Abstract
This study used 11C‐PBR28 positron emission tomography (PET) imaging to determine whether levels of 18‐kDa translocator protein (TSPO), an inflammation‐specific biomarker, are increased in frontotemporal lobar degeneration (FTLD) patients. 11C‐PBR28, 18F‐FDG, and 11C‐PIB brain PET scans, as well as magnetic resonance imaging (MRI), were conducted in four FTLD patients and 22 healthy controls. 11C‐PBR28 scans revealed that all FTLD patients showed increased TSPO binding versus controls. Significantly greater increases in TSPO were observed in the frontal, lateral temporal, parietal, and occipital cortices, topographically consistent with individual clinical phenotypes and with brain MRI and 18F‐FDG PET. Amyloid burden was not increased.
Introduction
Frontotemporal lobar degeneration (FTLD) is the second most frequent cause of presenile neurodegenerative dementia.1 Patients with clinical FTLD can present with one of three clinical syndromes: behavioral variant frontotemporal dementia (bvFTD), semantic dementia, and progressive nonfluent aphasia (PNFA).2 Animal studies have demonstrated that abnormal neuroimmune responses associated with gene mutations can cause inherited forms of FTLD, suggesting that neuroinflammation may play a pathogenic role.3, 4 Thus, positron emission tomography (PET) scans using radioligands specific to active components of the neuroinflammatory process could be useful for monitoring disease‐specific brain changes in living patients with FTLD.
Among the PET radioligands developed to quantify neuroinflammation, the most extensively studied in humans are those that bind to 18‐kDa translocator protein (TSPO), a mitochondrial protein that is highly expressed in activated microglia. Only two published PET studies have examined TSPO in FTLD. One used the prototypical TSPO radioligand 11C‐(R)‐PK11195 in patients with FTLD5 and the second used 11C‐DAA1106 in presymptomatic carriers of the FTLD genetic mutation.6 However, 11C‐(R)‐PK11195 has been shown to have low specific‐to‐nonspecific binding,7 and further studies using improved radioligands have not yet been performed in symptomatic FTLD patients.
Previous studies using the second‐generation TSPO radioligand 11C‐PBR28 found strong correlations between brain uptake and the clinical severity or progression of Alzheimer's disease (AD).8, 9 Another study found that the distribution of 11C‐PBR28 binding colocalized to neurodegeneration in different clinical subtypes of AD.10 Building on this work, this study sought to investigate whether 11C‐PBR28 binding was increased in FTLD patients in their relevant affected brain areas. The expected affected brain regions were identified by clinical findings, brain magnetic resonance imaging (MRI), and 18F‐fluorodeoxyglucose (FDG) PET scan.
Methods
Subjects
Subjects were recruited by the Molecular Imaging Branch of the National Institute of Mental Health, National Institutes of Health (NIMH‐NIH). All participants or their surrogate gave informed consent in accordance with the National Institutes of Health Combined Neurosciences Institutional Review Board. Four patients who met criteria for one of the clinical FTLD syndromes were recruited.2 Twenty‐two cognitively normal subjects from a previously described database11 were retrospectively demographically matched with the FTLD patients to serve as a control group; subjects with significant comorbid medical or psychiatric illnesses were excluded. TSPO‐binding affinity was determined by in vitro receptor binding to TSPO on leukocyte membranes,12 and low‐affinity binders were excluded. Although genetic testing was not available, none of the patients had a family history suggesting autosomal dominant inheritance.
Imaging procedures and analysis
Both FTLD patients and healthy controls underwent brain MRI as well as PET imaging with 11C‐PBR28. 18F‐FDG PET scans were obtained in all four patients with FTLD, and 11C‐Pittsburgh Compound B (PIB) PET scans were obtained in three of the four FTLD patients. 11C‐PIB PET data were also available for all healthy controls older than 45 years (N = 15), and were confirmed to be “PIB‐negative” using a dichotomizing method adapted from Jack and colleagues.13 Brain MRI, 18F‐FDG, 11C‐PIB, and 11C‐PBR28 PET procedures and preprocessing steps were performed as previously described.10
For region‐of‐interest (ROI)‐based analysis of 11C‐PBR28 PET images, the realigned PET images were coregistered to the MR images using SPM8 software (Wellcome Center for Human Neuroimaging, London, UK). A set of 83 predefined regions from the Hammers N30R83 maximum probability atlas was adjusted to the MRI scan of individual subjects using the PNEURO module of PMOD 3.9 (PMOD Technologies, Zurich, Switzerland).14 These were segmented from the spatially normalized individual MRIs and applied to the dynamic PET images. Partial volume effects were corrected within each PET image using a region‐based voxel‐wise method provided in PNEURO/PMOD.15 For better representation and noise reduction, the 83 ROIs were combined into 11 ROIs via weighted averaging. Total distribution volume (V T) in each ROI was calculated using the two‐tissue compartment model and corrected for free fraction of radioligand in plasma (f P). Data were also analyzed using a simplified ratio method as previously described, with cerebellum as a pseudoreference region.16 For visual comparison of TSPO distribution in the FTLD patients and two representative healthy controls, 11C‐PBR28 parametric images – where each voxel represents V T/f P – were generated using the Logan plot and arithmetic tools in PMOD.
For the three FTLD patients whose 11C‐PIB PET data were available, parametric images of 11C‐PIB distribution volume ratio were generated using the Logan reference model.17 In all FTLD patients, standardized uptake value ratio images of 18F‐FDG PET were generated using cerebellum as a reference region.
Statistical analysis
Data were analyzed using IBM SPSS Statistics 25 (Chicago, IL, USA). Demographic variables were compared using the Mann–Whitney U‐test and the Chi‐squared test. The ROI‐based comparisons of 11C‐PBR28 binding were conducted using the Mann–Whitney U‐test. To correct for TSPO‐binding affinity, each ROI value of 11C‐PBR28 V T/f P in mixed‐affinity binders was multiplied by 1.2 before comparison, based on a previous study that determined the ratio of 11C‐PBR28 binding for high‐affinity binders and mixed‐affinity binders.11 This correction was not conducted with the simplified ratio method because differences in TSPO‐binding affinity are cancelled out in the ratio.
Results
Subject characteristics
Demographic characteristics are described in Table 1. Three of the four FTLD patients met criteria for bvFTD18 and one had PNFA.2 No significant differences in age, sex, education level, or TSPO‐binding affinity was noted between the patient and control groups.
Table 1.
Demographic and clinical characteristics
| Variable | FTLD (n = 4) | HC (n = 22) | P value |
|---|---|---|---|
| Age (y) | 54.3 ± 6.9 | 54.4 ± 14.1 | 0.66* |
| Sex | 1F, 3M | 7F, 15 M | 1.00** |
| Education (y) | 17.0 ± 3.5 | 16.2 ± 2.6 | 0.66* |
| TSPO‐binding affinity type (HAB:MAB) | 2:2 | 8:14 | 0.63** |
| Mini‐Mental State Examination score | 17.0 ± 11.4 | 29.9 ± 0.3 | <0.001* |
Abbreviations: FTLD, frontotemporal lobar degeneration; HC, healthy control; TSPO, 18‐kDa translocator protein; HAB, high‐affinity binder; MAB, mixed‐affinity binder.
By Mann–Whitney U‐test;
By Chi‐squared test.
Patterns of TSPO binding
All four patients showed higher 11C‐PBR28 binding than healthy controls, regardless of TSPO‐binding affinity (Fig. 1). The frontotemporal cortex showed the most prominent increase despite substantial interindividual variability. In the ROI‐based analysis, FTLD patients had 1.4‐ to 1.8‐fold greater V T/f P in frontal cortex (P value ranged between 0.002 and 0.04) and 1.4‐ to 1.5‐fold greater V T/f P in lateral temporal, parietal, and occipital cortices (P value ranged between 0.02 and 0.03) than healthy controls. Results obtained using the simplified ratio method were similar except that no difference was observed in the occipital cortex (P = 0.66).
Figure 1.
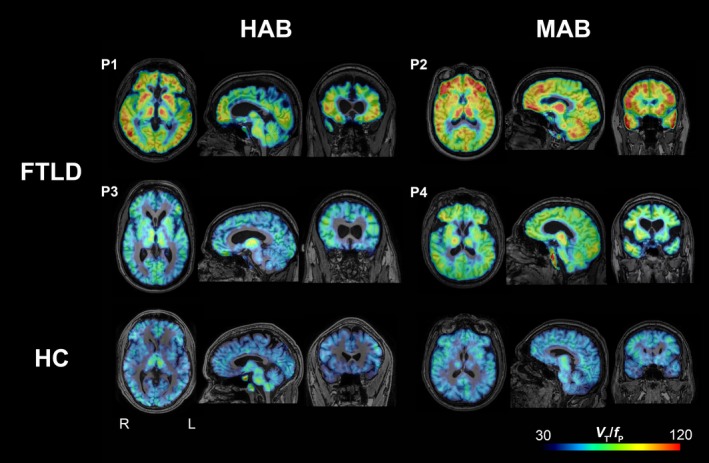
Parametric images of 11C‐PBR28 total distribution volume corrected by plasma‐free fraction (V T/f P) in four patients with frontotemporal lobar degeneration (FTLD) (P1, P2, P3, and P4) and two representative healthy control subjects (bottom row). When visually compared to healthy control subjects, FTLD patients had greater 11C‐PBR28 binding in frontotemporal cortices and other brain areas. Abbreviations: HAB, high‐affinity binder; MAB, mixed‐affinity binder; HC, healthy control.
Visual comparisons with brain MRI, 18F‐FDG PET, and 11C‐PIB PET
On T1‐weighted MR and 18F‐FDG PET images, all three patients with bvFTD showed significant atrophy and hypometabolism in the bilateral anterior frontal cortices, and the PNFA patient showed prominent atrophy and hypometabolism in the bilateral perisylvian areas (Fig. 2). None of the patients who underwent amyloid PET scans showed increased 11C‐PIB uptake in any gray matter region.
Figure 2.
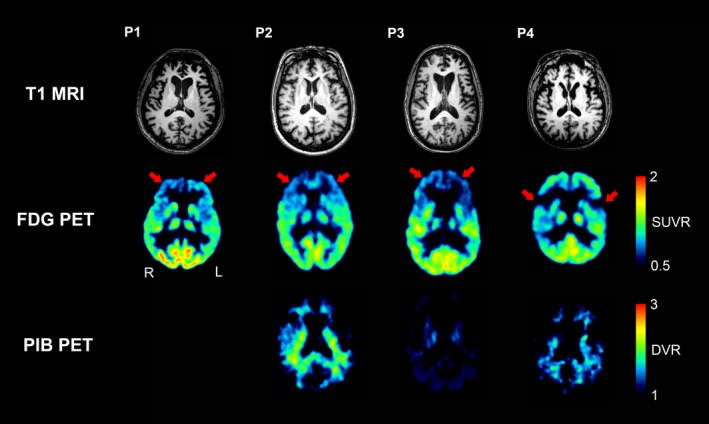
Brain magnetic resonance (MR), 18F‐FDG PET, and 11C‐PIB PET images of patients with frontotemporal lobar degeneration (FTLD). Patients 1, 2, and 3, who had behavioral variant frontotemporal dementia (P1, P2, and P3), showed atrophy and hypometabolism in the bilateral anterior frontal cortices on MRI and 18F‐FDG PET, respectively. Patient 4, who had progressive nonfluent aphasia (P4), showed bilateral perisylvian atrophy and hypometabolism on MRI and 18F‐FDG PET, respectively. 11C‐PIB uptake was not increased in these areas or other gray matter areas for any of the patients. 11C‐PIB PET imaging was not available for Patient 1. Abbreviations: FDG, fluorodeoxyglucose; PIB, Pittsburgh Compound B.
Discussion
In this study, 11C‐PBR28 binding was increased in the brain of FTLD patients although the amount of increase varied between patients. Regions of increased 11C‐PBR28 binding in FTLD patients corresponded with the expected neurodegeneration predicted by clinical phenotype as well as MRI and 18F‐FDG PET scans. Amyloid burden, as measured by 11C‐PIB, was not increased in any gray matter region in FTLD patients, consistent with the nonamyloid pathology of FTLD. The results also confirm that the simplified ratio method of 11C‐PBR28 developed for use in AD can be extended for use in FTLD.
In contrast to a previous study using 11C‐(R)‐PK11195 that found increased TSPO binding mainly in medial temporal, subcortical, and some frontal cortical regions,5 the present 11C‐PBR28 study found that the highest increases in binding were observed in neocortical regions – including frontal, lateral temporal, parietal, and occipital cortices – with no significant increase in medial temporal or subcortical regions.5 While these differences may be partially attributable to clinical and pathological heterogeneity among FTLD patients, 11C‐PBR28 would also be expected to be more sensitive and specific than 11C‐(R)‐PK11195 given the greater specific‐to‐nonspecific binding of 11C‐PBR28.7 Another study that used 11C‐DAA1106 imaging found the greatest increase in the occipital, posterior cingulate, and medial frontal cortex; these differences may be due to the fact that they included only presymptomatic MAPT mutation carriers.6
The distribution of in vivo TSPO binding in this study is consistent with previous postmortem findings demonstrating that microglial activation is increased in frontal and lateral temporal cortical gray and subcortical white matter in FTLD brain tissue, regardless of histological or genetic subtype.19 Other studies also found that different types of pathologies linked to tau and progranulin were commonly associated with abnormal microglial response.3, 20 In addition, major proteins and their coding genes associated with certain familial types of FTLD are thought to be functionally linked to microglial activity.3, 4 Taken together, the results suggest that, in FTLD, early heterogenous pathological processes involve a variety of genes and proteins, and that neuroinflammation may be a subsequent convergent process; this, in turn, may explain why diverse genotypes and proteinopathies result in the small number of common phenotypes seen in FTLD. In this regard, further investigations with larger patient populations are warranted to determine whether correlations exist between in vivo neuroinflammation measured by TSPO imaging and clinical severity or progression of FTLD. The hope is TSPO imaging may serve as a useful pharmacodynamic biomarker, enhancing the efficiency of early phase clinical trials for FTLD.
In summary, this study used an improved TSPO radioligand to confirm that neuroinflammation levels are increased in FTLD. The ability to use TSPO imaging to evaluate the in vivo status of neuroinflammation in FTLD is promising for future investigations into the underlying pathophysiology of this disorder and to the concomitant discovery of relevant therapeutic targets.
Author Contributions
VP, RI, and WK contributed to the conception and design of the study; MJK, MM, KJ, JS, CM, and SZ contributed to the acquisition and analysis of data; MJK, JS, RI, and WK drafted the text and prepared the figures and table.
Conflict of Interest
The authors have no other conflicts of interest to disclose, financial or otherwise.
Acknowledgment
The authors thank Ioline Henter (NIMH) for invaluable editorial assistance.
Funding information
This work was supported in part by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (IRP‐NIMH‐NIH; ZIAMH002795 and ZIAMH002793).
Funding Statement
This work was funded by National Institute of Mental Health grant IRP-NIMH-NIH; ZIAMH002795 and ZIAMH002793.
References
- 1. Mercy L, Hodges JR, Dawson K, et al. Incidence of early‐onset dementias in Cambridgeshire, United Kingdom. Neurology 2008;71:1496–1499. [DOI] [PubMed] [Google Scholar]
- 2. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 3. Yin F, Banerjee R, Thomas B, et al. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin‐deficient mice. J Exp Med 2010;207:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O'Rourke JG, Bogdanik L, Yanez A, et al. C9orf72 is required for proper macrophage and microglial function in mice. Science (New York, NY) 2016;351:1324–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cagnin A, Rossor M, Sampson EL, et al. In vivo detection of microglial activation in frontotemporal dementia. Ann Neurol 2004;56:894–897. [DOI] [PubMed] [Google Scholar]
- 6. Miyoshi M, Shinotoh H, Wszolek ZK, et al. In vivo detection of neuropathologic changes in presymptomatic MAPT mutation carriers: a PET and MRI study. Parkinsonism Relat Disord 2010;16:404–408. [DOI] [PubMed] [Google Scholar]
- 7. Fujita M, Kobayashi M, Ikawa M, et al. Comparison of four (11)C‐labeled PET ligands to quantify translocator protein 18 kDa (TSPO) in human brain: (R)‐PK11195, PBR28, DPA‐713, and ER176‐based on recent publications that measured specific‐to‐non‐displaceable ratios. EJNMMI Res 2017;7:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kreisl WC, Lyoo CH, McGwier M, et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer's disease. Brain 2013;136(Pt 7):2228–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kreisl WC, Lyoo CH, Liow JS, et al. (11)C‐PBR28 binding to translocator protein increases with progression of Alzheimer's disease. Neurobiol Aging 2016;44:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kreisl WC, Lyoo CH, Liow JS, et al. Distinct patterns of increased translocator protein in posterior cortical atrophy and amnestic Alzheimer's disease. Neurobiol Aging 2017;51:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paul S, Gallagher E, Liow JS, et al. Building a database for brain 18 kDa translocator protein imaged using [(11)C]PBR28 in healthy subjects. J Cereb Blood Flow Metab 2018;11:271678x18771250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kreisl WC, Jenko KJ, Hines CS, et al. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab 2013;33:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jack CR Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain 2008;131(Pt 3):665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gousias IS, Rueckert D, Heckemann RA, et al. Automatic segmentation of brain MRIs of 2‐year‐olds into 83 regions of interest. NeuroImage 2008;40:672–684. [DOI] [PubMed] [Google Scholar]
- 15. Thomas BA, Erlandsson K, Modat M, et al. The importance of appropriate partial volume correction for PET quantification in Alzheimer's disease. Eur J Nucl Med Mol Imaging 2011;38:1104–1119. [DOI] [PubMed] [Google Scholar]
- 16. Lyoo CH, Ikawa M, Liow JS, et al. Cerebellum can serve as a pseudo‐reference region in alzheimer disease to detect neuroinflammation measured with pet radioligand binding to translocator protein. J Nucl Med 2015;56:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound‐B. J Cereb Blood Flow Metab 2005;25:1528–1547. [DOI] [PubMed] [Google Scholar]
- 18. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134(Pt 9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lant SB, Robinson AC, Thompson JC, et al. Patterns of microglial cell activation in frontotemporal lobar degeneration. Neuropathol Appl Neurobiol 2014;40:686–696. [DOI] [PubMed] [Google Scholar]
- 20. Bhaskar K, Konerth M, Kokiko‐Cochran ON, et al. Regulation of tau pathology by the microglial fractalkine receptor. Neuron 2010;68:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


