Abstract
Background
A family history of premature coronary artery disease (CAD) is a well‐known risk factor for cardiovascular events.
Hypothesis
Atorvastatin may improve endothelial dysfunction (ED) in the first‐degree relatives (FDRs) of patients with premature CAD with ED.
Methods
Thirty‐five FDRs (median age, 52 years [interquartile range (IQR), 46–57 years], 21 male) of patients with premature CAD with ED were recruited in a prospective trial with a crossover double‐blind design: 6 weeks of treatment with atorvastatin 40 mg/day followed by placebo, or vice versa. After each treatment, the digital pulse wave amplitude was determined by EndoPAT to obtain the reactive hyperemia index (RHI), a measure for endothelial function. The primary outcome was the difference of RHI between atorvastatin and placebo treatment.
Results
Low‐density lipoprotein cholesterol was lower after atorvastatin compared with placebo treatment (124 [102–145] mg/dL vs 67 [50–73] mg/dL, P < 0.001). However, RHI was not different after atorvastatin compared with placebo treatment (1.9 [1.5–2.4] vs 1.9 [1.6–2.2], P = 0.902). Also, the augmentation index was similar after each treatment. These results were observed both in subjects who had indications for statin treatment (31%) and those who did not (69%) according to National Cholesterol Education Program Adult Treatment Panel III guidelines.
Conclusions
Despite improvement in the lipid profile, atorvastatin failed to improve ED in the FDRs of patients with premature CAD with ED. Although we identified those with ED in FDRs of patients with premature CAD as a high‐risk group for future cardiovascular events, atorvastatin treatment may not be a beneficial primary prevention strategy for this population.
Introduction
A family history of premature coronary artery disease (CAD) increases the risk of cardiovascular disease and is a well‐known risk factor for cardiovascular events. Thus, early implementation of primary prevention is essential for the prevention of CAD.1, 2, 3 Accordingly, screening of first‐degree relatives (FDRs) of patients with CAD has been recommended,4 but appropriate primary prevention for CAD is not performed in standard practice.5, 6 Furthermore, risk stratification and prevention strategies for cardiovascular events in FDRs have not been adequately studied.
To date, striking benefits have been reported with statin treatment in patients with a wide range of cholesterol levels in the setting of primary prevention.7 These findings may be due not only to the cholesterol‐lowering effects of statins, but also to their ability to protect against endothelial dysfunction (ED).8, 9, 10 Accumulating evidence suggests that ED is an early marker for atherosclerosis and can be detected prior to the structural vessel wall changes documented by angiography or ultrasound,11 and thus it can be reliably used as a surrogate for cardiovascular events.12, 13
Therefore, in this study, we explored whether statin treatment, as a proactive primary‐prevention strategy, would improve ED in the FDRs of patients with premature CAD who were identified to have ED.
Methods
Study Population
Between August 2009 and May 2010, a total of 132 asymptomatic FDRs of patients who had premature CAD were consecutively screened at Severance Cardiovascular Hospital by a phone call inviting them to our hospital for the evaluation of subclinical CAD. After a family history of patients with premature CAD was taken, participants who had a documented history of CAD were excluded. Premature CAD was defined as acute myocardial infarction before the age of 55 years for men or 65 years for women.14 Out of 132 subjects screened, 48 subjects (36.4%) were identified to have ED. Six subjects did not consent to participate in the study. Among the 42 subjects initially recruited, 7 subjects dropped out during the study period (4 for noncompliance and 3 for withdrawn consent).
Study Design
This study was randomized, placebo‐controlled, and double‐blinded, with a crossover design as illustrated in Figure 1. None of the investigators involved in clinical and laboratory assessments were aware of the subjects' treatment allocation at any time during the study. After screening, patients were randomly assigned following simple randomization procedures (computerized random numbers) to either therapy with atorvastatin 40 mg/day first or matching placebo first for 6 weeks. This treatment period was followed by a washout period of another 2 weeks, during which the patients did not receive any study medication. Study medication (atorvastatin 40 mg tablet or matching placebo; provided by Chong Kun Dang Pharmaceuticals Co., Seoul, Korea) was packaged and distributed by the pharmacy in Severance Cardiovascular Hospital. The tablets were identical in size, color, and taste to prevent unblinding of staff and patients. After each of 2 treatment periods (atorvastatin and placebo), assessment of endothelial function and lipid profile was performed. The primary outcome of this study was the difference of reactive hyperemic index (RHI) between atorvastatin and placebo treatment as a representation of improvement of endothelial function after treatment with atorvastatin relative to placebo.
Figure 1.
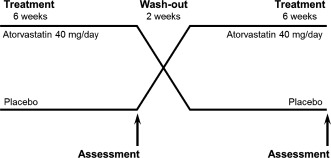
Study design. Subjects were assigned to 1 of 2 treatment arms and started on either atorvastatin or placebo. After a 2‐week washout period, they switched over to the other treatment.
The study protocol was approved by the institutional review board, and all patients gave written informed consent.
Endothelial Function Assessment
Endothelial function was measured using peripheral arterial tonometry (PAT) with the EndoPAT 2000 system (Itamar Medical, Caesarea, Israel) and quantified by the RHI in digital blood flow after arm occlusion.15, 16 In a previous study, RHI measured by EndoPAT closely paralleled coronary artery endothelial function and was shown to predict future vascular events in patients with CAD.17
After 5 minutes of baseline recording, a blood pressure (BP) cuff was inflated to suprasystolic pressure in the test arm. After 5 minutes of occlusion, the cuff was rapidly deflated and the PAT tracings were recorded. Reactive hyperemic index was determined as the ratio of PAT amplitude in the test arm to the control arm, averaged in 30‐second intervals of postcuff deflation, divided by the average PAT ratio measured for the 140‐second interval before cuff inflation. In the present study, ED was defined categorically by an RHI <2.0.18
Assessment of Risk Factors
All individuals provided demographic information, their medical history, and their medication profile. Smoking was defined as anyone who is a current or former smoker. Dyslipidemia was defined as high total cholesterol (TC; >200 mg/dL), high low‐density lipoprotein cholesterol (LDL‐C; >160 mg/dL), low high‐density lipoprotein cholesterol (HDL‐C; <35mg/dL for men, <40 mg/dL for women), and/or high triglycerides (TG), or current use of lipid‐lowering therapy. Participants were considered to have diabetes mellitus (DM) if they reported a history of DM and/or were receiving antidiabetic treatment or had a fasting plasma glucose level of ≥126 mg/dL. Hypertension was defined as a self‐reported history of hypertension and/or a history of antihypertensive medication use or a BP of ≥140/90 mm Hg at the time of the visit. All participants underwent a laboratory blood analysis that included TC, TG, HDL‐C, LDL‐C, fasting plasma glucose, and serum creatinine levels.
Statistical Analysis
Data are presented as the median (interquartile range [IQR]). The primary endpoint was analyzed using the Wilcoxon signed rank test because the difference in RHI values was not normally distributed. On the basis of a previous study, assuming a baseline RHI of 1.27 ± 0.37,15 a total of 32 patients (16 patients per group) were needed to detect a 30% change in the RHI with 80% power at the 0.05 significance level under a 2‐by‐2 crossover design.19 We therefore aimed to randomize a total of 42 subjects, predicting a dropout rate of 20% by 14 weeks, which would have left a final sample of 34 subjects for evaluation.
All analyses were conducted using SPSS version 18.0 software (SPSS Inc, Chicago, IL). A P value <0.05 was considered statistically significant.
Results
Baseline characteristics are provided in Table 1. Seventeen subjects were allocated to the atorvastatin‐first treatment arm and 18 subjects to the placebo‐first treatment arm. Compliance was 94%, as assessed by counting the returned medications. Atorvastatin was well tolerated in all study subjects. Despite having a family history of premature CAD and ED, 22 subjects (62%) had a low‐risk Framingham Risk Score (FRS) of <10%. In addition, the median LDL‐C level was 124 mg/dL (IQR, 101–148 mg/dL) and therefore well below the cutoff value for statin treatment according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP‐ATP III) guidelines.20 Eleven subjects (31%) were indicated for drug therapy according to this guideline.
Table 1.
Baseline Characteristics of Study Subjects
| Variables | N = 35 |
|---|---|
| Age, y | 52 (46–57) |
| Male sex | 21 (60) |
| Height, cm | 163 (159–171) |
| Weight, kg | 61 (57–71) |
| BMI, kg/m2 | 23.6 (21.8–25.5) |
| Waist circumference, cm | 33 (30–34) |
| DM | 2 (6) |
| Hypertension | 10 (29) |
| Smoking | |
| Nonsmoker | 15 (43) |
| Ex‐smoker | 12 (34) |
| Current smoker | 8 (23) |
| Dyslipidemia | 10 (29) |
| SBP, mm Hg | 128 (111–139) |
| DBP, mm Hg | 78 (70–83) |
| TC, mg/dL | 194 (173–224) |
| LDL‐C, mg/dL | 124 (101–148) |
| HDL‐C, mg/dL | 47 (36–54) |
| TG, mg/dL | 96 (75–154) |
| Glucose, mg/dL | 89 (85–98) |
| RHI | 1.6 (1.4–1.7) |
| AI | 21.5 (11.0–37.3) |
| Creatinine, mg/dL | 0.85 (0.70–0.94) |
| FRS | |
| <10% | 22 (62) |
| 10%–20% | 11 (31) |
| >20% | 2 (7) |
| Indicated for statin therapya | 11 (31) |
Abbreviations: AI, augmentation index; BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; FRS, Framingham Risk Score; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; NCEP‐ATP III, National Cholesterol Education Program Adult Treatment Panel III; RHI, reactive hyperemic index; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
Values are presented as median (interquartile range) or n (%).
According to the NCEP‐ATP III guidelines.20
Treatment with atorvastatin 40 mg/day over 6 weeks led to a significant reduction in LDL‐C levels (Table 2 and Figure 2). After atorvastatin treatment, the median LDL‐C was 67 mg/dL (IQR, 50–73 mg/dL) compared with a median of 124 mg/dL (IQR, 102–145 mg/dL) after placebo treatment (P < 0.001). Triglyceride levels were also lower after atorvastatin treatment compared with placebo (87 mg/dL [IQR, 62–118] vs 112 mg/dL [IQR, 76–177 mg/dL], P = 0.001; Figure 2). However, HDL‐C was not affected by atorvastatin treatment (46 mg/dL [IQR, 38–58 mg/dL] vs 46 mg/dL [IQR, 40–53 mg/dL], P = 0.657). Likewise, systolic and diastolic BP were not affected by atorvastatin treatment.
Table 2.
Endothelial Function and Lipid Profiles After Treatment
| Placebo | Atorvastatin | Differencea | P Valueb | |
|---|---|---|---|---|
| RHI | 1.9 (1.5–2.4) | 1.9 (1.6–2.2) | 0.0 (−0.3, 0.3) | 0.902 |
| AI | 29 (16–35) | 24 (17–33) | −1 (−8, 9) | 0.922 |
| LDL‐C, mg/dL | 124 (102–145) | 67 (50–73) | −60 (−74, −46) | <0.001 |
| HDL‐C, mg/dL | 46 (38–58) | 46 (40–53) | −1 (−5, 4) | 0.657 |
| TG, mg/dL | 112 (76–177) | 87 (62–118) | −25 (−57, −6) | <0.001 |
| TC, mg/dL | 192 (172–219) | 131 (113–142) | −66 (−50, −87) | <0.001 |
| SBP, mm Hg | 122 (109–132) | 117 (111–128) | −1 (−4, 11) | 0.917 |
| DBP, mm Hg | 75 (70–87) | 73 (67–83) | −2 (−6, 2) | 0.197 |
Abbreviations: AI, augmentation index; DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; RHI, reactive hyperemia index; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
Values are presented as median (interquartile range).
Values are the difference between atorvastatin and placebo treatment. b P value from a Wilcoxon signed rank test.
Figure 2.
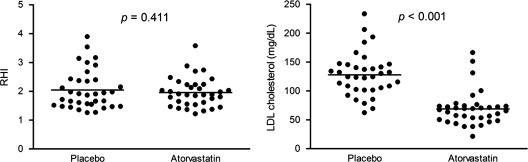
Comparison of the RHI and LDL‐C levels between placebo and atorvastatin treatment. The LDL‐C was significantly lower after atorvastatin treatment than after placebo. However, the RHI, which indicates endothelial function, was not different between the 2 groups. Each dot represents a unique subject; lines and error bars show the median and IQR. P values were from a Wilcoxon signed rank test. Abbreviations: IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; RHI, reactive hyperemic index.
However, the observed improvement in lipid profile did not lead to an improvement in ED. The RHI after atorvastatin treatment was not different from that after placebo treatment (1.9 [IQR, 1.6–2.2] vs 1.9 [IQR, 1.5–2.4], P = 0.902; Figure 2). Additionally, the proportion of subjects who normalized endothelial function (RHI ≥2) after atorvastatin and placebo treatment was not significantly different (40% vs 37%, P = 1.000) (Figure 3). The augmentation index after atorvastatin treatment was not different from that after placebo treatment (24 [IQR, 17–33] vs 29 [IQR 16–35], P = 0.922). In the subgroup analysis, these results were consistent both in subjects indicated for drug therapy (11 subjects, 31%) and subjects not indicated for drug therapy (24 subjects, 69%), according to the NCEP‐ATP III guidelines. In the subgroup of subjects indicated for drug therapy, the RHI after atorvastatin treatment was 1.7 (IQR, 1.5–2.4) and the RHI after placebo was 1.9 (IQR, 1.5–2.2), which was not statistically significant (P = 0.423). Similarly, in the subgroup not indicated for drug therapy, the RHI after atorvastatin treatment did not differ significantly from that after placebo treatment (1.9 [IQR, 1.6–2.2] vs 1.9 [IQR, 1.5–2.4], P = 0.502).
Figure 3.
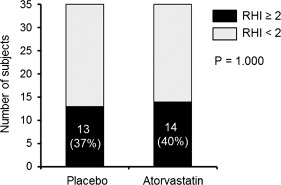
The proportion of subjects who normalized endothelial function after placebo and atorvastatin treatment. The proportion of subjects who normalized endothelial function (RHI ≥2) after atorvastatin and placebo treatment was not different (40% vs 37%, P = 1.000). Abbreviations: RHI, reactive hyperemic index.
Discussion
The principal finding of the present study was that despite an improvement in lipid profile, atorvastatin treatment failed to improve ED or arterial stiffness in the FDRs of patients with premature CAD with ED.
A family history of premature CAD is a well‐known risk factor for cardiovascular events and necessitates the implementation of aggressive primary prevention for CAD. Several studies have showed a higher proportion of subclinical coronary atherosclerosis in FDRs by several diagnostic methods such as carotid ultrasound, coronary artery calcium (CAC) score, and coronary CT angiography.1, 21, 22 However, despite the significance of a family history of premature CAD as a risk factor for cardiovascular events, there are few studies on risk stratification or prevention strategies for cardiovascular events in FDRs of patients with CAD. Therefore, we investigated whether statin treatment for primary prevention of CAD in FDRs with ED would improve the ED. Previous studies have demonstrated that statin therapy improves the endothelium‐dependent dilation of coronary and peripheral arteries in patients with hypercholesterolemia. Lovastatin significantly improved endothelium‐mediated responses in the coronary arteries after 6 months.8 Another study in patients with moderately elevated cholesterol levels showed that the vasodilator response to acetylcholine as determined by forearm blood flow was significantly increased after 1 month of treatment with simvastatin.9 In addition, in the Reduction of Cholesterol in Ischemia and Function of the Endothelium (RECIFE) trial, 6 weeks of pravastatin therapy (40 mg/d) increased flow‐mediated dilation compared with placebo in patients with acute coronary syndromes.10
However, in the present study, atorvastatin treatment failed to improve ED. Several factors might explain these discrepancies. The most important attributing factors may be the study population, as the baseline LDL‐C was relatively lower. Furthermore, even though we screened for patients with ED, our study subjects had a relatively mild degree of ED at baseline. Similar to our findings, another study showed that 6 months of cholesterol‐lowering therapy had no significant effect on coronary endothelial vasomotor function in a population of patients with CAD and mildly elevated cholesterol levels.23 In that study, the authors also suggested the reason for the lack of improvement in endothelial function was attributable to a lower baseline LDL‐C level and the mild degree of CAD. Therefore, these findings support the importance of adequate statin treatment for primary prevention. According to the current guidelines, statin therapy may not be indicated for the majority of subjects.20 In addition, these results raise the need to further stratify risk assessment among FDRs, because not all FDRs will have the same risk for future cardiovascular events, and to identify subjects who stand to benefit from statin therapies for primary prevention.7 Despite the fact that the outcome of the St. Francis Heart trial, which investigated the effect of atorvastatin 20 mg/day in asymptomatic individuals with a CAC score above the 80th percentile, did not reach statistical significance, a post hoc analysis showed that the combination of a positive family history and a CAC score above the 80th percentile was a subgroup within the primary‐prevention population that received greater benefit from statin treatment than the population at large.24
Our study has certain limitations. First, the number of subjects was relatively small, so subgroup analysis to identify subsets that may have benefited more from atorvastatin treatment could not be performed. However, the implementation of a crossover design potentiated its statistical power. Second, the addition of a second baseline assessment before the initiation of the second treatment period might have provided additional information and excluded carryover effects between the treatment phases. However, studies have suggested that withdrawal of statins causes acute termination of their beneficial effects.25, 26 Therefore, we believe that the 2‐week washout period was sufficient.27 Third, concomitant assessment of inflammatory markers such as high‐sensitivity C‐reactive protein could have been more helpful in identifying potential effects of atorvastatin on vascular inflammation in the studied populations. Fourth, endothelium‐independent increase of finger blood flow after administration of nitroglycerin was not measured. However, each patient served as his/her own control subject with the comparison of the hyperemic arm with the contralateral control arm, and the ratio of PAT amplitude has been widely used as a surrogate marker for ED.16, 17 Finally, other statins such as simvastatin or rosuvastatin might have different efficacies on anti‐inflammatory effects. Thus, our findings might be limited to atorvastatin.
Conclusion
Despite the improvement in lipid profile, atorvastatin treatment failed to improve ED or arterial stiffness in the FDRs of patients with premature CAD with ED. Although we specifically identified FDRs with ED as a subgroup at high risk for future cardiovascular events, atorvastatin treatment as a primary‐prevention strategy may not be beneficial in these subjects.
This work was supported by the National Research Foundation of Korea grant funded by the Korea government (MEST; no. 2012027176) and by the Chong Kun Dang Pharmaceutical Corp., Seoul, Korea. ClinicalTrials.gov: NCT00917527. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Nasir K, Michos ED, Rumberger JA, et al. Coronary artery calcification and family history of premature coronary heart disease: sibling history is more strongly associated than parental history. Circulation. 2004;110:2150–2156. [DOI] [PubMed] [Google Scholar]
- 2. Lloyd‐Jones DM, Nam BH, D'Agostino RB Sr, et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle‐aged adults: a prospective study of parents and offspring. JAMA. 2004;291:2204–2211. [DOI] [PubMed] [Google Scholar]
- 3. Andresdottir MB, Sigurdsson G, Sigvaldason H, et al. Fifteen percent of myocardial infarctions and coronary revascularizations explained by family history unrelated to conventional risk factors. The Reykjavik Cohort Study. Eur Heart J. 2002;23:1655–1663. [DOI] [PubMed] [Google Scholar]
- 4. Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–e636. [DOI] [PubMed] [Google Scholar]
- 5. Yanek LR, Moy TF, Blumenthal RS, et al. Hypertension among siblings of persons with premature coronary heart disease. Hypertension. 1998;32:123–128. [DOI] [PubMed] [Google Scholar]
- 6. Hengstenberg C, Holmer SR, Mayer B, et al. Siblings of myocardial infarction patients are overlooked in primary prevention of cardiovascular disease. Eur Heart J. 2001;22:926–933. [DOI] [PubMed] [Google Scholar]
- 7. Joshi PH, Chaudhari S, Blaha MJ, et al. A point‐by‐point response to recent arguments against the use of statins in primary prevention: this statement is endorsed by the American Society for Preventive Cardiology. Clin Cardiol. 2012;35:404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Treasure CB, Klein JL, Weintraub WS, et al. Beneficial effects of cholesterol‐lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481–487. [DOI] [PubMed] [Google Scholar]
- 9. O'Driscoll G, Green D, Taylor RR. Simvastatin, an HMG‐coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95:1126–1131. [DOI] [PubMed] [Google Scholar]
- 10. Dupuis J, Tardif JC, Cernacek P, et al. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes. The RECIFE (Reduction of Cholesterol in Ischemia and Function of the Endothelium) trial. Circulation. 1999;99:3227–3233. [DOI] [PubMed] [Google Scholar]
- 11. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 suppl 1):III27–III32. [DOI] [PubMed] [Google Scholar]
- 12. Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long‐term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. [DOI] [PubMed] [Google Scholar]
- 13. Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. [DOI] [PubMed] [Google Scholar]
- 14. Hunt SC, Williams RR, Barlow GK. A comparison of positive family history definitions for defining risk of future disease. J Chronic Dis. 1986;39:809–821. [DOI] [PubMed] [Google Scholar]
- 15. Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. [DOI] [PubMed] [Google Scholar]
- 16. Hamburg NM, Benjamin EJ. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med. 2009;19:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by non‐invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. [DOI] [PubMed] [Google Scholar]
- 18. Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Julious SA. Sample sizes for clinical trials with normal data. Stat Med. 2004;23:1921–1986. [DOI] [PubMed] [Google Scholar]
- 20. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection , Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 21. Wang TJ, Nam BH, D'Agostino RB, et al. Carotid intima‐media thickness is associated with premature parental coronary heart disease: the Framingham Heart Study. Circulation. 2003;108:572–576. [DOI] [PubMed] [Google Scholar]
- 22. Kang MK, Chang HJ, Kim YJ, et al. Prevalence and determinants of coronary artery disease in first‐degree relatives of premature coronary artery disease. Coron Artery Dis. 2012;23:167–173. [DOI] [PubMed] [Google Scholar]
- 23. Vita JA, Yeung AC, Winniford M, et al. Effect of cholesterol‐lowering therapy on coronary endothelial vasomotor function in patients with coronary artery disease. Circulation. 2000;102:846–851. [DOI] [PubMed] [Google Scholar]
- 24. Mulders TA, Sivapalaratnam S, Stroes ES, et al. Asymptomatic individuals with a positive family history for premature coronary artery disease and elevated coronary calcium scores benefit from statin treatment: a post hoc analysis from the St. Francis Heart Study. JACC Cardiovasc Imaging. 2012;5:252–260. [DOI] [PubMed] [Google Scholar]
- 25. Wassmann S, Faul A, Hennen B, et al. Rapid effect of 3‐hydroxy‐3‐methylglutaryl coenzyme a reductase inhibition on coronary endothelial function. Circ Res. 2003;93:e98–e103. [DOI] [PubMed] [Google Scholar]
- 26. Tsunekawa T, Hayashi T, Kano H, et al. Cerivastatin, a hydroxymethylglutaryl coenzyme a reductase inhibitor, improves endothelial function in elderly diabetic patients within 3 days. Circulation. 2001;104:376–379. [DOI] [PubMed] [Google Scholar]
- 27. Schneider MP, Schmidt BM, John S, et al. Effects of statin treatment on endothelial function, oxidative stress and inflammation in patients with arterial hypertension and normal cholesterol levels. J Hypertens. 2011;29:1757–1764. [DOI] [PubMed] [Google Scholar]


