Abstract
Background
Effective warfarin thromboprophylaxis requires maintaining anticoagulation within the recommended international normalized ratio (INR) range. INR testing rates and associations between testing and outcomes are not well understood.
Hypothesis
INR testing rates after hospitalization for acute decompensated heart failure are suboptimal, and testing is associated with lower risks of mortality and adverse clinical events.
Methods
We conducted a retrospective cohort study of patients who were long‐term warfarin users and were hospitalized for heart failure, had a medical history of atrial fibrillation or valvular heart disease, and were enrolled in fee‐for‐service Medicare. INR testing was defined as ≥1 outpatient INR test within 45 days after discharge. Using Cox proportional hazards models, we examined associations between testing and all‐cause mortality, all‐cause readmission, and adverse clinical events at 1 year.
Results
Among 8558 patients, 7722 (90.2%) were tested. After 1 year, tested patients had lower all‐cause mortality (23.5% vs 32.6%; P < 0.001) and fewer myocardial infarctions (2.0% vs 3.3%; P = 0.02). These differences remained significant after multivariable adjustment with hazard ratios of 0.72 (95% confidence interval [CI]: 0.63‐0.84; P < 0.001) and 0.58 (95% CI: 0.41‐0.83; P = 0.003), respectively. Differences in all‐cause readmission, thromboembolic events, ischemic stroke, and bleeding events were not statistically significant.
Conclusions
Postdischarge outpatient INR testing in patients with heart failure complicated by atrial fibrillation or valvular heart disease was high. INR testing was associated with improved survival and fewer myocardial infarctions at 1 year but was not independently associated with other adverse clinical events.
Introduction
Atrial fibrillation and valvular heart disease can complicate the management of patients with heart failure,1, 2, 3 and they affect up to 25% of these patients.4 To reduce thromboembolism risk, guidelines support the use of anticoagulation prophylaxis in patients with mechanical heart valves or with concurrent heart failure and atrial fibrillation.1, 2, 3
Warfarin is the mainstay of anticoagulation prophylaxis. Optimal benefit depends upon maintaining the international normalized ratio (INR) within an appropriate range, which requires regular testing. Guidelines recommend INR testing no less than every 4 weeks once warfarin dosing is stabilized.5 Monthly INR testing for stable warfarin users has been recommended as a quality measure.6
Patients with heart failure and concomitant atrial fibrillation or valvular heart disease often have complex treatment regimens that can be particularly challenging during the transition from hospital to home.7 Using data from a clinical registry linked with Medicare claims, we examined relationships between INR testing and 1‐year outcomes among long‐term warfarin users.
Methods
Data Sources
We obtained hospitalization data from the Acute Decompensated Heart Failure National Registry, which was established to study the characteristics, treatments, and inpatient outcomes of patients hospitalized with acute decompensated heart failure.8 More than 185 000 patients were enrolled in the registry between January 2001 and March 2006. Registry data included demographic characteristics, comorbid conditions, medications, and discharge disposition.
To analyze long‐term outcomes, we obtained fee‐for‐service Medicare standard analytic claim files from the US Centers for Medicare & Medicaid Services (CMS). The inpatient files contain hospital claims covered under Medicare Part A and include service dates and diagnosis and procedure codes. The denominator files include patient demographic characteristics, information about Medicare eligibility and enrollment, and death dates, if applicable. We obtained Medicare Part B carrier and outpatient facility claims to identify INR testing. We used indirect identifiers to link the registry records to the Medicare files using methods that have been described previously.9 Previous research has shown that older patients enrolled in the registry are representative of fee‐for‐service Medicare beneficiaries.10
Study Population
We included patients 65 years or older living in the United States who had a registry hospitalization linked to Medicare claims and were discharged alive to home. If a patient had multiple hospitalizations, we used the earliest as the index hospitalization. We used registry information to restrict the population to patients who were long‐term warfarin users at admission, received a warfarin prescription at discharge, and did not have warfarin contraindications or intolerance. To infer the primary indication for warfarin, we required patients to have a medical history of atrial fibrillation or valvular heart disease. (Presence of mechanical heart valves was not documented in the registry.) We further limited the population to patients discharged before December 2004, because the registry did not capture history of valvular heart disease after 2004. Finally, we required that patients were alive and enrolled in fee‐for‐service Medicare without hospitalizations for bleeding events, ischemic stroke, myocardial infarction, or thromboembolic events for at least 45 days after discharge from the index hospitalization.
Postdischarge Outpatient INR Testing
The study variable of interest was postdischarge outpatient INR testing, defined as a dichotomous variable (yes vs no). Patients were considered tested if they had 1 or more carrier or outpatient facility claims for the prothrombin time laboratory test (Current Procedural Terminology code 85610) or home INR monitoring instruction, equipment, or interpretation of results (Healthcare Common Procedure Coding System codes G0248, G0249, G0250) within 45 days after the index hospitalization. We used an ascertainment period of 45 days instead of the recommended 4 weeks to account for variation between planned and actual recall intervals. In a sensitivity analysis, we used a 90‐day ascertainment period.
Outcomes
We followed patients for 1 year after the end of the ascertainment period. Outcomes of interest were all‐cause mortality, all‐cause readmission, and inpatient admissions for adverse clinical events, including bleeding events, ischemic stroke, myocardial infarction, and thromboembolic events. We identified all‐cause mortality on the basis of death dates recorded in the Medicare denominator files, and we identified all‐cause readmission on the basis of any subsequent inpatient claim except transfers to or from another hospital and admissions for rehabilitation (diagnosis related group 462 or International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] diagnosis code V57.xx). We identified adverse clinical events on the basis of the primary ICD‐9‐CM diagnosis and/or procedure codes listed on any subsequent inpatient claim (see Supporting Information, Appendix, in the online version of this article).
Patient Characteristics
As shown in Table 1, baseline characteristics from the registry included demographic characteristics, medical history, results of the initial clinical evaluation, initial vital signs, laboratory test results, and discharge medications. For variables with low rates of missingness (ie, <5% of records), we imputed continuous variables to the overall median value and dichotomous variables to no. For evaluation of ejection fraction (14.7% missing), we created a categorical variable that included a category for missing. We used registry data to derive CHADS2 scores11 and to infer the primary indication for warfarin therapy, because outcomes can differ by warfarin indication.12 Using the comorbid conditions from the ADHERE registry, the CHADS2 score was created by adding 1 point each for the presence of congestive heart failure, hypertension, age 75 years or older, and diabetes mellitus and by adding 2 points for stroke or transient ischemic attack. All patients in the study had a CHADS2 score of at least 1, because all were admitted to the hospital with heart failure. We used Medicare data to determine geographic region, index hospitalization year, and length of stay and to flag hospitalizations longer than 7 days, which are associated with readmission among patients with heart failure.13
Table 1.
Characteristics of the Study Population
| Characteristic | INR Testing | P Value | |
|---|---|---|---|
| Tested, n = 7722 | Not Tested, n = 836 | ||
| Age, y, mean (SD) | 78.1 (6.7) | 77.5 (7.0) | 0.02 |
| Male, No. (%) | 3895 (50.4) | 456 (54.5) | 0.02 |
| Race, No. (%) | |||
| Black | 421 (5.5) | 83 (9.9) | <0.001 |
| White | 6842 (88.6) | 693 (82.9) | <0.001 |
| Other/unknown | 459 (5.9) | 60 (7.2) | 0.16 |
| US geographic region | |||
| Midwest | 2610 (33.8) | 237 (28.3) | 0.001 |
| Northeast | 1730 (22.4) | 172 (20.6) | 0.23 |
| South | 2983 (38.6) | 369 (44.1) | 0.002 |
| West | 399 (5.2) | 58 (6.9) | 0.03 |
| Medical history, No. (%) | |||
| Chronic renal insufficiency | 1863 (24.1) | 231 (27.6) | 0.03 |
| Coronary artery disease | 4970 (64.4) | 526 (62.9) | 0.41 |
| Diabetes mellitus | 2701 (35.0) | 339 (40.6) | 0.001 |
| Hypertension | 5466 (70.8) | 590 (70.6) | 0.90 |
| Myocardial infarction | 2352 (30.5) | 243 (29.1) | 0.41 |
| Peripheral vascular disease | 1484 (19.2) | 161 (19.3) | 0.98 |
| Stroke or transient ischemic attack | 1594 (20.6) | 177 (21.2) | 0.72 |
| CHADS2 score | |||
| Score, mean (SD) | 3.2 (1.2) | 3.2(1.1) | 0.57 |
| Score ≥2, No. (%) | 7349 (95.2) | 798 (95.5) | 0.71 |
| Warfarin indication, No. (%) | |||
| Atrial fibrillation | 4339 (56.2) | 555 (66.4) | <0.001 |
| Valvular heart disease | 844 (10.9) | 82 (9.8) | 0.32 |
| Atrial fibrillation and valvular heart disease | 2539 (32.9) | 199 (23.8) | <0.001 |
| Discharge medications, No. (%) | |||
| ACE inhibitor or ARB | 5224 (67.7) | 552 (66.0) | 0.34 |
| Aspirin | 2085 (27.0) | 218 (26.1) | 0.57 |
| β‐Blocker | 4909 (63.6) | 504 (60.3) | 0.06 |
| Clopidogrel | 309 (4.0) | 31 (3.7) | 0.68 |
| Diuretic | 7117 (92.2) | 752 (90.0) | 0.03 |
| Lipid‐lowering agent | 3035 (39.3) | 311 (37.2) | 0.24 |
| Index length of stay >7 days, No. (%) | 1237 (16.0) | 132 (15.8) | 0.86 |
| Hospitalized during ascertainment period, No. (%) | 1858 (24.1) | 267 (31.9) | <0.001 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; INR, international normalized ratio.
Statistical Analysis
To describe baseline characteristics of the study population, we present categorical variables as frequencies and continuous variables as means with standard deviations (SDs). We tested for differences between groups using χ2 tests for categorical variables and Kruskal‐Wallis tests for continuous variables.
We describe INR testing and number of INR tests within the 45‐day ascertainment period. We examined the unadjusted and multivariable adjusted relationships between patient characteristics and INR testing using modified Poisson models.14 We modeled INR testing as a function of patient characteristics and any hospitalization during the ascertainment period.
We report unadjusted outcome rates by INR testing. We used Kaplan‐Meier methods to estimate mortality and log‐rank tests to assess differences in mortality between groups. For all other outcomes, we used the cumulative incidence function, which accounts for the competing risk of death, to calculate cumulative incidence estimates, and Gray tests to assess differences between groups.
We used Cox proportional hazards models to examine associations between INR testing and outcomes. In multivariable analyses, we modeled each outcome as a function of INR testing and patient characteristics. We used robust standard errors to account for clustering of patients within hospitals. We censored data for patients if they enrolled in Medicare managed care, and for outcomes other than mortality, at the time of death. In a sensitivity analysis, we tested for an interaction between INR testing and warfarin indication, and between INR testing and the variables which we found to be predictors of INR testing. If the interaction was statistically significant, we ran a separate model for that outcome.
We used a significance level of 0.05 and 2‐sided tests for all hypotheses. We used SAS version 9.2 (SAS Institute Inc., Cary, NC) for all analyses. The institutional review board of the Duke University Health System approved the study.
Results
We identified 9301 patients from 264 hospitals who were 65 years or older living in the United States, were discharged alive to home, were on warfarin at admission, and had a history of atrial fibrillation or valvular heart disease. We excluded 178 patients who terminated fee‐for‐service Medicare coverage and 565 patients who died or were admitted to the hospital for adverse clinical events during the 45‐day ascertainment period, resulting in a final study population of 8558 patients from 262 hospitals. In addition to heart failure, 4894 (57.2%) had atrial fibrillation, 926 (10.8%) had valvular heart disease, and 2738 (32.0%) had both atrial fibrillation and valvular heart disease.
Of the 8558 patients, 7722 (90.2%) were tested within 45 days after discharge. Testing rates were slightly higher among patients with both atrial fibrillation and valvular heart disease (n = 2539 [92.7%]) than among those with only atrial fibrillation (n = 4339 [88.7%]) or valvular heart disease (n = 844 [91.1%]; P < 0.001 for overall group difference). Among tested patients, the average number of INR tests on separate days during the ascertainment period was 3.3 (SD, 1.9). In a sensitivity analysis using a 90‐day ascertainment period, 8093 (94.6%) patients were tested within 90 days after discharge.
Patients who were tested were slightly older, more likely to be white and male, and more likely to reside in the Midwest than in the South or West (Table 1). Tested patients were more likely to have concomitant atrial fibrillation and valvular heart disease, were less likely to have chronic renal insufficiency or diabetes or be hospitalized during the ascertainment period, and were more likely to be discharged on a diuretic.
In multivariable analysis, diabetes mellitus, dyspnea, hospitalization during the ascertainment period, ejection fraction, geographic region, index year, race, sex, and warfarin indication were associated with INR testing (Table 2). INR testing was negatively associated with black race, diabetes mellitus, dyspnea, hospitalization during the ascertainment period, index hospitalization year after 2001, male sex, and missing ejection fraction. Patients in the Midwest were more likely to be tested than those in the South, and patients who had valvular heart disease with or without atrial fibrillation were more likely to be tested that those who had only atrial fibrillation.
Table 2.
Predictors of Postdischarge Outpatient International Normalized Ratio Testing
| Variable | Unadjusted RR (95% CI) | P Value | Adjusted RR (95% CI) | P Value |
|---|---|---|---|---|
| Age, per 5 years | 1.01 (1.00‐1.01) | 0.04 | 1.00 (1.00‐1.01) | 0.14 |
| Male sex | 0.98 (0.97‐1.00) | 0.02 | 0.98 (0.97‐1.00) | 0.02 |
| Race | ||||
| Black | 0.92 (0.88‐0.96) | <0.001 | 0.93 (0.89‐0.97) | <0.001 |
| White | 1.00 [Reference] | 1.00 [Reference] | ||
| Other/unknown | 0.97 (0.94‐1.01) | 0.11 | 0.98 (0.95‐1.01) | 0.16 |
| US geographic region | ||||
| Midwest | 1.03 (1.01‐1.05) | <0.001 | 1.03 (1.01‐1.05) | <0.001 |
| Northeast | 1.02 (1.00‐1.04) | 0.02 | 1.02 (1.00‐1.04) | 0.05 |
| South | 1.00 [Reference] | 1.00 [Reference] | ||
| West | 0.98 (0.95‐1.02) | 0.31 | 0.98 (0.95‐1.02) | 0.35 |
| Medical history | ||||
| Chronic obstructive pulmonary disease | 0.99 (0.97‐1.00) | 0.16 | 0.99 (0.98‐1.01) | 0.49 |
| Chronic renal insufficiency | 0.98 (0.97‐1.00) | 0.03 | 0.99 (0.97‐1.01) | 0.29 |
| Coronary artery disease | 1.01 (0.99‐1.02) | 0.41 | 1.01 (0.99‐1.02) | 0.53 |
| Diabetes mellitus | 0.98 (0.96‐0.99) | 0.002 | 0.98 (0.97‐1.00) | 0.03 |
| Dyspnea | 0.98 (0.96‐1.01) | 0.18 | 0.98 (0.95‐1.00) | 0.04 |
| Fatigue | 1.01 (1.00‐1.03) | 0.10 | 1.01 (1.00‐1.03) | 0.13 |
| Hyperlipidemia | 1.02 (1.00‐1.03) | 0.02 | 1.02 (1.00‐1.03) | 0.07 |
| Hypertension | 1.00 (0.99‐1.02) | 0.90 | 1.00 (0.99‐1.02) | 0.68 |
| Myocardial infarction | 1.01 (0.99‐1.02) | 0.40 | 1.00 (0.99‐1.02) | 0.64 |
| Peripheral vascular disease | 1.00 (0.98‐1.02) | 0.98 | 1.00 (0.98‐1.02) | 0.95 |
| Rales | 1.00 (0.98‐1.01) | 0.64 | 1.00 (0.98‐1.01) | 0.59 |
| Stroke or transient ischemic attack | 1.00 (0.98‐1.01) | 0.72 | 1.00 (0.98‐1.01) | 0.64 |
| Warfarin indication | ||||
| Atrial fibrillation | 1.00 [Reference] | 1.00 [Reference] | ||
| Atrial fibrillation and valvular heart disease | 1.05 (1.03‐1.06) | <0.001 | 1.04 (1.02‐1.06) | <0.001 |
| Valvular heart disease | 1.03 (1.01‐1.05) | 0.02 | 1.03 (1.01‐1.05) | 0.01 |
| Discharge medications | ||||
| ACE inhibitor or ARB | 1.01 (0.99‐1.02) | 0.35 | 1.00 (0.98‐1.02) | 0.97 |
| Aspirin | 1.00 (0.99‐1.02) | 0.56 | 1.00 (0.98‐1.02) | 0.98 |
| β‐Blocker | 1.01 (1.00‐1.03) | 0.07 | 1.01 (1.00‐1.03) | 0.19 |
| Clopidogrel | 1.01 (0.97‐1.04) | 0.67 | 1.01 (0.97‐1.04) | 0.76 |
| Diuretic | 1.03 (1.00‐1.06) | 0.04 | 1.02 (0.99‐1.05) | 0.11 |
| Lipid‐lowering agent | 1.01 (0.99‐1.02) | 0.23 | 1.00 (0.98‐1.02) | 0.92 |
| Index length of stay >7 days | 1.00 (0.98‐1.02) | 0.86 | 1.00 (0.99‐1.02) | 0.65 |
| Hospitalized during ascertainment period | 0.96 (0.94‐0.98) | <0.001 | 0.96 (0.94‐0.98) | <0.001 |
| Index year | ||||
| 2001 | 1.00 [Reference] | 1.00 [Reference] | ||
| 2002 | 0.95 (0.92‐0.98) | 0.002 | 0.95 (0.92‐0.98) | 0.003 |
| 2003 | 0.96 (0.93‐0.99) | 0.008 | 0.96 (0.92‐0.99) | 0.007 |
| 2004 | 0.96 (0.93‐1.00) | 0.03 | 0.96 (0.93‐1.00) | 0.03 |
| Ejection fraction | ||||
| Mildly impaired (>40%) | 1.00 [Reference] | 1.00 [Reference] | ||
| Moderately impaired (26%–40%) | 1.00 (0.99‐1.02) | 0.64 | 1.00 (0.99‐1.02) | 0.60 |
| Severely impaired (≤25%) | 1.00 (0.98‐1.01) | 0.62 | 1.00 (0.98‐1.02) | 0.79 |
| Missing | 0.97 (0.94‐0.99) | 0.004 | 0.97 (0.95‐0.99) | 0.02 |
| Heart rate, bpm | ||||
| <80 | 1.00 [Reference] | 1.00 [Reference] | ||
| 80–100 | 1.01 (0.99‐1.02) | 0.50 | 1.01 (0.99‐1.02) | 0.39 |
| >100 | 1.00 (0.99‐1.02) | 0.68 | 1.01 (0.99‐1.03) | 0.46 |
| Hemoglobin, g/dL | ||||
| <9 | 1.03 (0.99‐1.06) | 0.15 | 1.04 (1.00‐1.08) | 0.05 |
| 9–11 | 0.99 (0.98‐1.01) | 0.49 | 1.00 (0.98‐1.01) | 0.65 |
| >11 | 1.00 [Reference] | 1.00 [Reference] | ||
| Serum creatinine, mg/dL | ||||
| <1.5 | 1.00 [Reference] | 1.00 [Reference] | ||
| 1.5–2.0 | 1.00 (0.98‐1.01) | 0.65 | 1.00 (0.98‐1.02) | 0.83 |
| >2.0 | 0.97 (0.95‐0.99) | 0.01 | 0.99 (0.96‐1.01) | 0.37 |
| Serum sodium, mEq/L | ||||
| <135 | 1.00 (0.98‐1.02) | 0.97 | 1.00 (0.98‐1.02) | 0.79 |
| 135–145 | 1.00 [Reference] | 1.00 [Reference] | ||
| >145 | 0.95 (0.89‐1.01) | 0.09 | 0.95 (0.90‐1.01) | 0.12 |
| Systolic blood pressure, mm Hg | ||||
| <110 | 0.99 (0.97‐1.01) | 0.45 | 1.00 (0.97‐1.02) | 0.70 |
| 110–150 | 1.00 [Reference] | 1.00 [Reference] | ||
| >150 | 0.98 (0.97‐1.00) | 0.06 | 0.99 (0.97‐1.00) | 0.08 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CI, confidence interval; RR, risk ratio.
Table 3 and Figures 1 and 2 show the unadjusted outcomes at 1 year. Rates of all‐cause readmission and all‐cause mortality were high, with 64% of patients readmitted and 24% dying within 1 year. Readmission rates did not differ by INR testing, but mortality was significantly lower among tested patients. Admissions for adverse clinical events were rare. The myocardial infarction rate was significantly lower among tested patients than nontested patients, but rates of bleeding events, ischemic stroke, and thromboembolic events did not differ between groups. After multivariable adjustment, the associations between INR testing and lower mortality and myocardial infarction admissions remained significant (Table 4).
Table 3.
Cumulative Incidence of Unadjusted Outcomes at 1 Year by Postdischarge INR Testing
| Outcome | INR Testing | P Value | |
|---|---|---|---|
| Tested, n = 7722 | Not Tested, n = 836 | ||
| All‐cause readmission, No. (%) | 4950 (64.4) | 542 (65.1) | 0.11 |
| All‐cause mortality, No. (%) | 1805 (23.5) | 270 (32.6) | <0.001 |
| Bleeding event, No. (%) | 340 (4.4) | 35 (4.2) | 0.82 |
| Ischemic stroke, No. (%) | 131 (1.7) | 11 (1.3) | 0.43 |
| Myocardial infarction, No. (%) | 153 (2.0) | 27 (3.3) | 0.02 |
| Thromboembolic event, No. (%) | 318 (4.1) | 37 (4.5) | 0.64 |
Abbreviation: INR, international normalized ratio.
Figure 1.
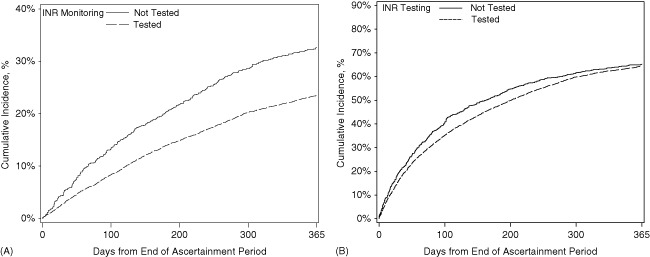
Cumulative incidence of (A) mortality and (B) readmissions by postdischarge outpatient international normalized ratio (INR) testing.
Figure 2.
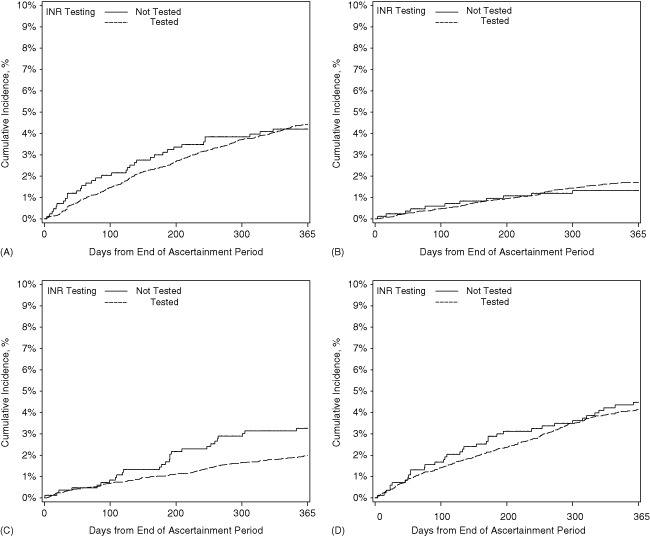
Cumulative incidence of (A) bleeding events, (B) ischemic stroke, (C) myocardial infarction, and (D) thromboembolic events by postdischarge outpatient international normalized ratio (INR) testing.
Table 4.
Associations Between Postdischarge International Normalized Ratio Testing and Outcomes
| Outcome | Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value |
|---|---|---|---|---|
| All‐cause readmission | 0.87 (0.79‐0.95) | 0.003 | 0.91 (0.83‐1.00) | 0.05 |
| All‐cause mortality | 0.67 (0.59‐0.77) | <0.001 | 0.72 (0.63‐0.84) | <0.001 |
| Bleeding event | 0.97 (0.69‐1.36) | 0.85 | 0.95 (0.68‐1.35) | 0.79 |
| Ischemic stroke | 1.19 (0.61‐2.31) | 0.61 | 1.24 (0.63‐2.43) | 0.54 |
| Myocardial infarction | 0.57 (0.40‐0.81) | 0.002 | 0.58 (0.41‐0.83) | 0.003 |
| Thromboembolic event | 0.86 (0.59‐1.24) | 0.41 | 0.87 (0.60‐1.27) | 0.47 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
In a sensitivity analysis, within each model we tested for an interaction between INR testing and diabetes mellitus, dyspnea, geographic region, hospitalization during the ascertainment period, index year, race, sex, and warfarin indication. We found a significant interaction between testing and hospitalization during the ascertainment period in the myocardial infarction model. The association between testing and myocardial infarction remained significant among the 2125 patients who were hospitalized during the ascertainment period (adjusted hazard ratio, 0.31; 95% confidence interval [CI]: 0.15‐0.62; P = 0.001), but was not significant among the 6433 patients who were not (adjusted hazard ratio, 0.78; 95% CI: 0.45‐1.35; P = 0.37).
Discussion
Outpatient INR testing in the United States has not been well described. Most studies have focused on the effectiveness of patient self‐testing15, 16 or on patients in special settings or quality‐improvement programs.17, 18 Previous studies found that the average time between INR tests among patients with nonvalvular atrial fibrillation was 25.3 days,19 and that 10.3% of Medicare beneficiaries with atrial fibrillation did not have a test in the 90 days after the index hospitalization.20
Our data complement previous research by providing insight into anticoagulation management practices during the transition from hospital to home among Medicare beneficiaries with heart failure complicated by atrial fibrillation or valvular heart disease. In our study, postdischarge outpatient INR testing rates were greater than 90% and increased to almost 95% when we extended the ascertainment period to 90 days. These findings suggest that most physicians are following guideline recommendations even during the challenging period of transition from hospital to home.
The lower rate of INR testing among black patients is consistent with previous research20, 21 and may be attributable in part to psychosocial and socioeconomic factors. Health illiteracy is higher among patients in racial/ethnic minority populations and is associated with poorer understanding of the recommended frequency of INR testing.22 The higher testing rate among patients with valvular heart disease also substantiates previous research.12 Regional variation in testing is a novel finding. CMS did not issue a national coverage determination for INR testing until late 2002, but prior local coverage determinations did not vary by region. However, access to care, availability of specialized anticoagulation clinics, practice patterns, and other factors may vary by region. More research is needed to explore this finding.
Our study provides valuable insight into associations between INR testing and outcomes in a real‐world setting. Although several studies have examined the association between the frequency of INR testing and time in therapeutic range,23, 24 to our knowledge, only 1 study has examined associations with adverse clinical events. Birman‐Deych et al20 found that Medicare beneficiaries with atrial fibrillation who received an INR test within 90 days after hospital discharge were significantly less likely to experience ischemic stroke. In our study, INR testing was not independently associated with stroke, but stroke was rare, occurring in less than 2% of the population. For this and other reasons, some researchers have cautioned against the utility of clinical event rates as measures of the quality of anticoagulation management.25, 26
The observed association between INR testing and improved survival in the absence of associations with stroke, bleeding events, and thromboembolic events has 2 potential explanations. As in similar studies,27, 28 admissions for adverse clinical events were rare, limiting our ability to detect associations. INR testing may be a marker of general adherence with heart failure therapies and follow‐up, both of which may contribute to improved survival but would not necessarily affect other measured outcomes.
Our study has several limitations. We were unable to measure outpatient medication use because the Medicare prescription drug benefit did not begin until 2006. Some patients may have discontinued warfarin or been nonadherent to therapy, especially patients who did not undergo INR testing, which has been used elsewhere as a proxy for warfarin adherence and persistence.20, 29, 30 However, this risk is mitigated by the fact that discontinuation rates level off in the third through fifth years after initiating therapy,29 and warfarin persistence rates are higher among older patients and patients with heart failure.21, 30, 31 We could not account for the intermediate outcome of time in therapeutic range. We could not account for potential barriers to testing, such as travel distance, appointment scheduling, lack of transportation, and illness or disability. We limited the analysis to fee‐for‐service Medicare beneficiaries hospitalized between 2001 and 2004 who were long‐term warfarin users and survived 45 days after discharge without adverse clinical events. Results may not be generalizable to more recent time periods, Medicare managed care enrollees, younger patients, new warfarin users, or patients who experienced adverse clinical events or death shortly after discharge. Finally, although we used rich clinical registry data, there may be residual unmeasured confounding.
Conclusion
Postdischarge outpatient INR testing rates in patients with heart failure complicated by atrial fibrillation or valvular heart disease were high. INR testing was associated with improved survival and fewer myocardial infarction events at 1 year, but was not independently associated with other adverse clinical events.
Supporting information
Appendix S1. Definitions of Adverse Clinical Events
Acknowledgments
Damon M. Seils, MA, Duke University, provided editorial assistance and prepared the manuscript.
Winslow Klaskala, PhD, MS, is deceased.
This study was supported by a research agreement between Duke University and Johnson & Johnson.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. [DOI] [PubMed] [Google Scholar]
- 2. Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. [DOI] [PubMed] [Google Scholar]
- 3. Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1–e142. [DOI] [PubMed] [Google Scholar]
- 4. Mountantonakis SE, Grau‐Sepulveda MV, Bhatt DL, et al. Presence of atrial fibrillation is independently associated with adverse outcomes in patients hospitalized with heart failure: an analysis of get with the guidelines‐heart failure. Circ Heart Fail. 2012;5:191–201. [DOI] [PubMed] [Google Scholar]
- 5. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence‐based clinical practice guidelines (8th edition). Chest. 2008;133:160S–198S. [DOI] [PubMed] [Google Scholar]
- 6. Phillips KW, Ansell J. Outpatient management of oral vitamin K antagonist therapy: defining and measuring high‐quality management. Expert Rev Cardiovasc Ther. 2008;6:57–70. [DOI] [PubMed] [Google Scholar]
- 7. Bixby MB, Konick‐McMahon J, McKenna CG. Applying the transitional care model to elderly patients with heart failure. J Cardiovasc Nurs. 2000;14:53–63. [DOI] [PubMed] [Google Scholar]
- 8. Adams KF Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216. [DOI] [PubMed] [Google Scholar]
- 9. Hammill BG, Hernandez AF, Peterson ED, et al. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kociol RD, Hammill BG, Fonarow GC, et al. Generalizability and longitudinal outcomes of a national heart failure clinical registry: comparison of Acute Decompensated Heart Failure National Registry (ADHERE) and non‐ADHERE Medicare beneficiaries. Am Heart J. 2010;160:885–892. [DOI] [PubMed] [Google Scholar]
- 11. Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 12. Rose AJ, Ozonoff A, Henault LE, et al. Anticoagulation for valvular heart disease in community‐based practice. Thromb Haemost. 2010;103:329–337. [DOI] [PubMed] [Google Scholar]
- 13. Krumholz HM, Parent EM, Tu N, et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99–104. [PubMed] [Google Scholar]
- 14. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 15. Connock M, Stevens C, Fry‐Smith A, et al. Clinical effectiveness and cost‐effectiveness of different models of managing long‐term oral anticoagulation therapy: a systematic review and economic modelling. Health Technol Assess. 2007;11:iii–iv, ix–66. [DOI] [PubMed] [Google Scholar]
- 16. Bloomfield HE, Krause A, Greer N, et al. Meta‐analysis: effect of patient self‐testing and self‐management of long‐term anticoagulation on major clinical outcomes. Ann Intern Med. 2011;154:472–482. [DOI] [PubMed] [Google Scholar]
- 17. Aspinall SL, Zhao X, Handler SM, et al. The quality of warfarin prescribing and monitoring in Veterans Affairs nursing homes. J Am Geriatr Soc. 2010;58:1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Desmond D, Kogan P, Underwood S, et al. Performance improvement in managed long‐term care: improving warfarin medication management. Home Healthc Nurse. 2009;27:150–159. [DOI] [PubMed] [Google Scholar]
- 19. Ansell J, Hollowell J, Pengo V, et al. Descriptive analysis of the process and quality of oral anticoagulation management in real‐life practice in patients with chronic non‐valvular atrial fibrillation: the international study of anticoagulation management (ISAM). J Thromb Thrombolysis. 2007;23:83–91. [DOI] [PubMed] [Google Scholar]
- 20. Birman‐Deych E, Radford MJ, Nilasena DS, et al. Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke. 2006;37:1070–1074. [DOI] [PubMed] [Google Scholar]
- 21. Arnsten JH, Gelfand JM, Singer DE. Determinants of compliance with anticoagulation: a case‐control study. Am J Med. 1997;103:11–17. [DOI] [PubMed] [Google Scholar]
- 22. Fang MC, Machtinger EL, Wang F, et al. Health literacy and anticoagulation‐related outcomes among patients taking warfarin. J Gen Intern Med. 2006;21:841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Samsa GP, Matchar DB. Relationship between test frequency and outcomes of anticoagulation: a literature review and commentary with implications for the design of randomized trials of patient self‐management. J Thromb Thrombolysis. 2000;9:283–292. [DOI] [PubMed] [Google Scholar]
- 24. Dolan G, Smith LA, Collins S, et al. Effect of setting, monitoring intensity and patient experience on anticoagulation control: a systematic review and meta‐analysis of the literature. Curr Med Res Opin. 2008;24:1459–1472. [DOI] [PubMed] [Google Scholar]
- 25. Rose AJ, Berlowitz DR, Frayne SM, et al. Measuring quality of oral anticoagulation care: extending quality measurement to a new field. Jt Comm J Qual Patient Saf. 2009;35:146–155. [DOI] [PubMed] [Google Scholar]
- 26. Witt DM. Quality measures and benchmarking for warfarin therapy. J Thromb Thrombolysis. 2011;31:242–248. [DOI] [PubMed] [Google Scholar]
- 27. Hernandez AF, Hammill BG, Kociol RD, et al. Clinical effectiveness of anticoagulation therapy among older patients with heart failure and without atrial fibrillation: findings from the ADHERE registry linked to Medicare claims. J Card Fail. 2013;19:401–407. [DOI] [PubMed] [Google Scholar]
- 28. Hess PL, Greiner MA, Fonarow GC, et al. Outcomes associated with warfarin use in older patients with heart failure and atrial fibrillation and a cardiovascular implantable electronic device: findings from the ADHERE registry linked to Medicare claims. Clin Cardiol. 2012;35:649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fang MC, Go AS, Chang Y, et al. Warfarin discontinuation after starting warfarin for atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2010;3:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pamboukian SV, Nisar I, Patel S, et al. Factors associated with non‐adherence to therapy with warfarin in a population of chronic heart failure patients. Clin Cardiol. 2008;31:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gallagher AM, Rietbrock S, Plumb J, et al. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis? J Thromb Haemost. 2008;6:1500–1506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Definitions of Adverse Clinical Events


