hyperlipidemia, % | 50.1 | 52 | 45.1 | 0.009 |
| Diabetes mellitus, % | 23.4 | 23.6 | 23 | 0.4 |
| CKD, % | 0.2 | 0.2 | 0.2 | 0.5 |
| CDFH, % | 19.4 | 20.9 | 15.3 | 0.008 |
| Smoking, % | 34.1 | 38.5 | 22.3 | <0.001 |
| Previous MI, % | 16.1 | 17 | 13.5 | 0.05 |
| Previous heart failure, % | 5.2 | 5.0 | 5.6 | 0.3 |
| Thrombolysis, % | 3.9 | 4.7 | 1.8 | 0.004 |
| 1° PCI, % | 27.2 | 29 | 22.3 | 0.005 |
| SAP on admission, mm Hg | 137 (26) | 137 (22) | 138 (32) | 0.3 |
| HR, bpm | 79 (19) | 78 (18) | 81 (20) | 0.003 |
| Killip >1, % | 18.7 | 16.9 | 23 | 0.004 |
| Peak CK, U/L | 1481 (1465) | 1575 (1912) | 1205 (1115) | <0.001 |
| Plasma glucose, mg/dL | 149 (71) | 148 (70) | 151 (68) | 0.2 |
| Total cholesterol, mg/dL | 197 (47) | 195 (465) | 203 (51) | 0.03 |
| HDL‐C, mg/dL | 44 (13) | 43 (23) | 55 (37) | 0.001 |
| LDL‐C, mg/dL | 117 (42) | 115 (41) | 118 (51) | 0.2 |
| Triglycerides, mg/dL | 186 (134) | 195 (141) | 157 (103) | 0.001 |
| VT‐VF, % | 0.7 | 1 | 0.2 | 0.06 |
| Reinfarction, % | 0.3 | 0.2 | 0.7 | 0.1 |
| Heart failure, % | 7.5 | 6.6 | 10 | 0.01 |
| Mortality, % | 4.1 | 3.3 | 6.2 | 0.006 |
Abbreviations: CDFH, cardiovascular disease family history; CK, creatine kinase; CKD, chronic kidney disease; HDL‐C, high‐density lipoprotein cholesterol; HR, heart rate; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; PCI, percutaneous coronary intervention; SAP, systolic arterial pressure; STEMI, ST‐segment elevation myocardial infarction; VF, ventricular fibrillation; VT, ventricular tachycardia.
P value is for the comparison between males and females. Data are presented as mean (standard deviation) except where otherwise indicated.
Figure 1.
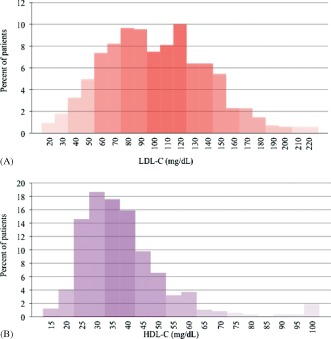
Lipoprotein levels histograms for patients admitted with myocardial infarction. (A) LDL‐C levels. (B) HDL‐C levels. Abbreviations: HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
Only 13% of patients were on statin therapy before their MI, and 58% received the medication very early after admission. The remaining 29% of patients were neither using nor were started on statins during the early hours following MI. As shown in Figure 2, very early use of statins increased progressively throughout the study period, whereas early prescription of other commonly used drugs after MI did not change during the same period of time.
Figure 2.
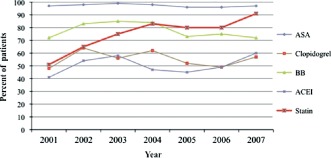
Changes in prescription patterns during the first 24 hours for myocardial infarction patients throughout the study period (2001–2007). Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ASA, aspirin; BB, β‐blocker.
Given that the majority of patients were not receiving statins at the time of admission, we focused on statin‐naïve patients to analyze the influence of its initiation on the very early phase following MI. Even though average LDL‐C levels were below the threshold of 130 mg/dL, lipid levels were significantly higher among patients who were prescribed statins within the first 24 hours after admission (Table 2). Of note, 77% of patients with LDL‐C ≥100 mg/dL on admission were prescribed statins within the first 24 hours vs 57% of patients with LDL‐C <100 mg/dL at entry (P < 0.001). This association was maintained after multivariate analysis (OR: 2.5, 95% CI: 1.8‐3.5) for early statin prescription in patients with LDL‐C ≥100 mg/dL. Thus, two‐thirds of patients were given statins within the first 24 hours after admission (group A), and one‐third did not receive these drugs soon after MI (group B). Of the 422 group B patients, 234 (55%) were discharged on statins, with 82% of those patients initiating therapy on the day of discharge.
Table 2.
Demographic, Cardiovascular, and Clinical Characteristics of Statin Naïve Patients With Myocardial Infarction on Admission, According to Statin Use
| Group | Very Early Start, Group A, (n = 850) | No Very Early Start, Group B (n = 422) | P Value |
|---|---|---|---|
| Age, y | 62 (13) | 67 (14) | <0.001 |
| Women, % | 26.7 | 31 | 0.001 |
| STEMI, % | 57 | 54 | <0.001 |
| Hypertension, % | 55.5 | 60 | <0.001 |
| Hyperlipidemia, % | 48 | 40 | <0.001 |
| Diabetes mellitus, % | 21 | 24 | 0.008 |
| CKD, % | 0.2 | 0.2 | 0.2 |
| CDFH, % | 20.5 | 17 | 0.1 |
| Smoking, % | 37 | 28 | <0.001 |
| Previous MI, % | 10 | 15 | <0.001 |
| Previous heart failure, % | 5.5 | 3 | <0.001 |
| Thrombolysis, % | 4 | 3 | 0.7 |
| 1° PCI, % | 30 | 25 | 0.002 |
| SAP on admission, mm Hg | 138 (26) | 135 (29) | 0.01 |
| HR, bpm | 79 (18) | 82 (19) | 0.001 |
| Killip >1, % | 17 | 33 | 0.01 |
| Peak CK, U/L | 1572 (1981) | 1492 (1695) | <0.001 |
| Plasma glucose, mg/dL | 146 (66) | 153 (79) | 0.04 |
| Total cholesterol, mg/dL | 208 (46) | 183 (43) | <0.001 |
| HDL‐C, mg/dL | 46 (22) | 47 (37) | 0.1 |
| LDL‐C, mg/dL | 125 (53) | 105 (37) | <0.001 |
| Triglycerides, mg/dL | 190 (133) | 186 (43) | 0.05 |
| VT‐VF, % | 1 | 0.2 | 0.3 |
| Reinfarction, % | 0.2 | 0.4 | 0.5 |
| Heart failure, % | 5 | 11 | <0.001 |
| Mortality, % | 1.8 | 8.5 | <0.001 |
Abbreviations: CDFH, cardiovascular disease family history; CK, creatine kinase; CKD, chronic kidney disease; HDL‐C, high‐density lipoprotein cholesterol; HR, heart rate; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; PCI, percutaneous coronary intervention; SAP, systemic arterial pressure; STEMI, ST‐segment elevation myocardial infarction; VF, ventricular fibrillation; VT, ventricular tachycardia.
P value is for the comparison between group A and group B. Data are presented as mean (SD) except where indicated otherwise. Very early start was defined as initiation of statins within the first 24 hours after MI.
Overall in‐hospital mortality was 4.1%, but differed among the statin‐naïve groups: 1.8% for group A and 8.5% for group B (P < 0.001). In patients with STEMI, the difference in in‐hospital mortality was even more pronounced: 2.0% and 11.8% for groups A and B, respectively (P < 0.001). For non‐STEMI patients, in‐hospital mortality was 1.7% for group A and 5.1% for group B (P = 0.05). After adjusting for confounding variables, the OR for overall in‐hospital mortality was 0.96 (95% CI: 0.93‐0.98) for group A vs B (Figure 3). In STEMI patients the adjusted OR was 0.93 (95% CI: 0.89‐0.96) (Figure 3). In the full model, which included the propensity score for statin prescription, the OR for in‐hospital mortality for group A vs B was 0.971 (95% CI: 0.944‐0.999, P = 0.04) (Figure 3).
Figure 3.
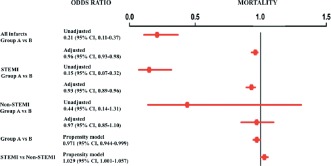
Unadjusted, adjusted, and propensity score odds ratios for in‐hospital mortality among myocardial infarction patients. Group A were patients who received very early initiation of statins (ie, within the first 24 hours after myocardial infarction). Group B were patients who did not receive very early initiation of statins. Abbreviations: CI, confidence interval; STEMI, ST‐segment elevation myocardial infarction.
Patients with previous MI (235 patients) had an average LDL‐C of 107 mg/dL, a value considered inappropriate for secondary prevention according to current guidelines.4 In fact, only 15% of these patients presented with LDL‐C below 70 mg/dL, which is currently suggested to be the goal. Interestingly, 65% of patients with previous MI were statin naïve at the moment of presentation, and a significant difference in in‐hospital mortality was found between those statin naïve patients who received very early statins in comparison with those who did not (2.2% vs 12%, P < 0.001). No differences in life‐threatening cardiac arrhythmias (1% and 0.2% for groups A and B, respectively; P = 0.1) or nonfatal reinfarction (0.2% and 0.4%, respectively, P = 0.5) were observed. However, group B patients had a higher incidence of heart failure during their hospitalization (11% vs 5%; P < 0.001). Killip class > 1 at admission remained significantly associated with mortality in the full propensity model, with an OR of 1.035 (95% CI: 1.002‐1.069, P = 0.03).
Discussion
This observational study provides useful information about several issues related to MI in Chile and perhaps in Latin America, an under‐represented region in large cardiovascular trials. In this population, cholesterol levels in patients suffering MI are characterized by a near‐normal LDL‐C and low HDL‐C pattern. These values are similar to those seen in the general Chilean adult population, as reported in 2 national health surveys conducted during 2003 and 2010.10, 11 There, reported mean levels for total‐C, LDL‐C, and HDL‐C were 189 mg/dL, 113 mg/dL, and 47 mg/dL, respectively. The global INTERHEART study found that abnormal lipids and smoking were the main risk factors for MI and contributed to two‐thirds of the population attributable risk (PAR).1 Of note, only 11% of the INTERHEART population came from Latin America. In the Latin American substudy of INTERHEART, lipid levels had a lower weight as risk factor; however, marked differences among participating countries were found: the PAR for the ratio of apolipoprotein (Apo) B to ApoA‐1 was larger in Argentina (67.6%) and Brazil (57.0%) than in Chile (35.2%).12
Our data provide important insights about the early initiation of statin therapy following MI. Although we analyzed an academic hospital where activities are subject to a high level of scrutiny, one‐third of MI patients did not receive statins in the first hours after their event. However, over the course of the study period, the very early use of statins steadily increased, reaching its highest rate in 2007. By 2007, nevertheless, almost 10% of patients did not receive statins in the immediate aftermath of MI, despite widespread awareness of large trials such as the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE‐IT) and Myocardial Ischemia Reduction with Acute Cholesterol Lowering (MIRACL) trials.13, 14 Large national registries have clearly shown that the administration of statins during the early phase of acute MI has consistently increased.15, 16 Data from the United States National Registry of Myocardial Infarction show that in 2000, 40% of patients did not receive statins during the first 24 hours following MI.17 Later, in 2009, the same registry reported that more than 80% of patients with a new MI were discharged on statins.18 Similarly, a French MI registry has shown that only 12% of patients did not receive statins during an acute coronary syndrome (ACS).19 In our population, there appears to be a relationship between LDL‐C on admission and the probability of receiving early statins. Those patients with LDL‐C considered unacceptable for secondary prevention (ie, LDL‐C >100 mg/dL) were more commonly administered very early statins compared with those patients who presented with lower levels.
This registry also shows that very early administration of statins, in addition to other evidence‐based therapies, during the course of MI is associated with a small but significant reduction in in‐hospital mortality. This association persisted after adjustment for confounding variables and probability of receiving statin therapy. Clinical trials (eg, PROVE‐IT, MIRACL, and Incremental Decrease in End Points Through Aggressive Lipid Lowering [IDEAL]) that evaluated the effects of statins initiated 3 to 7 days after ACS showed that the administration of these drugs was associated with a significant reduction in adverse cardiovascular events and long‐term mortality.13, 14, 20 Meta‐analyses including the major trials evaluating the effects of intensive statin use following ACS have demonstrated similar results.21, 22
Similar to our findings, a thorough analysis of more than 300 000 patients from an MI database in the United States, performed by Fonarow and colleagues, showed significantly lower rates of early complications and in‐hospital mortality among patients treated with statins within the first 24 hours of presentation.17 On the other hand, a later report that evaluated the influence of treatment initiated during the first 2 days after admission showed no significant effect at 4 and 12 months after discharge.23
There are several possible mechanisms that could help us understand the association between statins administered in the early phase of MI and in‐hospital mortality. Beneficial effects on endothelial function and modulating properties over inflammation cascades are thought to be responsible for the stabilization of the atherosclerotic plaque induced by statins.24, 25, 26 Timing and dose of statin therapy appear to influence its impact on inflammatory and endothelial responses during MI.27 Moreover, a meta‐analysis showed that postprocedural myocardial infarctions, particularly after percutaneous coronary interventions, are reduced by preprocedural use of statins.28 Statin therapy is also associated with reductions in the activity of a number of coagulation factors (ie, tissue factor, prothrombin, factor VII), which may reduce stability of the fibrin clots or clot formation.29, 30
Trained personnel completed our prospective database rigorously and with strict criteria. Nevertheless, the observational nature of this study and the small sample size constitute limitations. To minimize this bias, a comprehensive statistical analysis was performed to obtain consistent and reliable results. We limited our analysis to only 1 of the participating hospitals of the GEMI. This decision was made to homogenize the laboratory data, because at the time of the study different laboratory techniques to determine the lipid profile were utilized in Chilean hospitals. Unfortunately, the GEMI registry does not provide data about the dose and type of statins prescribed to patients. Thus, the possible influence that different doses of these drugs could have on in‐hospital mortality could not be addressed in the present study.
Conclusion
Low HDL‐C was the main lipid disturbance in our studied population. Two‐thirds of patients received statins during the early hours following MI, and this strategy was more commonly used in patients with higher LDL‐C levels on admission and throughout the study period. Finally, we found an association that supports the cardioprotective effects of statins when used in the first 24 hours after MI. However, the design of our study did not allow us to establish a direct relationship between very early statin use and reduction in in‐hospital mortality. Therefore, prospective clinical trials to evaluate this hypothesis are of great clinical interest. The findings from our registry support strategies that encourage very early use of statins in patients with acute MI.
Acknowledgment
The authors thank Emily K. Donovan for her assistance in the preparation of this manuscript.
Dr. Giugliano is an investigator participating in clinical trials as a member of the TIMI Study Group, which receives research grant support from Amgen and Merck. Dr. Giugliano has received honoraria for consulting and/or CME lectures from Amgen, Merck, and Sanofi.
References
- 1. Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 2. Heart Protection Study Collaborative Group . MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet. 2002;360:7–22.12114036 [Google Scholar]
- 3. Kritharides L. Reducing low‐density lipoprotein cholesterol—treating to target and meeting new European goals. Eur Heart J Suppl. 2004;6(suppl A):A12–A18. [Google Scholar]
- 4. National Institutes of Health, US Department of Health and Human Services . Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive Summary. Bethesda, MD: National Institutes of Health; 2001. NIH publication 01–3670.
- 5. Sachdeva A, Cannon C, Deedwania P, et al.; GWTG Steering Committee and Hospitals. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J. 2009;157:111–117.e2. [DOI] [PubMed] [Google Scholar]
- 6. Laufs U, La Fata V, Plutzky J, et al. Upregulation of endothelial nitric oxide synthase by HMG‐CoA reductase inhibitors. Circulation. 1998;97:1129–1135. [DOI] [PubMed] [Google Scholar]
- 7. Patti G, Pasceri V, Colonna G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA‐ACS randomized trial. J Am Coll Cardiol. 2007;49:1272–1278. [DOI] [PubMed] [Google Scholar]
- 8. Corbalan R, Nazzal C, Prieto JC, et al. Reduction of myocardial infarction mortality in Chilean hospitals [in Spanish]. Rev Med Chile. 2002;130:368–378. [PubMed] [Google Scholar]
- 9. Austin PC. Primer on statistical interpretation or methods report card on propensity‐score matching in the cardiology literature from 2004 to 2006: a systematic review. Circ Cardiovasc Qual Outcomes. 2008;1:62–67. [DOI] [PubMed] [Google Scholar]
- 10. Minsal. Encuesta Nacional de Salud Chile, 2003. http://epi.minsal.cl/epi/html/invest/ENS/ENS.htm. Accessed October 20, 2012.
- 11. Minsal. Encuenta Nacional de Salud Chile, 2010. Available at: http://www.minsal.gob.cl/portal/url/item/bcb03d7bc28b64dfe040010165012d23.pdf. Accessed October 20, 2012.
- 12. Lanas F, Avezum A, Bautista LE, et al.; INTERHEART Investigators in Latin America. Risk factors for acute myocardial infarction in Latin America: the INTERHEART Latin American study. Circulation. 2007;115:1067–1074. [DOI] [PubMed] [Google Scholar]
- 13. Cannon CP, Braunwald E, McCabe CH, et al.; Pravastatin or Atorvastatin Evaluation and Infection Therapy—Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 14. Schwartz GG, Olsson AG, Ezekowitz MD, et al.; Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711–1718. [DOI] [PubMed] [Google Scholar]
- 15. Carey IM, DeWilde S, Shah SM, et al. Statin use after first myocardial infarction in UK men and women from 1997 to 2006: Who started and who continued treatment? Nutr Metab Cardiovasc Dis. 2012;22:400–408. [DOI] [PubMed] [Google Scholar]
- 16. Helin‐Salmivaara A, Lavikainen PT, Korhonen MJ, et al. Pattern of statin use among 10 cohorts of new users from 1995 to 2004: a register‐based nationwide study. Am J Manag Care. 2010;16:116–122. [PubMed] [Google Scholar]
- 17. Fonarow GC, Wright RS, Spencer FA, et al.; National Registry of Myocardial Infarction 4 Investigators. Effect of statin use within the first 24 hours of admission for acute myocardial infarction on early morbidity and mortality. Am J Cardiol. 2005;96:611–616. [DOI] [PubMed] [Google Scholar]
- 18. Fonarow GC, French WJ, Frederick PD; National Registry of Myocardial Infarction Investigators. Trends in the use of lipid‐lowering medications at discharge in patients with acute myocardial infarction: 1998 to 2006. Am Heart J. 2009;157:185–194.e2. [DOI] [PubMed] [Google Scholar]
- 19. Ferrieres J, Bataille V, Leclercq F, et al. Patterns of statin prescription in acute myocardial infarction: the French registry of Acute ST‐elevation or non‐ST‐elevation Myocardial Infarction (FAST‐MI). Atherosclerosis. 2009;204:491–496. [DOI] [PubMed] [Google Scholar]
- 20. Pedersen TR, Faergeman O, Kastelein JJ, et al.; Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group. High‐dose atorvastatin vs usual‐dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. [DOI] [PubMed] [Google Scholar]
- 21. Hulten E, Jackson JL, Douglas K, et al. The effect of early, intensive statin therapy on acute coronary syndrome: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2006;166:1814–1821. [DOI] [PubMed] [Google Scholar]
- 22. Cannon CP, Steinberg BA, Murphy SA, et al. Meta‐analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–445. [DOI] [PubMed] [Google Scholar]
- 23. Li YH, Wu HL, Yang YH, et al. Effect of early versus late in‐hospital initiation of statin therapy on the clinical outcomes of patients with acute coronary syndrome. Int Heart J. 2007;48:677–688. [DOI] [PubMed] [Google Scholar]
- 24. Ray KK, Cannon CP. The potential relevance of the multiple lipid‐independent (pleiotropic) effects of statins in the management of acute coronary syndromes. J Am Coll Cardiol. 2005;46:1425–1433. [DOI] [PubMed] [Google Scholar]
- 25. Blanco‐Colio LM, Munoz‐Garcia B, Martin‐Ventura JL, et al. 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitors decrease Fas ligand expression and cytotoxicity in activated human T lymphocytes. Circulation. 2003;108:1506–1513. [DOI] [PubMed] [Google Scholar]
- 26. Shimada K, Miyauchi K, Daida H. Early intervention with atorvastatin modulates TH1/TH2 imbalance in patients with acute coronary syndrome: from bedside to bench. Circulation. 2004;109:e213–e214. [DOI] [PubMed] [Google Scholar]
- 27. Sposito AC, Santos SN, de Faria EC, et al. Timing and dose of statin therapy define its impact on inflammatory and endothelial responses during myocardial infarction. Arterioscler Thromb Vasc Biol. 2011;31:1240–1246. [DOI] [PubMed] [Google Scholar]
- 28. Winchester D, Wen X, Xie L, et al. Evidence of pre‐procedural statin therapy a meta‐analysis of randomized trials. J Am Coll Cardiol. 2010;56:1099–1109. [DOI] [PubMed] [Google Scholar]
- 29. Undas A, Brummel KE, Musial J, et al. Simvastatin depresses blood clotting by inhibiting activation of prothrombin, factor V, and factor XIII and by enhancing factor Va inactivation. Circulation. 2001;103:2248–2253. [DOI] [PubMed] [Google Scholar]
- 30. Ray KK, Cannon CP. Pathological changes in acute coronary syndromes: the role of statin therapy in the modulation of inflammation, endothelial function and coagulation. J Thromb Thrombolysis. 2004;18:89–101. [DOI] [PubMed] [Google Scholar]


