Abstract
Background
A high prevalence of Pulmonary Hypertension (PH) in sickle cell disease (SCD) has been reported in several studies. However, few studies that describe the hemodynamics have actually measured pulmonary artery occlusive pressure (PAOP). Furthermore, even PAOP has been shown to be unreliable in discriminating pulmonary artery hypertension from pulmonary venous hypertension. We prospectively examined the accuracy of PAOP using simultaneous left ventricular end diastolic pressure (LVEDP) measurement as the gold standard.
Hypothesis
In patients with SCD, PAOP may not reflect LVEDP leading to over‐diagnosis of PAH.
Methods
We prospectively examined hemodynamic data on 26 patients with SCD, at a large academic center, from 2009 through 2011. These patients underwent simultaneous PAOP and LVEDP measurements.
Results
We tested 106 adult SCD patients with 2‐D Echocardiography for evaluation of PH. Of the 106 patients, 43 (41%) were found to have a tricuspid regurgitant jet velocity ≥ 2.5 m/sec. Of these 43, 26 patients underwent right heart catheterization (RHC) and simultaneous measurement of LVEDP. Twelve patients among the 106 (11.1%) patients were found to have PH. Eight of these (7.5 %) had PAH by PAOP criteria but only 4/106 (3/7%) had PAH by LVEDP criteria. PAOP significantly underestimated the LVEDP in both the PH group and group with normal hemodynamics (p=0.00004). BNP, and creatinine levels significantly increased in PAH group (p< 0.02, 0.01, 0.03). PAOP misclassified 50% of patients in this sickle cell disease cohort. In conclusion, PAOP may underestimate LVEDP in sickle cell patients with pulmonary hypertension and can lead to misclassification.
Introduction
Many previous studies have reported that pulmonary hypertension (PH) is a frequent complication of sickle cell disease (SCD).1, 2 Echocardiography‐based studies have estimated the prevalence of PH to be around 20% to 30%2, 3 ,and have indicated that patients with pulmonary hypertension have higher mortality and a worse prognosis.3, 4 A large National Institutes of Health‐sponsored prospective study that defined PH as a tricuspid regurgitant jet velocity (TRV) >2.5 m/s, found the prevalence of PH to be 32% and the risk of mortality 10%.5 Other studies have revealed even higher mortality rates of 17% and 10% vs 2% and 1% for controls.3, 6 Echocardiography does not reliably define the presence of PH, and when present, does not differentiate between pulmonary artery hypertension (PAH) or pulmonary venous hypertension (PVH). The gold standard for making the diagnosis of PAH is a right heart catheterization (RHC).7 Few studies have evaluated the prevalence of PH in sickle cell disease by right heart catheterization.3, 8, 9 Additionally, in screening programs for PH in other high‐risk populations, the use of an echocardiogram alone to define PH resulted in a substantial number of false‐positive diagnoses when RHC was used for confirmation.10 In a study of hospitalized patients recovering from acute crisis,3 10 of the 20 patients (50%) were found to have PVH (mean pulmonary artery [PA] pressure ≥25 mm Hg and pulmonary artery occlusion pressure [PAOP] pressures >15 mm Hg). Another study of sickle cell patients not in crisis revealed that 46% of patients diagnosed with PH by echocardiographic criteria (TRV of 2.5 m/s or more) and RHC had PVH.11 The same group also showed that diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease, indicating the importance of defining this complication.12 Diastolic dysfunction was implicated in one‐third of patients with PH who had a tricuspid regurgitant (TR) jet >2.5 m/s. Recently, it has been shown that the true prevalence of PAH in sickle cell patients is 6%, as confirmed by RHC using PAOP criteria of ≤15 mm Hg and mean PA pressure of ≥25 mm Hg.9 This procedure is now recommended by international guidelines as the standard of care.9
Furthermore, another recently published study of PH patients who did not have SCD suggested that almost 50% of patients meeting criteria for PAH based on PAOP had increased left ventricular end‐diastolic pressure (LVEDP).13 PAOP also consistently underestimated LVEDP. This suggests that patients with PVH may be erroneously labeled as PAH even with RHC. This misclassification of disease may result in the inappropriate use of PAH‐specific therapies that are not only costly but have also been shown to cause increased morbidity in patients with PVH.14, 15, 16 A National Institutes of Health study was terminated prematurely when patients with SCD and PH sildenafil group demonstrated increased pain crises.22 This information highlights the need to better define the causes of PH in SCD to more appropriately treat this condition.
The objective of the study was to determine the extent of discrepancy between PAOP and LVEDP in sickle cell patients undergoing RHC for evaluation of PH.
Methods
Study Design
All adult patients (18 years or older) with SCD (HbSS) followed in a single adult comprehensive sickle cell center (Brody School of Medicine) were screened with echocardiography for the presence of PH from January 2009 to February 2011. Patients with an elevated TRV of ≥2.5 m/s were referred for evaluation of PH and were consented to participate in the study. Hemodynamic data were prospectively collected. The study was approved by University Medical Center Institutional Review Board (UMCIRB #09‐0781). Patients with TRV of 2.5 mm Hg or more with symptoms undergoing RHC were invited, and written consent was obtained. The study was performed on SCD patients in their normal state in a stable outpatient setting. Outpatient stable setting is defined as absence of major pain crisis requiring admission or emergency room visit within the last 4 weeks. All echocardiograms were performed within 3 months of catheterization, and patients with significant left heart disease (ejection fraction <40%, valvular heart disease, cardiomyopathy, congenital heart disease, or known significant coronary artery disease) were excluded.
If patients did not clinically need left heart catheterization, based on risk assessment by a board‐certified cardiologist, a simultaneous LVEDP was obtained via right radial access with a 5 F Tiger catheter (Terumo Inc., Somerset, NJ). If clinically indicated, a coronary angiogram was performed with the same 5 F Tiger catheter. Cardiac output was computed by the Fick equation. The protocol is based on our clinical experience of underestimation of LVEDP by PAOP in sickle cell patients and other supporting data.3, 14, 25 All patients with unexplained dyspnea on exertion (New York Heart Association class 2 or above) with TRV of 2.5 m/s or more had a more comprehensive workup for PH by a board‐certified pulmonologist, including pulmonary function tests, ventilation perfusion scan, computed tomography scan of the chest, and polysomnography (if indicated clinically). Blood tests routinely used for the care of these patients were reviewed.
A patient was considered to have PH if mean PA pressures were ≥25 mm Hg at rest during catheterization. Patients were considered to have PVH if the PAOP and/or LVEDP pressure was >15 mm Hg.9 PAH was defined as mean PA pressure of 25 mm Hg or more and LVEDP was 15 mm Hg or less.
Hemodynamic Measurements
Cardiac catheterization was performed by an interventional cardiologist and a pulmonologist with PAH expertise. Hemodynamic measurements were made using a standard protocol under conscious sedation. RHC was performed through the right femoral venous access with a 7 F Bard balloon‐tipped catheter (Bard Medical, Covington, GA) under fluoroscopic guidance. The catheter was advanced into the PAOP position first, and the pressure was recorded. PAOP was confirmed by an oximetry and manual waveform analysis in the end‐expiratory phase. The balloon was then deflated, and the PA pressures were recorded in the end‐expiratory phase and manually interpreted. Pressures were recorded in the right ventricle and right atrium, sequentially as well, with the withdrawal of the catheter. A simultaneous LVEDP was obtained through right radial access with a 5 F Tiger catheter (Terumo Inc.). If clinically indicated, a coronary angiogram was performed with the same 5 F Tiger catheter. Cardiac output was computed by the Fick equation.
Pulmonary function tests and 6‐minute walk tests were measured using Medgraphics Elite DX model #830001‐008 (MGC Diagnostics Corp., Saint Paul, MN) and continuous pulse oximetry according to American Thoracic Society recommendations.17, 18, 19
Statistical Analysis
Data were presented in a step‐down procedure (Duncan's multiple range test) when noncongruent as baseline demographics, laboratory profiles, pulmonary characteristics, RHC. and echocardiography findings. A one‐way analysis of variance (ANOVA) model was used to compare means between groups (H0:μa = μb = μc; a: mPAP<25mmHg, b: PAOP ≤ 15mmHg | ≥25mmHg, c: PAOP>15mmHg | ≥25mmHg). Post hoc pairwise multiple comparisons were evaluated using the Ryan‐Einot‐Gabriel‐Welsch procedure. Multivariable log‐normal plots were used to assess the underlying normality and heterogeneity of the data. When appropriate, a log or square root transformation was applied to the data. The association between row and column variables was tested using Fisher exact test, assuming a conditional hypergeometric distribution. All analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC).
Results
A total of 106 SCD patients were referred as outpatients for 2‐dimensional echocardiogram testing from the adult sickle cell clinic when in their steady state. Patients with TRV ≥2.5 m/s and dyspnea were referred to our PH clinic. Of the 106 patients, 63 were found to have a TR jet of <2.5 m/s and were excluded from our study and did not undergo full PH evaluation. Of the remaining 43 patients (40.5%), 26 patients (24.5%) underwent RHC (Figure 1). Three of these patients either had left ventricular ejection fraction (LVEF) <40% or significant valvular abnormalities and were excluded. Fourteen patients either did not agree to have further evaluation with catheterizations or did not follow up for the procedure.
Figure 1.
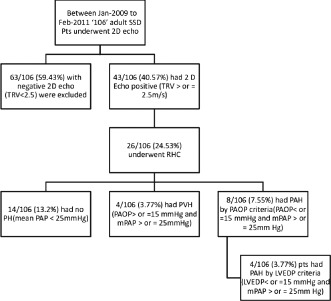
Flow chart of patient recruitment.
Of the patients who underwent catheterization, 14 of 106 (13.2 %) were found to have normal PA pressures. The remaining 12 of 106 patients (11.3%) were found to have increased pulmonary pressures consistent with PH. Eight of these patients (7.5%) had PAH by PAOP criteria. When the 8 patients with PAH were defined by LVEDP criteria alone, 4 of the patients were found to have PVH. Using LVEDP criteria, the prevalence of PAH in our group of 63 patients with an elevated TR jet flow ≥2.5 m/s was found to be 3.7% (Figure 2).
Figure 2.
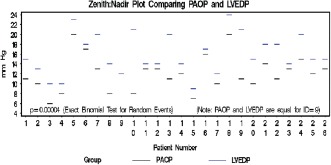
Comparison between left ventricular end‐diastolic pressures (LVEDP) and pulmonary artery occlusion pressure (PAOP) measured simultaneously in all patients undergoing right heart catheterization.
The positive predictive value of echocardiograms to accurately diagnose PH with TR jet velocity of ≥2.5 m/s was 46% (12 of 26 patients).
When the patients were compared based on the catheterization‐based diagnosis (Tables 1 and 2), no significant differences were found in the 3 groups (no PH, PAH, or PVH) except for brain natriuretic peptide (BNP) levels (P = 0.02). The patient demographics appeared comparable among the 3 groups (Table 1). BNP levels were increased in the true PAH group and even more elevated in the PVH group.
Table 1.
Baseline Characteristics of the Groups
| Baseline Characteristics | Group 1, n = 14a | Group 2, n = 4b | Group 3, n = 8c | P Value |
|---|---|---|---|---|
| Age, y, no., mean ± SD (range) | 14, 45 ± 13, (25–65) | 4, 45 ± 20, (24–72) | 8, 43 ± 10, (27–62) | NS |
| Sex, n (%) | ||||
| Male | 4 (29) | 2 (50) | 1 (13) | 0.34 |
| Female | 10 (71) | 2 (50) | 7 (88) | |
| BMI, no., mean ± SD (range) | 13, 26 ± 5.3, 26 (17–37) | 4, 24 ± 4.3, (19–28) | 8, 26 ± 12, (15–55) | NS |
| Hospital admission (5 years), no., mean ± SD (range), | 11, 6.1 ± 8.3, (0–30) | 4, 12 ± 7.7, (2.0–20) | 8, 6.3 ± 4.3, (1.0–15) | NS |
| NYHA status, n (%) | ||||
| II | 10 (71) | 2 (50) | 5 (63) | 0.86 |
| III | 4 (29) | 2 (50) | 3 (38) | |
| Hydroxyurea, n (%) | ||||
| No | 8 (57) | 2 (50) | 2 (25) | 0.31 |
| Yes | 6 (43) | 2 (50) | 6 (75) |
Abbreviations: BMI, body mass index; LVEDP, Left ventricular end diastolic pressure; mPAP, Mean pulmonary artery pressure; NS, not significant; NYHA, New york heart association; PAOP, Pulmonary artery occlusion pressure; PAH, pulmonary arterial hypertension; PCWP, pulmonary capillary wedge pressure; PHTN, pulmonary hypertension; PVH, pulmonary venous hypertension; SD, standard deviation.
No PHTN:mPAP<25mmHg,
PAH:mPAP25, PAOP≥15 and LVEDP ≥ 15mmHg,
PVH:mPAP25 and LVEDP>15mmHg. Group 1: No pulmonary hypertension Group 2: pulmonary arterial hypertension (based on LVEDP) Group 3: pulmonary venous hypertension (based on LVEDP).
Table 2.
Laboratory Characteristics Between the Two Groups
| Lab Profile | Group 1, n = 14, No., Mean ± SD (Range)a | Group 2, n = 4, No., Mean ± SD (Range)b | Group 3, n = 8, No., Mean ± SD (Range)c | P Value |
|---|---|---|---|---|
| RBC (∩106) | 14, 2.7 ± 0.87 (1.6–4.7) | 4, 2.5 ± 1.0 (2.0–4.0) | 8, 2.4 ± .54 (1.7–3.2) | NS |
| Reticulocyte count | 13, 7.8 ± 2.8 (115–283) | 4, 24 ± 1.5 (0.44–4.1)d | 8, 10 ± 5.1 (2.9–19)e | 0.001 |
| Hb, g/dL | 14, 8.5 ± 1.8 (5.2–12.2) | 4, 8.2 ± 1.9 (6.4–11) | 8, 8 ± 1.6 (6.4–11) | NS |
| Albumin | 14, 4.2 ± .38 (3.6–4.7) | 4, 3.6 ± .60 (3.0–4.4)d | 8, 4.1 ± .37 (3.6–4.6)e | 0.032 |
| WBC, ×1000 | 14, 10.0 ± 4.0 (6.4–22) | 4, 9.0 ± 5.5 (2.7–16) | 8, 11 ± 3.0 (8.2–17) | NS |
| BUN, mg/dL | 14, 13 ± 10 (5.0–45) | 4, 22 ± 10, (7.0–29) | 8, 17 ± 14 (5.0–47) | NS |
| Creatinine, mg/dL | 14, 0.80 ± 0.25 (0.40–1.2) | 4, 2.4 ± 1.5 (0.58–4.2)d | 8, 1.3 ± 1.3 (0.43–4.3)e | 0.030 |
| LDH, multiple ULN | 9, 773 ± 497 (308–1672) | 3, 294 ± 139 (156–434) | 8, 767 ± 444 (213–1532) | NS |
| Total bilirubin, mg/dL | 14, 2.7 ± 1.6 (0.90–6.3) | 4, 1.8 ± 1.7 (0.40–4.3) | 8, 3.0 ± 1.5 (1.0–5.8) | NS |
| BNP | 4, 11 ± 8.6 (4.0–23) | 2, 764 ± 112 (685–843)d | 6, 682 ± 960 (13–2492)e | 0.011 |
Abbreviations: BNP, B‐type natriuretic peptide; BUN, blood urea nitrogen; HG, hemoglobin; LDH, lactate dehydrogenase; LVEDP, left ventricular end diastolic pressure; NS, not significant; PCWP, pulmonary capillary wedge pressure; RBC, red blood cell count; SD, standard deviation; WBC, white blood cell count.
No PHTN:mPAP<25mmHg,
PAH:mPAP25, PCWP≥15 and LVEDP ≥ 15mmHg,
PVH:mPAP25 and LVEDP>15mmHg,
†P < 0.05 for the comparisons with group 1, ‡P < 0.05 for the comparisons with group 2.
We also compared the patients in the 3 categories (normal pulmonary pressures, PAH, and PVH) based first on the PAOP criteria and then on LVEDP criteria. Among patients classified according to LVEDP criteria, TRV in PAH patients was significantly increased (3.5 ± 0.82 vs 2.9 ± 0.30) when compared with PVH or patients with normal hemodynamics (P = 0.05). This was not the case when patients were classified by PAOP criteria, where there was no difference between the groups.
Twenty of the 26 patients (77%) were found to have left atrial enlargement on echocardiogram, suggesting concomitant left heart dysfunction, though this observation did not help to differentiate the patients with PH from patients with normal hemodynamics (P = 0.31).
RHC Data
Mean PA pressures were increased in the PAH and the PVH groups compared with the group with normal pressures. Right atrial pressure was higher in the PAH (13.5 ± 7.2 mm Hg) and the PVH (12 ± 4.4 mm Hg) groups as compared to the group with normal pressure (6.4 ± 2.3). Pulmonary vascular resistance (PVR) was highest in the PAH group (2.5 ± 0.92 Wood units), followed by the PVH group (2.1 ± 0.88), and then the normal group (1.4 ± 0.37). There was no difference in the LVEF in the 3 groups. CO was high in all 3 groups, with no significant differences (Table 3).
Table 3.
Hemodynamic Characteristics of the Groups
| Right Heart Catheterization | No Pulmonary HTN, No., Mean ± SD, Median (Range) | Pulmonary Arterial HTN, No., Mean ± SD, Median (Range) | Pulmonary Venous HTN, No., Mean ± SD, Median (Range) | P Value |
|---|---|---|---|---|
| Systolic PAP, mm Hg | 14, 31 ± 4.2, 31 (24–38) | 4, 42 ± 6.7, 41 (37–51)a | 8, 49 ± 15, 42 (38–74)a | 0.0006 |
| Diastolic PAP, mm Hg | 14, 12 ± 4.4, 13 (7.0–24) | 4, 20 ± 6.6, 19 (12–28) | 8, 20 ± 7.0, 21 (8.0–28)a | 0.011 |
| Mean PAP, mm Hg | 14, 20 ± 3.4, 21 (15–24) | 4, 30 ± 4.2, 30 (25–34)a | 8, 33 ± 8.9, 30 (25–48)a | <0.0001 |
| PCWP, mm Hg | 14, 10 ± 2.4, 11 (6–13) | 4, 12 ± 1.4, 13 (10–13) | 8, 16 ± 3.5, 16 (11–20)a, b | 0.001 |
| LVEDP, mm Hg | 14, 14 ± 3.8, 14 (9.0–21) | 4, 15 ± 0.50, 15 (14–15) | 8, 20 ± 2.6, 19 (17–24)a, b | 0.0017 |
| Mean RAP, mm Hg | 14, 6.4 ± 2.3, 6.5 (3.0–10.0) | 4, 13.5 ± 7.2, 11 (8–24)a | 8, 12 ± 4.4, 13 (5.0–18)a | 0.0016 |
| Pulmonary vascular resistance, Wood units | 14, 1.4 ± 0.37, 1.3 (.84–2.1) | 4, 2.5 ± 0.92, 2.4 (1.6–3.5)a | 8, 2.1 ± 0.88, 1.8 (1.4–4.1)a | 0.003 |
| Left ventricular ejection fraction | 14, 64 ± 8.5, 64 (52–80) | 4, 59 ± 15, 62 (39–75) | 8, 59 ± 12, 61 (35–72) | NS |
| CO, L/min | 14, 7.3 ± 1.5, 7.7 (3.7–9.8) | 4, 7.3 ± 1.4, 7.5 (5.4–8.7) | 8, 7.2 ± 1.3, 7.3 (5.6–9.1) | NS |
| CI, L/min/m2 | 14, 4.0 ± 0.17, 4.0 (3.8–4.1) | 4, 4.0 ± 0.17, 3.8 (3.7–4.1) | 7, 3.9 ± 0.44, 3.8 (3.4–4.6) | NS |
Abbreviations: CO, Cardiac output; CI, Cardiac index; HTN, hypertension; LVEDP, left ventricular end diastolic pressure; NS, not significant; PAP, pulmonary artery pressure; PAOP, pulmonary artery occlusion pressure; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure; SD, standard deviation.
††P < 0.05 for the comparisons with group 1,
‡P < 0.05 for the comparisons with group 2.
PAOP vs LVEDP
PAOP consistently underestimated LVEDP in the PAH group (P = 0.002) and the entire group (P = 0.0001). Half (50%) of the patients who were defined as having PAH by PAOP criteria did not have PAH by LVEDP criteria. The mean difference between LVEDP and PAOP in the PH group (12 patients) was 4.63 mm Hg and 3.80 mm Hg, respectively, in the entire group (26 patients) (Figure 2)
Clinical Outcome
Hospital admissions and minicrises (defined as painful crises at home that were resolved by medications and rest alone) were not different in any group whether they were classified by PAOP criteria or LVEDP criteria. We also did not find any difference between those prescribed hydroxyurea in any group. Compliance with hydroxyurea treatment, however, was not measured. During the 24‐month follow‐up, 2 patients in the study group died. Both patients had pulmonary hypertension (1 PAH and 1 PVH).
Figure 3.
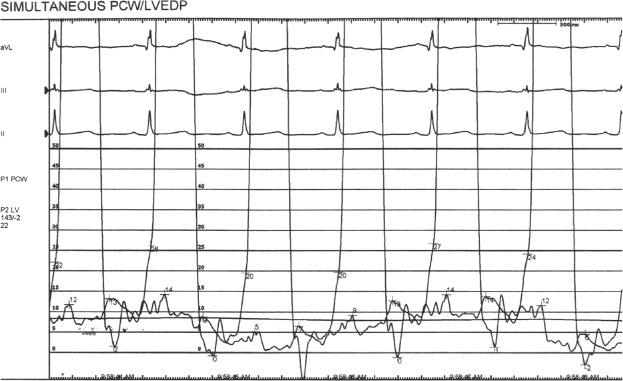
Figure demonstrating PAOP and LVEDP hemodynamic tracings from a representative patient showing end expiratory PAOP of 14 mmHg and LVEDP of 20 mmHg.
Figure 4.
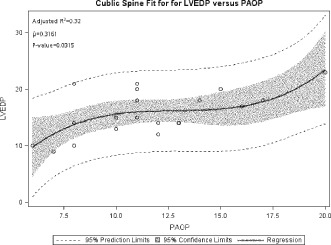
Figure comparing LVEDP vs. PAOP for non‐PH and PH patients as a linear regression analysis or as a paired‐subjects line graph.
Polysomnography
Of the 26 patients who underwent hemodynamic evaluation, 22 had overnight polysomnography. Five of the 22 patients had obstructive sleep apnea (OSA). The mean apnea‐hypopnea index (AHI) of patients with OSA was 18 compared with the AHI of 3 in the non‐OSA group. There was no significant difference between the groups for OSA. There was no difference between the groups for nocturnal oxygen desaturations or desaturation on exertion (6‐minute walk test).
Laboratory Data
When the groups were classified based on LVEDP criteria, we found that the PAH group had significantly lower albumin levels (3.6 ± 0.60 g/dL) compared to the PVH group (4.1 ± 0.37 g/dL) and the normal group (4.2 ± 0.38 g/dL). The serum creatinine level was found to be significantly increased in the PAH group (2.4 ± 1.5 mg/dL) compared to the PVH group (1.3 ± 1.3 mg/dL) and normal group (0.80 ± 0.25 mg/dL) (P = 0.03). The PVH group also had significantly increased serum creatinine levels than patients with normal pulmonary pressures. We also found that reticulocyte percentage was significantly increased in the PAH group (24 ± 1.5) compared with the PVH group (10 ± 5.1) and normal pressure group (7.8 ± 2.8). Reticulocyte percentage in the PVH group was lower but still significantly higher than the normal pressure group (Table 2).
Pulmonary Function Testing
Pulmonary function testing (PFT) data was available on 21 patients. Of the 21 patients, 14 patients had restrictive defects (defined as Forced expiratory volume in 1 second (FEV1) / Forced vital capacity (FVC) ratio of >70% and total lung capacity of <80%), though most of these defects were mild. Three patients had isolated diffusion capacity defects; 1 had an obstructive defect, 1 had mixed defects, and 2 had normal PFTs. Mean diffusion capacity in the PH patient group was not significantly different than the non‐PH patient group.
Ventilation/Perfusion Scan
No patients in our cohort were found to have any evidence of chronic thromboembolic disease on ventilation perfusion scans.
Discussion
Our study revealed that use of PAOP criteria overestimates PAH as compared to LVEDP criteria (7.5% vs 3.5%). The study found significant difference between the LVEDP and PAOP pressures measured in the SCD patients undergoing RHC. We found that 50% (4/8) of patients had to be reclassified when LVEDP criteria was used for diagnosis of PAH. This is the first study to report a discrepancy between LVEDP and PAOP measurements in a sickle cell patient cohort. As in previous studies, our study confirms that PH in SCD patients is mostly of the postcapillary nature (PVH).9, 24 However, these previous studies used PAOP criteria and did not measure simultaneous LVEDP.
We found a significant discrepancy in simultaneously measured PAOP and LVEDP in SCD patients undergoing RHC. Our studies showed that PAOP consistently underestimated LVEDP in the entire group and not just in the PH group. This also resulted in reclassification of 4/8 (50%) patients. These findings are consistent with a prior study by Halpern et al in a non‐SCD population.
Prior studies have revealed PAOP is subject to significant errors in measurement and interpretation.25, 26 Studies have also shown that as the PAOP increases (over 10 mm Hg) the correlation between PAOP and left atrial pressures is subject to considerable error.26 There are several reasons that could explain the differences between the PAOP and LVEDP. The relationship between PAOP and LVEDP is not linear but curvilinear. After 10 mm Hg pressure there is considerable variation between the 2 pressures.27 PAOP poorly reflects LVEDP, because it does not accurately follow the late diastolic pressure rise due to left atrial contraction or pericardial pressures that impact LVEDP.27
A digital method of measuring the PAOP may also cause discrepancy25; however, in our study manual measurements were made at the end‐expiratory phase. PVR has been shown to be low in patients with SCD.11, 28 In our study, the PVR was relatively increased in both PAH and PVH, which is consistent with prior observations.28
The low positive predictive value of echocardiograms for prevalence of PH in our study (46%) is consistent with data from other centers (25% to 46%).9, 14, 24 The slight variation among studies may be reflective of sample size differences and patient selection criteria.
BNP was elevated in both the PAH and PVH groups compared to normal controls but was not significantly different between the PAH and PVH groups. This finding is similar to prior studies that revealed elevated BNP levels in PH; however, BNP was not able to differentiate pre‐ and postcapillary PH.20, 21 We also found that patients classified as PAH by LVEDP criteria, (group 2) was found to have significantly low albumin levels. The reticulocyte percentage was increased in the PAH group as was the serum creatinine level. Elevation of serum creatinine has been shown to be associated with PH in prior studies.11
In contrast to pediatric patients, we found that adult SCD patients have a more restrictive pattern on pulmonary function testing, as noted in prior studies.11 PH patients, combined as a group, had significant restrictive defects (P = 0.04) compared to SCD patients with normal pulmonary pressures.
The strength of the study is the prospective consecutive collection of data with simultaneous measurements of PAOP and LVEDP. Hemodynamic measurements were taken in a stable outpatient setting, as pulmonary pressures have been shown to increase during vaso‐occlusive pain crisis.8, 21 Also, patients with significant valvular or left heart disease on echocardiogram were excluded from the study.
There are several limitations in this study. We had a limited number of patients due to the targeted population. The patients were recruited from the pulmonary clinic; therefore, there may have been a referral bias. A significant number of patients (n = 17) with TRV of 2.5 m/s or more did not undergo RHC; therefore, this is a conservative estimate of the prevalence. Some of the laboratory data were collected retrospectively and were not timed with the RHC. Many of the reticulocyte percent values taken during hospital admission for pain crisis may not have represented a stable state.
Conclusion
Our study reports a discrepancy of PAOP and LVEDP in SCD patients, which may affect the classification of PH in this cohort, with subsequent implications on management. We found that the prevalence of PAH is increased (3.7%) in SCD patients, which is elevated compared to the prevalence of idiopathic PAH. The majority of PH patients appear to have PVH. A larger cohort study is needed to confirm the findings in our study. We recommend that sickle cell patients with PAOP above 10 cm of H2O pressure on RHC undergo verification by LVEDP measurement to avoid misclassification.
Acknowledgments
The authors acknowledge with gratitude the contribution Lynne Bair, social worker and data manager in the adult sickle cell clinic, for her support and services in completing this study.
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing. Sunil Sharma, MD, had access to and takes responsibility for the integrity of the data and the accuracy of the data analysis. All of the authors contributed to data collection, analysis, drafting of the manuscript, and final approval.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Haque AK, Gokhale S, Rampy BA, Adegboyega P, Duarte A, Saldana MJ. Pulmonary hypertension in sickle cell hemoglobinopathy: A clinicopathologic study of 20 cases. Hum Pathol. 2002; 33:1037–1043. [DOI] [PubMed] [Google Scholar]
- 2. Sutton LL, Castro O, Cross DJ, Spencer JE, Lewis JF. Pulmonary hypertension in sickle cell disease. Am J Cardiol. 1994;74:626–628. [DOI] [PubMed] [Google Scholar]
- 3. Castro O. Systemic fat embolism and pulmonary hypertension in sickle cell disease. Hematol Oncol Clin North Am. 1996;10:1289–1303. [DOI] [PubMed] [Google Scholar]
- 4. Powars D, Weidman JA, Odom‐Maryon T, Niland JC, Johnson C. Sickle cell chronic lung disease: Prior morbidity and the risk of pulmonary failure. Medicine (Baltimore). 1988;67:66–76. [PubMed] [Google Scholar]
- 5. Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. [DOI] [PubMed] [Google Scholar]
- 6. Ataga KI, Moore CG, Jones S, Olajide O, Strayhorn D, Hinderliter A, Orringer EP. Pulmonary hypertension in patients with sickle cell disease: A longitudinal study. Br J Haematol. 2006;134:109–115. [DOI] [PubMed] [Google Scholar]
- 7. Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez‐Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Guidelines for the diagnosis and treatment of pulmonary hypertension: The task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (ESC) and the european respiratory society (ERS), endorsed by the international society of heart and lung transplantation (ISHLT). Eur Heart J. 2009;30:2493–2537. [DOI] [PubMed] [Google Scholar]
- 8. Machado RF, Mack AK, Martyr S, Barnett C, Macarthur P, Sachdev V, Ernst I, Hunter LA, Coles WA, Nichols JP, Kato GJ, Gladwin MT. Severity of pulmonary hypertension during vaso‐occlusive pain crisis and exercise in patients with sickle cell disease. Br J Haematol. 2007; 136: 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parent F, Bachir D, Inamo J, A hemodynamic study of pulmonary hypertension in sickle cell disease. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011; 365: 44–53. [DOI] [PubMed] [Google Scholar]
- 10. Hachulla E, Gressin V, Guillevin L, Carpentier P, Diot E, Sibilia J, Kahan A, Cabane J, Francès C, Launay D, Mouthon L, Allanore Y, Tiev KP, Clerson P, de Groote P, Humbert M. Early detection of pulmonary arterial hypertension in systemic sclerosis: A french nationwide prospective multicenter study. Arthritis Rheum. 2005; 52: 3792–3800. [DOI] [PubMed] [Google Scholar]
- 11. Anthi A, Machado RF, Jison ML, Taveira‐Dasilva AM, Rubin LJ, Hunter L, Hunter CJ, Coles W, Nichols J, Avila NA, Sachdev V, Chen CC, Gladwin MT. Hemodynamic and functional assessment of patients with sickle cell disease and pulmonary hypertension. Am J Respir Crit Care Med. 2007;175: 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sachdev V, Machado RF, Shizukuda Y, Rao YN, Sidenko S, Ernst I, St Peter M, Coles WA, Rosing DR, Blackwelder WC, Castro O, Kato GJ, Gladwin MT. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007;49:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Halpern SD, Taichman DB. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end‐diastolic pressure. Chest. 2009;136: 37–43. [DOI] [PubMed] [Google Scholar]
- 14. Soto FJ. Pulmonary venous hypertension: A diagnostic and therapeutic dilemma. Advances in Pulmonary Hypertension . Winter 2007;6:168–174. Available from: http://www.phaonlineuniv.org/Journal/Vol6No4Winter07/PulmonaryVenousHypertension. Accessed 11/6/2001. [Google Scholar]
- 15. Oudiz RJ. Pulmonary hypertension associated with left‐sided heart disease. Clin Chest Med. 2007;28: 233–41. [DOI] [PubMed] [Google Scholar]
- 16. Benza RL, Tallaj JA. Pulmonary hypertension out of proportion to left heart disease. Advances in Pulmonary Hypertension. Spring 2006;5: 21–29. Available from: http://www.phaonlineuniv.org/Journal/Vol5No1Spring06/PHandLeftHeartDisease. Accessed 11/6/2011. [Google Scholar]
- 17. Standardization of spirometry , 1994 update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152: 1107–1136. [DOI] [PubMed] [Google Scholar]
- 18. American thoracic society. single‐breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique‐‐1995 update. Am J Respir Crit Care Med. 1995;152: 2185–2198. [DOI] [PubMed] [Google Scholar]
- 19. Brooks D, Solway S, Gibbons WJ. ATS statement on six‐minute walk test. Am J Respir Crit Care Med. 2003; 167:1287. [DOI] [PubMed] [Google Scholar]
- 20. Machado RF, Anthi A, Steinberg MH, Bonds D, Sachdev V, Kato GJ, Taveira‐DaSilva AM, Ballas SK, Blackwelder W, Xu X, Hunter L, Barton B, Waclawiw M, Castro O, Gladwin MT; N‐terminal pro‐brain natriuretic peptide levels and risk of death in sickle cell disease. JAMA. 2006;296: 310–318. [DOI] [PubMed] [Google Scholar]
- 21. Mekontso Dessap A, Leon R, Habibi A, Nzouakou R, Roudot‐Thoraval F, Adnot S, Godeau B, Galacteros F, Brun‐Buisson C, Brochard L, Maitre B. Pulmonary hypertension and cor pulmonale during severe acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 2008;177: 646–653. [DOI] [PubMed] [Google Scholar]
- 22. Machado RF, Barst RJ, Yovetich NA, Hassell KL, Kato GJ, Gordeuk VR, Gibbs JS, Little JA, Schraufnagel DE, Krishnamurti L, Girgis RE, Morris CR, Rosenzweig EB, Badesch DB, Lanzkron S, Onyekwere O, Castro OL, Sachdev V, Waclawiw MA, Woolson R, Goldsmith JC, Gladwin MT. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011;118: 855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA. 2012 Mar 28;307: 1254–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fonseca GH, Souza R, Salemi VM, Jardim CV, Gualandro SF. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur Respir J. 2012;39: 112–118. [DOI] [PubMed] [Google Scholar]
- 25. Ryan JJ, Rich JD, Thiruvoipati T, Swamy R, Kim GH, Rich S. Current practice for determining pulmonary capillary wedge pressure predisposes to serious errors in the classification of patients with pulmonary hypertension. Am Heart J. 2012 Apr;163(4): 589–594 [DOI] [PubMed] [Google Scholar]
- 26. Walston, A , Kendall E. Comparison of pulmonary wedge and left atrial pressure in man. American Heart Journal. 1973: 159–164. [DOI] [PubMed] [Google Scholar]
- 27. Pinsky MR. Clinical significance of pulmonary artery occlusion pressure. Intensive Care Med. 2003;29(2):175–178. [DOI] [PubMed] [Google Scholar]
- 28. Miller AC, Gladwin MT. Pulmonary complications of sickle cell disease. Am J Respir Crit Care Med Vol 185, Iss. 11, pp 1154–1165, Jun 1, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


