Abstract
Background
Smaller coronary artery diameter portends worse outcomes after coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI). The suggestion that women have smaller coronary artery diameters than men has not been validated by a large‐scale study.
Hypothesis
We sought to confirm a gender difference with respect to coronary artery diameter, even after accounting for body habitus and left ventricular mass (LVM).
Methods
From 4200 subjects evaluated for cardiovascular disease by computed tomography angiography, we selected 710 subjects (383 males, 327 females) with coronary artery calcium (CAC) scores <100, eliminating patients with artery remodeling. Diameters of the left main (LM), left anterior descending (LAD), left circumflex (CX), and right coronary arteries (RCA), were measured. Measurements were compared using a 2‐sample t test and the multiple regression model, accounting for body habitus and LVM.
Results
After adjusting for age, race, weight, height, body mass index, body surface index, LVM, and CAC, women have smaller diameters in the LM (males 4.35 mm, females 3.91 mm), LAD (males 3.54 mm, females 3.24 mm), CX (males 3.18, females 2.75 mm), and RCA (males 3.70 mm, females 3.26 mm) (P < 0.001). This difference is not related to body habitus or LVM.
Conclusions
Gender significantly influences artery diameter of the LM, LAD, CX, and RCA. This may warrant gender specific approaches during PCI and CABG. As neither body habitus nor LVM relate to the difference in coronary artery diameter, our study encourages a search for inherent differences between genders that can account for this difference.
Introduction
In the United States during 2010, an estimated 492 000 patients underwent percutaneous coronary intervention (PCI), whereas a total of 219 000 patients underwent coronary artery bypass grafting (CABG) procedures.1 Coronary vessels with small luminal diameters are associated with substantially increased risk of in‐hospital mortality with CABG.2, 3 Smaller coronary artery diameter is also associated with lower rates of procedural success and increased rates of subsequent in‐hospital major events with PCI.4 Following both PCI and CABG, females demonstrate an increased risk of mortality and morbidity. This includes higher bleeding rates, more thrombotic events, lower graft patency, and higher readmission rates from procedural complications, heart failure, or unstable angina.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 The difference in outcomes between genders has been attributed to women having smaller coronary artery diameters, more comorbidities at presentation, and a higher requirement of emergent surgeries.17, 18, 19, 20, 21 Studies supporting the idea that women have smaller coronary arteries have been limited by small sample size, measurement of only proximal artery segments, inclusion of a highly symptomatic cohort, or employment of intravascular ultrasound (IVUS), which may cause vasospasm.22, 23, 24, 25, 26 Furthermore, some studies have made the assumption that coronary diameter is likely related to body habitus. In a large sample size with fewer symptoms and lower atherosclerotic disease burden, we sought to confirm gender differences in coronary diameter using multidetector computed tomography (MDCT). In addition, we were interested in whether or not this proposed difference was related to body habitus or left ventricular mass (LVM).
Methods
Study Population
We collected data on 4200 subjects who were referred for evaluation of possible coronary artery disease (CAD) and who underwent coronary computed tomography angiography (CTA). Only those with coronary artery calcium scores (CAC) below 100 were included, hoping to eliminate subjects with potential coronary artery remodeling.27, 28, 29 Subjects with poor‐quality imaging, whether it was because of motion artifact or because of tortuous coronary vessels, were eliminated. This criterion narrowed our sample size to 710. Patient demographics studied included age, race, height, weight, body surface index (BSI), body mass index (BMI), LVM, calcium score, cholesterol, diabetes, hypertension, family history of CAD, and smoking.
Study Protocol
CAC Scanning
Using electrocardiograph (ECG)‐triggering at 75% of the R‐R interval, 30 to 40 contiguous slices were taken. Presence of calcium in a coronary artery was defined by detection of a density of >130 Hounsfield units (HU) in ≥3 contiguous pixels (>1 mm2) overlying a coronary artery.
Cardiac CTA
β‐Blockers were administered to patients (57%) with a heart rate greater than 65 bpm. A test intravenous (IV) bolus of 15 mL of contrast agent, followed by 20 mL of saline flush (rate of 4.5 mL/s), followed by 1 puff of nitrate (400 µg) was administered to assess blood transit time from the arm to the ascending aorta. Using a power injector, retrospective ECG‐gated cardiac CTA was performed with a triphasic injection sequence. This sequence included 50 mL of nonionic IV contrast material (Iopamidol 370) injected at a rate of 5.0 mL, followed by 50 mL of 60% contrast and saline mixture, followed by 50 mL of saline flush. Contrast was injected through a 18‐ to 20‐gauge angiocatheter in the antecubital vein. ECG‐triggered dose modulation was applied in each case, with 400 to 600 mA in the 60% to 80% R‐R interval, and 250 to 350 mA for the rest of the cardiac cycle (81% to 59% of the next cycle). The mean radiation dose was 11.9 ± 1.2 mSv. Cardiac data were reconstructed from 5% to 95% of R‐R interval.
Data Acquisition
A 64‐slice MDCT scanner (Lightspeed VCT; General Electric Healthcare Technologies, Milwaukee, WI) was used for all patients. Imaging started 1 inch above the ostium of the left main coronary artery and continued to 1 inch below the inferior aspect of the heart. The prospective CTA was completed at 75% of the R‐R interval. The following imaging and reconstruction parameters were applied: collimation 40 × 0.625 mm; 120 kVp; 220–670 mAs; pitch 0.18–0.24; rotation time 0.35 seconds; matrix 512 × 512; pixel size 0.39 mm2, and mean effective radiation dose of 1 to 2 mSv (8.0–11.5 mSv). Coronary vessels were reviewed (AW Volume Share; General Electric Healthcare Technologies) and volume renderings and curved multiplanar reformations were done. Each vessel was deemed normal (no stenosis and CAC 0), nonobstructive CAD (luminal stenosis 1%–49%), or obstructive CAD (luminal stenosis >50%). Vessels 1.5 mm in diameter or larger were assessed. Two skilled cardiologists blinded to the clinical data assessed the coronary arteries separately.
Measurements (CAC, LVM, Coronary Diameter)
First, the CAC score was calculated using the Agatston scoring method.30 Second, to calculate LVM, epicardial and endocardial segmentation (including papillary muscles) was completed. Eight‐ to 15‐slice levels were traced, and the remaining slice levels were computed automatically by the workstation computers. We calculated the 3‐dimensional total LV volume (LVM + LV cavity) and LV cavity by using the modified Simpson method (sum of the cross‐sectional volumes). LVM in milliliters was calculated based on the following formula: LVM = (total LV volume − LV cavity volume). The result was multiplied by 1.05 g/mL (myocardial tissue density) to obtain LVM in grams.
Finally, to calculate coronary artery diameter, manual measurements were made using axial data and thin‐slab reconstructions. The window level during measurements was 300 to 400 HU, with an 800 to 1000 HU window width. Bifurcations of the coronary tree were used as landmarks. The left main artery (LM) was measured at 3 arbitrary points before bifurcation. The left anterior descending (LAD) and left circumflex (CX) arteries were measured at 3 arbitrary points within the 10‐mm proximal segment after bifurcation from the LM. The right coronary artery (RCA) was measured at 3 arbitrary points within the 10‐mm proximal segment. We attempted to take these 3 arbitrary measurements at equidistant points (Figure 1). The 3 measurements of each artery were then averaged.
Figure 1.
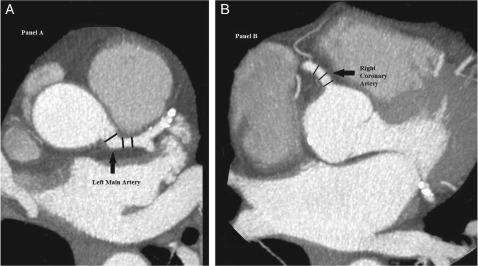
Coronary artery diameter measurements. Examples of coronary artery diameter measurements taken in the left main artery 10 mm before bifurcation (panel A, left) and the right coronary artery within the 10‐mm proximal segment (panel B, right) are shown. In a similar way, measurements are taken from the left anterior descending artery and the left circumflex artery (both within the proximal 10‐mm segment after bifurcation from the left main artery). The 3 measurements taken in each artery are averaged to produce the final diameter data for that particular coronary artery.
Analysis
To compare baseline characteristics of the study population, we used the Student t test for continuous variables and the χ2 test for categorical values. The nonparametric test was applied to compare total calcium scores between genders. To address the relationship of coronary artery lumen size with gender, we used the multiple regression model. Subsequently, both crude and adjusted models were analyzed. Covariates in our multivariate model included age, race, height, weight, BSI, BMI, LVM, calcium score, cholesterol, diabetes, hypertension, family history of CAD, and smoking.
Results
Clinical Characteristics
The inclusion criterion was met by 710 subjects, of which 54% were males (n = 383) and 46% were females (n = 327). Caucasians were the most dominant race/ethnicity represented in our sample size, followed by Hispanics, Asians, African Americans, and others. On average, men were 5 years younger, 13 cm taller, 14 kg heavier, had a 0.25 m2 larger body surface area, and had a 44‐g larger LVM. When compared to their male counterparts, hypertension was seen in 10% more women and diabetes was seen in 9% more women. There was no significant difference between genders in terms of BMI, hyperlipidemia, and smoking. The median CAC score for women and men was 0 and 10, respectively (summarized in Table 1).
Table 1.
Baseline Characteristics of the Total Study Population
| Characteristic | Women, n = 327 | Men, n = 383 | P Value |
|---|---|---|---|
| Age, y | 61 ± 12 | 56 ± 12 | <0.001 |
| Race/ethnicity | 0.037 | ||
| Caucasian | 90 (49.7) | 146 (58.4) | |
| Hispanic | 45 (24.9) | 39 (15.6) | |
| African‐American | 15 (8.3) | 19 (7.6) | |
| Asian | 29 (16.0) | 35 (14.0) | |
| Others | 2 (1.1) | 11 (4.4) | |
| Height, cm | 162.28 ± 7.44 | 175.77 ± 9.96 | <0.001 |
| Weight, kg | 72.52 ± 16.60 | 86.53 ± 16.31 | <0.001 |
| BMI, kg/m2 | 27.56 ± 5.82 | 28.01 ± 4.71 | 0.378 |
| BSA, m2 | 1.80 ± 0.22 | 2.05 ± 0.23 | <0.001 |
| Hypertension, n (%) | 97 (53.9) | 102 (43.6) | 0.038 |
| Hyperlipidemia, n (%) | 96 (53.0) | 111 (47.0) | 0.224 |
| Diabetes, n (%) | 49 (27.2) | 43 (18.3) | 0.03 |
| Smoking, n (%) | 12 (7.9) | 17 (8.9) | 0.764 |
| Total calcium scores, median (25%, 75%) | 0 (0, 16) | 10 (0, 50) | <0.001 |
| LVM, g | 118.64 ± 39.95 | 162.40 ± 42.41 | <0.001 |
Abbreviations: BMI, body mass index; BSA, body surface index; LVM, left ventricular mass.
Coronary Diameter
The association of coronary artery lumen size with gender, based on the crude and adjusted model, is shown in Table 2. Both before and after adjustment for the covariates of age, LVM, BSI, BMI, height, weight, race, total calcium scores, hypertension, diabetes, high cholesterol, family history of CAD, and smoking, multivariate analysis showed that gender is a strong independent predictor of vessel size. Women have smaller coronary artery diameters in all vessels (P < 0.001). In females, the LM diameter is 0.44 mm smaller, the LAD artery diameter is 0.30 mm smaller, the CX artery diameter is 0.43 mm smaller, and the RCA diameter is 0.44 mm smaller than males. This translates to an 11.25% larger LM, 9.26% larger LAD, 15.64% larger CX, and 13.50% larger RCA in males vs females. There is no significant relationship between coronary artery diameter and other clinical factors such as age, race, height, weight, BSI, BMI, LVM, calcium score, cholesterol, diabetes, hypertension, family history of CAD, and smoking (P < 0.001).
Table 2.
Adjusted Association of Coronary Artery Lumen Size With Gender
| Artery | Gender | No. | Lumen Size | Size Δ, Unadjusted Model | Size Δ, Adjusted Modela | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | β (SE) | 95% CI | P Value | β (SE) | 95% CI | P Value | |||
| LM, mm | Men | 382 | 4.35 ± 0.82 | Referent | Referent | ||||
| Women | 327 | 3.91 ± 0.67 | −0.44 (0.06) | −0.55 to −0.33 | <0.001 | −0.42 (0.08) | −0.59 to −0.26 | <0.001 | |
| LAD, mm | Men | 358 | 3.54 ± 0.67 | Referent | Referent | ||||
| Women | 320 | 3.24 ± 0.58 | −0.29 (0.05) | −0.39 to −0.20 | <0.001 | −0.30 (0.07) | −0.44 to −0.16 | <0.001 | |
| CX, mm | Men | 373 | 3.18 ± 0.71 | Referent | Referent | ||||
| Women | 324 | 2.75 ± 0.64 | −0.43 (0.05) | −0.53 to −0.33 | <0.001 | −0.45 (0.08) | −0.61 to −0.29 | <0.001 | |
| RCA, mm | Men | 377 | 3.70 ± 0.70 | Referent | Referent | ||||
| Women | 326 | 3.26 ± 0.65 | −0.44 (0.05) | −0.54 to −0.34 | <0.001 | −0.46 (0.07) | −0.60 to −0.31 | <0.001 | |
Abbreviations: CI, confidence interval; CX, left circumflex; LAD, left anterior descending; LM, left main; SE, standard error; RCA, right coronary artery; SD, standard deviation.
β is the regression coefficient (lumen size different compared to referent) from the regression model.
Adjusted for age, weight, height, body surface index, body mass index, race, total calcium scores, hypertension, diabetes, high cholesterol, family history of coronary artery disease, smoking, and left ventricular hypertrophy.
Discussion
In 2010, over 700 000 patients underwent either PCI or CABG.1 There is a reported increased morbidity and mortality in females associated with both CABG and PCI.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 This discrepancy has been attributed to the fact that women have smaller coronary artery diameters than men, with the assumption that smaller coronary arteries may be correlated to smaller body habitus.17, 18, 19, 20, 21 Our study was founded on the belief that thoroughly understanding the nature of coronary vessels can help the surgeon or interventionalist better prepare for the procedure, consequently reducing the associated morbidity and mortality.
Previous research attempting to gain insight into gender differences in coronary artery diameter has been limited by confounding variables, small sample size, and inability to measure all coronary arteries. Studies involving an invasive angiogram cohort may be confounded by collection bias, because these patients are likely to be more symptomatic when compared to a more moderate risk cohort. Studies employing IVUS are limited to measuring only proximal segment measurements, and these measurements may be inaccurate due to vessel manipulation and resultant vasospasm.22, 23, 24, 25, 26 Noninvasive imaging, because it is more readily available and allows for evaluation of the entire coronary tree without manipulation, should be the reference standard when evaluating coronary diameter.
Here we provide strong evidence using data from over 700 patients demonstrating that women have smaller coronary artery diameters in all vessels, and this difference is not significantly related to weight, height, BSI, BMI, and LVM. We restricted our study population to those with CAC scores of 0 to 100 in attempt to report findings in a cohort that is at low risk for cardiovascular disease (CVD).31 Because age, race, cholesterol, diabetes, hypertension, family history of CAD, and smoking could influence coronary diameter, it is interesting to note that correcting for all of these factors did not affect the study outcome.
Understanding gender differences in atherosclerosis and coronary disease is of paramount importance. We know that CVD tends to develop almost a decade later in women than in men, yet it is the leading cause of death in women. Despite advances in medical therapy, death from CVD is increasing in middle‐aged women (age 35–54 years), and incidence of CVD, although decreasing in men, has stayed the same in women.10, 32 Clearly, there is a knowledge gap that needs to be filled to improve these unsettling trends. We feel that our results supplement current medical knowledge by definitively showing that both body habitus and LVM have no significant relationship to coronary artery diameter. This study, in turn, may encourage gender‐specific approaches to CVD treatment. Recognizing that women start with smaller luminal diameters, and are therefore subject to greater changes in luminal area from the same degree of atherosclerosis occurring in male counterparts, may support a more aggressive primary prevention strategy in females.
Perhaps the atypical presentation of women with acute coronary syndrome and the poor periprocedural outcomes can be explained by a difference in the nature and size of blood vessels.5, 10, 33 It is clear that the size disparity between men and women is not solely related to body habitus or LVM, and just because men are heavier, taller, or have bigger hearts, does not imply that they also have larger coronary diameters. We hope that our study leads to investigations behind the intrinsic differences between males and females, whether biochemical or hormonal, that explain such anatomic variance.34, 35, 36, 37 Insight into the mechanism behind vasculature differences between genders may lead to translational research that allows new therapies and interventions to be planned.
Limitations
Our study was limited by certain factors. The administration of vasoactive medications prior to CTA could have influenced diameter measurements, but we expect that both genders would be equally affected, and this should not influence the study outcome. Furthermore, complete data regarding indications for the CTA were not collected, and this could have helped gain insight into characteristics of our patient population. Last, diabetes was seen in more women, and hypertension was seen in more men included in our study; however, the differences were not statistically significant.
Conclusion
Women have smaller coronary artery diameters than males, even after accounting for differences in body habitus and LVM.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Connor NJ, Morton JR, Birkmeyer JD, et al. Effect of coronary artery diameter in patients undergoing coronary bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation. 1996;93:652–655. [DOI] [PubMed] [Google Scholar]
- 3. Bjork VO, Ekestrom S, Henze A, et al. Early and late patency of aortocoronary vein grafts. Scand J Thorac Cardiovasc Surg. 1981;15:11–21. [DOI] [PubMed] [Google Scholar]
- 4. Schunkert H, Harrell L, Palacios IF. Implications of small reference vessel diameter in patients undergoing percutaneous coronary revascularization. J Am Coll Cardiol. 1999;34:40–48. [DOI] [PubMed] [Google Scholar]
- 5. Stramba‐Badiale M, Fox KM, Priori SG, et al. Cardiovascular diseases in women: a statement from the policy conference of the European Society of Cardiology. Eur Heart J. 2006;27:994–1005. [DOI] [PubMed] [Google Scholar]
- 6. Woods SE, Noble G, Smith JM, et al. The influence of gender in patients undergoing coronary artery bypass graft surgery: an eight‐year prospective hospitalized cohort study. J Am Coll Surg. 2003;196:428–434. [DOI] [PubMed] [Google Scholar]
- 7. Malenka DJ, O'Rourke D, Miller MA, et al. Cause of in‐hospital death in 12,232 consecutive patients undergoing percutaneous transluminal coronary angioplasty. The Northern New England Cardiovascular Disease Study Group. Am Heart J. 1999;137(4 pt 1):632–638. [DOI] [PubMed] [Google Scholar]
- 8. Vaccarino V, Abramson JL, Veledar E, et al. Sex differences in hospital mortality after coronary artery bypass surgery: evidence for a higher mortality in younger women. Circulation. 2002;105:1176–1181. [DOI] [PubMed] [Google Scholar]
- 9. Alexander KP, Chen AY, Newby LK, et al. Sex differences in major bleeding with glycoprotein IIb/IIIa inhibitors: results from the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) initiative. Circulation. 2006;114:1380–1387. [DOI] [PubMed] [Google Scholar]
- 10. Maas AH, Appelman YE. Gender differences in coronary heart disease. Neth Heart J. 2010;18:598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Argulian E, Patel AD, Abramson JL, et al. Gender differences in short‐term cardiovascular outcomes after percutaneous coronary interventions. Am J Cardiol. 2006;98:48–53. [DOI] [PubMed] [Google Scholar]
- 12. Cantor WJ, Miller JM, Hellkamp AS, et al. Role of target vessel size and body surface area on outcomes after percutaneous coronary interventions in women. Am Heart J. 2002;144:297–302. [DOI] [PubMed] [Google Scholar]
- 13. Abramov D, Tamariz MG, Sever JY, et al. The influence of gender on the outcome of coronary artery bypass surgery. Ann Thorac Surg. 2000;70:800–805; discussion 806. [DOI] [PubMed] [Google Scholar]
- 14. Guru V, Fremes SE, Austin PC, et al. Gender differences in outcomes after hospital discharge from coronary artery bypass grafting. Circulation. 2006;113:507–516. [DOI] [PubMed] [Google Scholar]
- 15. Kim C, Redberg RF, Pavlic T, et al. A systematic review of gender differences in mortality after coronary artery bypass graft surgery and percutaneous coronary interventions. Clin Cardiol. 2007;30:491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mehilli J, Kastrati A, Bollwein H, et al. Gender and restenosis after coronary artery stenting. Eur Heart J. 2003;24:1523–1530. [DOI] [PubMed] [Google Scholar]
- 17. Khan SS, Nessim S, Gray R, et al. Increased mortality of women in coronary artery bypass surgery: evidence for referral bias. Ann Intern Med. 1990;112:561–567. [DOI] [PubMed] [Google Scholar]
- 18. Greenberg MA, Mueller HS. Why the excess mortality in women after PTCA? Circulation. 1993;87:1030–1032. [DOI] [PubMed] [Google Scholar]
- 19. Ellis SG, Roubin GS, King SB III, et al. Angiographic and clinical predictors of acute closure after native vessel coronary angioplasty. Circulation. 1988;77:372–379. [DOI] [PubMed] [Google Scholar]
- 20. Kelsey SF, James M, Holubkov AL, et al. Results of percutaneous transluminal coronary angioplasty in women. 1985‐1986 National Heart, Lung, and Blood Institute's Coronary Angioplasty Registry. Circulation. 1993;87:720–727. [DOI] [PubMed] [Google Scholar]
- 21. Christakis GT, Weisel RD, Buth KJ, et al. Is body size the cause for poor outcomes of coronary artery bypass operations in women? J Thorac Cardiovasc Surg. 1995;110:1344–1356; discussion 1356‐1348. [DOI] [PubMed] [Google Scholar]
- 22. Dodge JT Jr, Brown BG, Bolson EL, et al. Lumen diameter of normal human coronary arteries. Influence of age, sex, anatomic variation, and left ventricular hypertrophy or dilation. Circulation. 1992;86:232–246. [DOI] [PubMed] [Google Scholar]
- 23. Yang F, Minutello RM, Bhagan S, et al. The impact of gender on vessel size in patients with angiographically normal coronary arteries. J Interv Cardiol. 2006;19:340–344. [DOI] [PubMed] [Google Scholar]
- 24. Dickerson JA, Nagaraja HN, Raman SV. Gender‐related differences in coronary artery dimensions: a volumetric analysis. Clin Cardiol. 2010;33:E44–E49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kucher N, Lipp E, Schwerzmann M, et al. Gender differences in coronary artery size per 100 g of left ventricular mass in a population without cardiac disease. Swiss Med Wkly. 2001;131(41‐42):610–615. [DOI] [PubMed] [Google Scholar]
- 26. Nissen SE. Application of intravascular ultrasound to characterize coronary artery disease and assess the progression or regression of atherosclerosis. Am J Cardiol. 2002;89:24B–31B. [DOI] [PubMed] [Google Scholar]
- 27. Zeb I, Budoff MJ. MESA: the NIH‐sponsored study that validates atherosclerosis imaging for primary prevention. Curr Atheroscler Rep. 2011;13:353–358. [DOI] [PubMed] [Google Scholar]
- 28. Alexopoulos N, Raggi P. Calcification in atherosclerosis. Nat Rev Cardiol. 2009;6:681–688. [DOI] [PubMed] [Google Scholar]
- 29. Jain A, McClelland RL, Polak JF, et al. Cardiovascular imaging for assessing cardiovascular risk in asymptomatic men versus women: the multi‐ethnic study of atherosclerosis (MESA). Circ Cardiovasc Imaging. 2011;4:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 31. Raggi P, Gongora MC, Gopal A, et al. Coronary artery calcium to predict all‐cause mortality in elderly men and women. J Am Coll Cardiol. 2008;52:17–23. [DOI] [PubMed] [Google Scholar]
- 32. Towfighi A, Zheng L, Ovbiagele B. Sex‐specific trends in midlife coronary heart disease risk and prevalence. Arch Intern Med. 2009;169:1762–1766. [DOI] [PubMed] [Google Scholar]
- 33. Pilote L, Dasgupta K, Guru V, et al. A comprehensive view of sex‐specific issues related to cardiovascular disease. CMAJ. 2007;176:S1–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Knot HJ, Lounsbury KM, Brayden JE, et al. Gender differences in coronary artery diameter reflect changes in both endothelial Ca2+ and ecNOS activity. Am J Physiol. 1999;276(3 pt 2):H961–H969. [DOI] [PubMed] [Google Scholar]
- 35. Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. [DOI] [PubMed] [Google Scholar]
- 36. Cavasin MA, Sankey SS, Yu AL, et al. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;284:H1560–H1569. [DOI] [PubMed] [Google Scholar]
- 37. Gabel SA, Walker VR, London RE, et al. Estrogen receptor beta mediates gender differences in ischemia/reperfusion injury. J Mol Cell Cardiol. 2005;38:289–297. [DOI] [PubMed] [Google Scholar]


