Abstract
Obesity is a major correlate of cardiovascular disease. Weight loss improves cardiovascular risk factors and has the potential to improve outcomes. Two drugs, phentermine plus topiramate and lorcaserin, have recently been approved by the US Food and Drug Administration for the indication of obesity; a third, bupropion plus naltrexone, is under consideration for approval. In clinical trials, these drugs cause weight loss and improve glucose tolerance, lipid profile, and, with the exception of bupropion plus naltrexone, blood pressure. However, their effect on cardiovascular outcomes is unknown. In defining appropriate roles for these drugs in preventive cardiology, it is important to remember the checkered history of drugs for obesity. New weight‐loss drugs share the serotonergic and sympathomimetic mechanisms that proved harmful in the cases of Fen‐Phen and sibutramine, respectively, albeit with significant differences. Given these risks, randomized cardiovascular outcomes trials are needed to establish the safety, and potential benefit, of these drugs. This review will discuss the history of pharmacotherapy for obesity, existing efficacy and safety data for the novel weight‐loss drugs, and issues in the design of postapproval clinical trials.
Introduction
Advances in prevention—smoking‐cessation campaigns, statin therapy, and tight blood pressure (BP) control, among others—have contributed to decreases in the burden of coronary artery disease over the past several decades. However, the increasing prevalence of obesity and obesity‐associated diseases like type 2 diabetes mellitus (T2DM) have tempered these gains.1 Despite recent data suggesting a plateau, rates of obesity remain substantially higher than a few decades ago.2 Intensive lifestyle interventions have produced clinically relevant weight loss,3 but modest interventions that are feasible in the primary‐care setting are less successful.4 Many efforts to develop an effective and safe weight‐loss pill have failed. In fact, the history of obesity pharmacotherapy has been notable for cardiovascular side effects, not benefits. In 2012, the US Food and Drug Administration (FDA) approved 2 medications for weight loss, the selective 5‐HT2C agonist lorcaserin and the combination pill phentermine plus topiramate; a third, bupropion plus naltrexone, is under consideration for approval at the time of this writing.5 The mechanisms of these drugs are in some cases similar to obesity pills of the past. The clinical and regulatory communities must now weigh the benefits and potential risks of these medications and also design further studies. This article will frame these questions in the context of the history of obesity medications and discuss the importance of clinical‐trial research in this area.
The Checkered History of Obesity Pharmacotherapy
There is a long history of unsafe drugs for obesity. Experimentation with desiccated thyroid began in the 1890s; patients experienced symptoms of hyperthyroidism.6 The pyretic dinitrophenol was first associated with weight loss in a 1933 case series of 9 patients. By the next year, >100 000 Americans had taken the drug; thousands of them would suffer blindness or fatal hyperthermia before regulators halted its sale in 1938.7 “Rainbow pills”—a nonstandard combination of amphetamine, thyroid hormone, and diuretics for weight loss and β‐blockers and benzodiazepines to manage side effects—were widely prescribed at profitable specialty clinics from the 1940s to the 1970s, despite evidence of harm.6 Even after FDA regulators sharply limited the use of prescription amphetamines, their use continued as dietary supplements.6 One amphetamine, phenylpropanolamine, has been associated with hemorrhagic stroke in young women.8
This pattern of rapid adoption of inadequately tested medications continued in the 1990s with fenfluramine. The use of Fen‐Phen, the combination of 2 previously approved medications, fenfluramine and phentermine, rapidly increased after a 1992 study showed that they induce sustained weight loss.9 In 1996, American physicians wrote >18 million prescriptions for fenfluramine.10 In 1997, Connolly and colleagues first reported right‐sided and left‐sided valvular regurgitation associated with fenfluramine; the glistening white histopathology was similar to carcinoid or ergotamine‐induced valve disease.10 These results were soon generalized to fenfluramine's purportedly safer stereoisomer dexfenfluramine.11 Both medications were also associated with dramatically increased rates of pulmonary hypertension.12 They were withdrawn from the market in 1997. Many patients filed lawsuits against drug manufacturers; one manufacturer, Wyeth, set aside as much as $22 billion to cover liability.13
Rimonabant introduced a novel mechanism of action, cannabinoid inverse agonism. Clinical trials showed weight loss and improvement in metabolic parameters, but they also showed depression and anxiety.14 The European Medicines Agency approved the drug, but FDA regulators did not.15 Rimonabant for Prevention of Cardiovascular Events (CRESCENDO), a long‐term cardiovascular outcomes trial, was terminated after revealing an increased rate of serious psychiatric side effects including suicide at mean follow‐up of 14 months.16
Sibutramine, a selective serotonin and norepinephrine reuptake inhibitor, combined 2 previously effective but potentially dangerous mechanisms. Approved by FDA regulators in 1997, sibutramine induced weight loss and improved lipid profile and glucose tolerance, but it also increased BP and pulse rate in clinical trials.17 After physicians prescribed the drug for longer than a decade, the 2010 Sibutramine Cardiovascular Outcomes Trial (SCOUT), a randomized cardiovascular outcomes study in patients with cardiovascular disease, diabetes mellitus (DM), or both, found that sibutramine caused a greater rate of cardiovascular events.18 In post hoc analysis by an FDA regulator, changes in BP did not predict these events, leaving the possibility of an unidentified mechanism of harm.19 Some felt this signal for harm in high‐risk patients should not be generalized to lower‐risk patients, who might lose weight without an increase in cardiovascular risk.18 Others felt that patients with subclinical cardiovascular disease would be difficult to identify.15 Ultimately, sibutramine was voluntarily removed from the market in 2010.15
Potential Cardiovascular Benefits of Weight Loss
Despite this checkered history, interest in obesity pharmacotherapy has continued because of the association between weight and cardiovascular risk. No study has explicitly demonstrated that weight loss improves cardiovascular outcomes or mortality. In observational studies, weight and mortality are robustly associated; 5‐kg/m2 increments in body mass index (BMI) over 25 are associated with 30% increased risk of death from ischemic heart disease and 20% increased all‐cause mortality.20 One nonrandomized study found that bariatric‐surgery patients had 24% lower all‐cause mortality than prospectively matched controls at 10‐year follow‐up.21 This study design, however, does not prove causation.
Weight loss improves cardiovascular risk factors in randomized controlled trials; the greatest effect occurs in DM (Figure 1). In the Finnish Diabetes Prevention Study, an intensive lifestyle intervention reduced the incidence of T2DM by 58% in patients with obesity and elevated blood glucose.3 In the Xendos trial, the addition of the gastrointestinal lipase inhibitor Orlistat to a lifestyle intervention decreased incidence of DM by 30% at 4‐year follow‐up in high‐risk patients; these patients lost on average just 2.8 kg more weight than patients in the placebo group.22 Among patients with DM, bariatric surgery reduced glycated hemoglobin (HbA1c) level and number of DM medications at 3‐year follow‐up in the recent Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) trial.23 Other risk factors also improve with weight loss. Weight loss due to lifestyle change,24 Orlistat,22 or bariatric surgery25 reduces systolic blood pressure (SBP). Bariatric surgery increased high‐density lipoprotein cholesterol (HDL‐C) levels, decreased triglyceride levels, and had no effect on low‐density lipoprotein cholesterol (LDL‐C) levels in the STAMPEDE trial.23 In one nonrandomized study, women who lost weight after completing a calorie‐restriction protocol were found to have decreased C‐reactive protein.26
Figure 1.
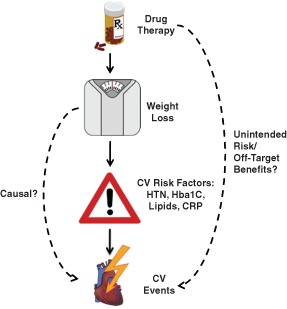
Theoretical relationship between drug therapy, weight loss, cardiovascular risk factors, and cardiovascular events. Solid arrows indicate known causal relationships. Dashed arrows indicate potential relationships. Abbreviations: CRP, C‐reactive protein; CV, cardiovascular; HbA1c, glycated hemoglobin; HTN, hypertension.
This strong relationship between weight and prognosis has been called into question for high‐risk patients by 2 observations. First, population studies have found that patients with moderate obesity live longer after diagnosis of coronary artery disease or heart failure.27 This association, the so‐called obesity paradox, persists after controlling for age and other risk factors. Possible mechanisms include greater nutritional reserve, greater lean body mass, or lower thromboxane production in patients with higher BMI.27 More likely, unmeasured confounding explains the obesity paradox. Patients who developed coronary artery disease despite normal weight might have greater non–obesity‐related risk‐factor burden. In addition, lower body weight is associated with chronic illness and frailty. Second, in the Look AHEAD (Action for Health in Diabetes) trial, weight loss did not improve cardiovascular outcomes in patients with diabetes. Patients randomized to a lifestyle intervention maintained lost weight throughout the study period but did not have a significantly lower rate of cardiovascular events at 10‐year follow‐up.28 One possible explanation for this result is that the intervention may have been administered too late in the disease process in these patients, resulting in events not being modifiable. There is no randomized trial to date showing that a weight‐loss regimen decreases the rate of cardiovascular events.
Potential Benefits of Modern Pharmacotherapy for Obesity
Phentermine Plus Topiramate
Phentermine plus topiramate causes weight loss and improvements in cardiovascular risk factors (Table 1). This combination pill has been tested in a range of dosages, from 3 mg phentermine plus 23 mg topiramate (low dose) to 15 mg phentermine plus 92 mg topiramate (high dose). In the Controlled‐Release Phentermine/Topiramate in Severely Obese Adults (EQUIP) trial, patients with BMI >35 but no weight‐related comorbidities were provided with an office‐based lifestyle intervention and randomized to high‐dose, low‐dose, and placebo groups.29 Patients in the high‐dose and low‐dose treatment groups lost 10.9% and 5.1% of body weight at 1 year, respectively, whereas those receiving placebo lost 1.6% of body weight. Sixty‐seven percent, 45%, and 17% of patients lost ≥5% of body weight in the high‐dose, low‐dose, and placebo groups, respectively.29 The study population in the Effects of Low‐Dose, Controlled‐Release, Phentermine Plus Topiramate Combination on Weight and Associated Comorbidities in Overweight and Obese Adults (CONQUER) trial included patients with lower BMI (minimum 27) but ≥22 obesity‐related comorbidities.30 Weight loss at 1 year was similar; 7.8% of body weight with an intermediate dosage of 7 mg phentermine plus 46 mg topiramate and 9.8% of body weight with the high‐dose formulation, compared with 1.2% of body weight in the placebo group.30 A subset of CONQUER subjects continued to receive drug or placebo for a second year as part of the Two‐Year Sustained Weight Loss and Metabolic Benefits With Controlled‐Release Phentermine/Topiramate in Obese and Overweight Adults (SEQUEL) study.31 Mean weight increased slightly in all groups during the second year, but the difference between the treatment and placebo groups persisted.31 In both the EQUIP and CONQUER populations, high‐dose treatment (and in some cases lower doses) improved SBP and diastolic blood pressure (DBP), LDL‐C and HDL‐C levels, and fasting serum glucose relative to placebo.29, 30 At 2‐year follow‐up of SEQUEL, phentermine plus topiramate reduced HbA1c in patients with DM at baseline and reduced rates of progression to DM in patients who did not have DM at baseline.31
Table 1.
Effects of Drug Treatment on Cardiovascular Risk Factors Compared With Placebo
| Phentermine/Topiramate29, 30, 31 | Lorcaserin32, 33, 34 | Buproprion/Naltrexone35, 36, 37, 38 | |
|---|---|---|---|
| Weight | ↓↓ | ↓ | ↓ |
| BP | ↓ | ↓ | ↑ |
| Heart rate | ↑ | ↓ | ↑ |
| HDL‐C | ↑ | ↑ | ↑ |
| LDL‐C | ↓ | ↓ | ↓ |
| Triglycerides | ↓ | ↓ | ↓ |
| Fasting glucose | ↓ | ↓ | ↓ |
| HbA1c in T2DM | ↓ | ↓ | ↓ |
| hs‐CRP | ↓ | ↓ | ↓ |
Abbreviations: BP, blood pressure; hs‐CRP, C‐reactive protein; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; T2DM, type 2 diabetes mellitus.
Arrows indicate that drug treatment compared with placebo caused a statistically significant change in outcome variable in ≥1 randomized, controlled clinical trial.
Lorcaserin
Lorcaserin induced less weight loss than phentermine plus topiramate in 3 phase III clinical trials (Table 1).32, 33, 34 The Behavioral Modification and Lorcaserin for Obesity and Overweight Management (BLOOM) and Behavioral Modification and Lorcaserin Second Study for Obesity Management (BLOSSOM) trials studied patients without DM, whereas Behavioral Modification and Lorcaserin for Obesity and Overweight Management in Diabetes Mellitus (BLOOM‐DM) was restricted to patients with DM. All patients received a lifestyle intervention and either lorcaserin or placebo. Mean weight loss in patients receiving the FDA‐approved dosage of 10 mg twice daily exceeded placebo by approximately 3% of body weight (BLOOM, 5.8% vs 2.2%; BLOSSOM, 5.8% vs 2.8%; BLOOM‐DM, 4.5% vs 1.5%). More patients in the treatment group lost ≥5% of baseline body weight (BLOOM, 47% vs 20%; BLOSSOM, 47% vs 25%; BLOOM‐DM, 37% vs 16%).32, 33, 34 In BLOOM, patients who continued therapy for a second year maintained most of their weight loss, but those who switched to placebo at 1 year regressed to the weight of the original placebo group.34 In BLOOM, patients receiving lorcaserin had lower SBP and DBP, pulse rate, LDL‐C and total cholesterol levels, insulin resistance, and C‐reactive protein levels than those receiving placebo.34 The BLOSSOM results were consistent with BLOOM and also showed an increase in HDL‐C and a decrease in apolipoprotein B level.32 The magnitude of improvements was small; for example, in BLOOM, SBP was on average 0.6 mm Hg lower in patients receiving lorcaserin.34 Among patients with DM in the BLOOM‐DM trial, lorcaserin improved HbA1c more than placebo (−0.8 vs −0.3).33
Bupropion Plus Naltrexone
Bupropion plus naltrexone, which has not been approved by the FDA at the time of this writing, has demonstrated weight‐loss efficacy in 4 phase III clinical trials (Table 1). This combination pill has been tested in 2 dosages, 360 mg of bupropion together with either 16 mg or 32 mg of naltrexone. The Contrave Obesity Research (COR)‐I and COR‐II trials both randomized low‐ or intermediate‐risk patients to bupropion plus naltrexone or placebo; all patients received a low‐intensity lifestyle intervention. COR‐I studied both dose combinations, whereas COR‐II studied only bupropion plus naltrexone 32 mg.35, 36 The COR‐BMOD (Behavior Modification) trial enrolled a similar population but utilized a more intensive, group‐based lifestyle intervention.37 The COR‐Diabetes trial was restricted to patients with T2DM and utilized a low‐intensity lifestyle intervention similar to COR‐I and COR‐II.38 Bupropion plus naltrexone caused 4% to 5% of body weight more weight loss than placebo in patients without DM and approximately 3% more in patients with DM. The proportion of patients achieving ≥5% weight loss was greater in the treatment group of all 4 trials. Bupropion plus naltrexone improved HDL‐C, insulin, and C‐reactive protein levels; BP was transiently increased and at 1 year was lower than baseline but greater than in the placebo group.35, 36, 37, 38 In patients with DM, bupropion plus naltrexone reduced HbA1c more than placebo (−0.6% vs −0.2%).38
Potential for Cardiovascular Harm
Phentermine Plus Topiramate
The cardiovascular safety of phentermine plus topiramate merits particular scrutiny because other drugs that share phentermine's sympathomimetic mechanism cause cardiovascular harm. Sibutramine increases incidence of myocardial infarction (MI) and stroke.18 Some but not all studies of stimulants prescribed for attention deficit hyperactivity disorder have reported a greater rate of cardiovascular events.39 Dobutamine in ambulatory management of heart failure caused greater mortality in 1 small, unpublished study.40 β‐Blockade, the opposite of adrenergic stimulation, is indicated for secondary prevention of MI.41 Like other sympathomimetic drugs, phentermine plus topiramate increases heart rate in clinical trials.30 The mechanism by which adrenergic stimulation causes cardiovascular events is likely multifactorial, a combination of chronotropy, inotropy, increased BP, and other effects on cardiac and endothelial tissue.
Phentermine plus topiramate may prove to be the exception—a safe sympathomimetic—either because it decreases BP or because the benefits of weight loss offset the effect of sympathomimetic stimulation. Analysis of the CONQUER trial using the Framingham risk model finds reductions in 10‐year risk of coronary heart disease of 0.5% (P < 0.005) for intermediate‐dose and 0.7% (P < 0.0001) for high‐dose treatment.42 However, this analysis must be interpreted cautiously because the Framingham model was not designed or validated as an outcome for therapy, and it does not include heart rate. A similar analysis of sibutramine using the Framingham model by Lauterbach and Evers in 2000 also predicted cardiovascular benefit that was not demonstrated subsequently in clinical trials.43 Due to the multiple effects of sympathomimetic stimulation, risk‐factor analysis is insufficient to assess safety.
Post hoc analyses of MI, stroke, and cardiovascular death events during phase III trials found a hazard ratio (HR) of 0.84 (95% confidence interval [CI]: 0.26‐2.64).42 Treatment groups had a significantly lower rate of events in the broadest composite outcome evaluated, all cardiovascular and neurovascular serious adverse events (HR: 0.54; 95% CI: 0.29‐0.98), though this outcome included noncardiac chest pain.42 Larger trials are necessary to assess cardiovascular safety with greater certainty.
Lorcaserin
Given the history of fenfluramine, a 5‐HT2 agonist, it is important review the distinct effects of 5‐HT2 receptor subtypes and lorcaserin's specificity for the 5‐HT2C G‐protein coupled receptor. Activation of the 5‐HT2C receptor promotes anorexia by activating central melanocortin pathways.44 5‐HT2C knockout mice are obese and insulin resistant; the cause of their obesity is hyperphagia, not altered metabolism.45 One mouse model suggests that 5‐HT2C signaling may also enhance glucose tolerance independently of its effect on body weight.46 The 5‐HT2B receptor, by contrast, is expressed on cardiac cells and has been implicated as the cause of fenfluramine‐associated valvulopathy and pulmonary hypertension.47, 48 The antiparkinsonian drugs pergolide and cabergoline activate the 5‐HT2B receptor and are associated with valvulopathy; but lisuride, which activates 5‐HT2A and 5‐HT2C but not 5‐HT2B, is not associated with valvular damage.47 5‐HT2B agonism activates the Gq signaling pathway and causes excessive valve cell division, overgrowth, and dysfunction.47 Avoiding 5‐HT2A cross‐reactivity is also important, as this receptor is implicated in psychosis.45 Despite the homology of these 3 receptors, lorcaserin activates 5‐HT2C with 18‐fold selectivity over 5‐HT2A and 104‐fold selectivity over 5‐HT2B in in vitro assays.45 It has no appreciable activity at other serotonin receptors or receptors for other biogenic amines.45 This preclinical profile suggests that lorcaserin should not cause valvulopathy or psychiatric side effects because of its high 5‐HT2C selectivity.
Clinical trials to date show a numerically greater rate of valvulopathy in patients receiving lorcaserin that does not reach statistical significance. The relative risk for new valvulopathy—defined by the FDA as mild aortic or moderate mitral regurgitation—was 1.16 (95% CI: 0.81‐1.67). In the treatment group, 2.37% of patients developed valvulopathy, compared with 2.04% in the placebo group.49 Most new regurgitation was either trace or mild; no patients reported symptoms of valvular regurgitation or underwent heart‐valve surgery.50 Additional echocardiograms were obtained at 2‐year follow‐up in the BLOOM trial; inclusion of these data in time‐to‐event analysis lowers the HR to 1.09 (95% CI: 0.83‐1.44).51 Ascertainment bias may explain some or all of the observed signal; in the Framingham Offspring Study, mitral regurgitation was easier to detect in lower‐BMI patients,52 such as those in the treatment group. Mitral, not aortic, regurgitation was responsible for the numerically greater rate of valvulopathy in the lorcaserin group; this observation supports the possibility of ascertainment bias. Placebo‐group data also suggest possible ascertainment bias; valvulopathy rates were lower higher in patients who lost weight.50 The available data exclude the dramatic increases in valvulopathy seen with fenfluramine. However, it is not possible to exclude a mild adverse effect due to the studies' limited duration and statistical power. There was no evidence of increased pulmonary artery pressure or depression in patients receiving lorcaserin.50
Post hoc analysis of the BLOOM and BLOSSOM trials shows numerically lower rates of cardiovascular death, MI, hospitalization for unstable angina, or stroke in patients treated with lorcaserin that were not statistically significant (odds ratio: 0.63, 95% CI: 0.19‐2.12).51
Bupropion Plus Naltrexone
Cardiovascular safety concerns for buproprion plus naltrexone are similar to phentermine plus topiramate due to the sympathomimetic mechanism of bupropion. Like sibutramine, bupropion inhibits reuptake of norepinephrine from the synaptic cleft; it has been reported to cause hypertension.53 Naltrexone could also contribute to increased heart rate or hypertension through inhibition of endogenous opioids. In phase III clinical trials, bupropion plus naltrexone increased heart rate and BP more than placebo.35, 36, 37, 38 These data suggest that the sympathomimetic effect of this medication may be greater than the effect of phentermine plus topiramate. Too few cardiovascular events occurred in COR‐I, COR‐II, and COR‐BMOD to draw conclusions.53
Guidelines
The 2013 American Heart Association/American College of Cardiology guidelines for the management of obesity highlight the roles of diet, exercise, and bariatric surgery, but they are appropriately vague about the role of pharmacotherapy. The treatment algorithm indicates pharmacotherapy may be considered in patients with BMI ≥30 or BMI ≥27 with comorbidity who have not responded to lifestyle intervention alone. There is no evidence‐based recommendation to prescribe any drug to any specific subset of patients.54
Standard of Safety: Regulatory and Clinical
Tightening FDA standards for cardiovascular safety of metabolic drugs have shaped the assessment of obesity pharmacotherapy. The first FDA guidance document for obesity drugs, published in 1996, required a 1‐year placebo‐controlled trial of 1500 subjects, with a subset continuing for a second year of open‐label drug exposure.15 One member of the FDA's advisory panel proposed requiring trials powered for mortality or cardiovascular risk‐factor outcomes, but such trials were considered impractical at that time.15 The 2004 update to this document maintained similar principles but increased the size of the required clinical trial to 3000 patients receiving active drug and 1500 receiving placebo.15 These trials, which included generally young patients, had low rates of cardiovascular events and therefore could not assess cardiovascular benefit or harm with any confidence.
In the years that followed, revelations of harm caused by sibutramine and reports of concern with the oral hypoglycemic rosiglitazone led regulators to adopt stricter standards for exclusion of cardiovascular risk. The 2008 FDA guidance document for DM drugs requires applicants to exclude a 1.8‐fold increased risk of major adverse cardiovascular events (MACE), defined as cardiovascular death, MI, or stroke, prior to approval and a 1.3‐fold increased risk in postapproval trials.55 In 2012, the FDA advisory committee recommended a similar concept for obesity drugs, though upper‐bound HRs were not specified.56 At the time, lorcaserin and phentermine plus topiramate were under evaluation for FDA approval based on the previous rules, but their completed phase III trials had not been designed to assess cardiovascular outcomes. Both drugs were approved with requirements for postapproval cardiovascular outcomes trials.51, 57
The European Medicines Agency has shifted its guidelines in a similar way. The most recent formal guideline, published in 2007, requires only demonstration of weight loss, not effect on morbidity or mortality, for approval.58 However, in a concept paper published in September 2012, the agency recommended revision of this guideline to include more rigorous assessment of cardiovascular and psychiatric outcomes in light of the experience with sibutramine and rimonabant.58
Upcoming trials must be designed not only to meet regulatory standards, but also to help physicians understand cardiovascular risks and guide therapy. Post hoc review of MACE in phase III trials of the 3 recent obesity drugs found a low event rate of approximately 0.5%.56 At this rate, very large trials would be required to meet the regulatory standard and provide clinically relevant information. Two strategies have been proposed to manage trial size in practice. First, assessment of broader cardiovascular endpoints including events such as unstable angina, revascularization, or heart failure could reduce sample size. Debate exists regarding the clinical importance of such events and whether all may be meaningfully impacted by weight loss. Second, trials may be enriched with high‐risk patients. The SCOUT and CRESCENDO trials restricted enrollment to patients with either existing cardiovascular disease or major cardiovascular risk factors. These trials observed annual placebo group MACE rates of 2.9% and 3.5%, respectively.16, 18 This strategy may limit the generalizability of results to the larger population of young, low‐risk patients.
Similar trials for lorcaserin and bupropion plus naltrexone are ongoing at the time of this writing. The Light Study of bupropion plus naltrexone (NCT01601704) has completed enrollment of exclusively high‐risk patients. The Cardiovascular and Metabolic Effects of Lorcaserin in Overweight and Obese Patients–Thrombolysis in Myocardial Infarction (CAMELLIA‐TIMI 61) trial of lorcaserin (NCT02019264) will test for differences in rates of MACEs, DM, and new valvular regurgitation in a high‐risk population. These trials offer the potential to define a role for pharmacotherapy in the evidence‐based treatment of obesity. Scientifically, they will provide prospective, randomized data about the relationship between obesity, weight loss, and cardiovascular outcomes.
Conclusion
Weight is associated with cardiovascular events and mortality, and weight loss due to lifestyle change or bariatric surgery improves cardiovascular risk factors. However, nutritional changes and exercise are difficult to sustain, and surgery carries significant risks. Thus, pharmacologic therapy for obesity has great potential to improve cardiovascular health. The FDA has evaluated 3 new medications for the indication of obesity in the past several years. The mechanisms of these drugs are distinct from but similar to those that have proved harmful in the past. The history of obesity pharmacotherapy teaches us that extrapolation from improvements in risk factors is insufficient for establishing safety. Cardiovascular outcomes trials are necessary to evaluate formally the risks and benefits of these therapies.
Dr. Wiviott receives funds for research through Brigham and Women's Hospital from Eisai Pharmaceuticals, AstraZeneca, and Merck. Dr. Wiviott has received consulting fees or speaking honoraria from Arena Pharmaceuticals, AstraZeneca, the Boston Clinical Research Institute, Bristol‐Myers Squibb, Daiichi‐Sankyo, Eli Lilly, ICON Medical Imaging, Johnson & Johnson, Medscape, and WebMD.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. [DOI] [PubMed] [Google Scholar]
- 2. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wadden TA, Volger S, Tsai AG, et al. Managing obesity in primary care practice: an overview with perspective from the POWER‐UP study. Int J Obes (Lond). 2013;37(suppl 1):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yanovski SZ, Yanovski JA. Long‐term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen PA, Goday A, Swann JP. The return of rainbow diet pills. Am J Public Health. 2012;102:1676–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colman E. Dinitrophenol and obesity: an early twentieth‐century regulatory dilemma. Regul Toxicol Pharmacol. 2007;48:115–117. [DOI] [PubMed] [Google Scholar]
- 8. Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343:1826–1832. [DOI] [PubMed] [Google Scholar]
- 9. Weintraub M, Sundaresan PR, Madan M, et al. Long‐term weight control study, I (weeks 0 to 34): the enhancement of behavior modification, caloric restriction, and exercise by fenfluramine plus phentermine versus placebo. Clin Pharmacol Ther. 1992;51:586–594. [DOI] [PubMed] [Google Scholar]
- 10. Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine‐phentermine. N Engl J Med. 1997;337:581–588. [DOI] [PubMed] [Google Scholar]
- 11. Weissman NJ, Tighe JF Jr, Gottdiener JS, et al; Sustained‐Release Dexfenfluramine Study Group. An assessment of heart‐valve abnormalities in obese patients taking dexfenfluramine, sustained‐release dexfenfluramine, or placebo. N Engl J Med. 1998;339:725–732. [DOI] [PubMed] [Google Scholar]
- 12. Abenhaim L, Moride Y, Brenot F, et al; International Primary Pulmonary Hypertension Study Group . Appetite‐suppressant drugs and the risk of primary pulmonary hypertension. N Engl J Med. 1996;335:609–616. [DOI] [PubMed] [Google Scholar]
- 13. Lenzner R, Maiello M. The $22 billion gold rush. Forbes March 24, 2006. [PubMed] [Google Scholar]
- 14. Pi‐Sunyer FX, Aronne LJ, Heshmati HM, et al. Effect of rimonabant, a cannabinoid‐1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO‐North America: a randomized controlled trial. JAMA. 2006;295:761–775. [DOI] [PubMed] [Google Scholar]
- 15. Colman E. Food and Drug Administration's Obesity Drug Guidance Document: a short history. Circulation. 2012;125:2156–2164. [DOI] [PubMed] [Google Scholar]
- 16. Topol EJ, Bousser MG, Fox KA, et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo‐controlled trial. Lancet. 2010;376:517–523. [DOI] [PubMed] [Google Scholar]
- 17. James WP, Astrup A, Finer N, et al; STORM Study Group . Effect of sibutramine on weight maintenance after weight loss: a randomised trial. Sibutramine Trial of Obesity Reduction and Maintenance. Lancet. 2000;356:2119–2125. [DOI] [PubMed] [Google Scholar]
- 18. James WP, Caterson ID, Coutinho W, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905–917. [DOI] [PubMed] [Google Scholar]
- 19. Colman E. Slides presented at: Meeting of the Endocrinologic and Metabolic Drugs Advisory Committee: Adelphi, MD; September 15, 2010.
- 20. Whitlock G, Lewington S, Sherliker P, et al. Body‐mass index and cause‐specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. [DOI] [PubMed] [Google Scholar]
- 22. Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155–161. [DOI] [PubMed] [Google Scholar]
- 23. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—3‐year outcomes. N Engl J Med. 2014;370:2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aucott L, Rothnie H, McIntyre L, et al. Long‐term weight loss from lifestyle intervention benefits blood pressure?: a systematic review. Hypertension. 2009;54:756–762. [DOI] [PubMed] [Google Scholar]
- 25. Ikramuddin S, Korner J, Lee WJ, et al. Roux‐en‐Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309:2240–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tchernof A, Nolan A, Sites CK, et al. Weight loss reduces C‐reactive protein levels in obese postmenopausal women. Circulation. 2002;105:564–569. [DOI] [PubMed] [Google Scholar]
- 27. Hainer V, Aldhoon‐Hainerova I. Obesity paradox does exist. Diabetes Care. 2013;36(suppl 2):S276–S281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allison DB, Gadde KM, Garvey WT, et al. Controlled‐release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gadde KM, Allison DB, Ryan DH, et al. Effects of low‐dose, controlled‐release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo‐controlled, phase 3 trial. Lancet. 2011;377:1341–1352. [DOI] [PubMed] [Google Scholar]
- 31. Garvey WT, Ryan DH, Look M, et al. Two‐year sustained weight loss and metabolic benefits with controlled‐release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo‐controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fidler MC, Sanchez M, Raether B, et al. A one‐year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067–3077. [DOI] [PubMed] [Google Scholar]
- 33. O'Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo‐controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM‐DM study. Obesity (Silver Spring). 2012;20:1426–1436. [DOI] [PubMed] [Google Scholar]
- 34. Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo‐controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. [DOI] [PubMed] [Google Scholar]
- 35. Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity‐related risk factors (COR‐II). Obesity (Silver Spring). 2013;21:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR‐I): a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2010;376:595–605. [DOI] [PubMed] [Google Scholar]
- 37. Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR‐BMOD trial. Obesity (Silver Spring). 2011;19:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained‐release/bupropion sustained‐release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36:4022–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Westover AN, Halm EA. Do prescription stimulants increase the risk of adverse cardiovascular events?: a systematic review. BMC Cardiovasc Disord. 2012;12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dies F, Krell M, Whitlow P. Intermittent dobutamine in ambulatory outpatients with chronic cardiac failure (abstract). Circulation. 1986;74(suppl II):S38.
- 41. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. [DOI] [PubMed] [Google Scholar]
- 42. Food US and Drug Administration . Clinical briefing document, QNEXA (phentermine/topiramate). Presented at: Endocrinologic and Metabolic Drugs Advisory Committee Meeting: Silver Spring, MD; February 22, 2012.
- 43. McMahon FG, Weinstein SP, Rowe E, et al. Sibutramine is safe and effective for weight loss in obese patients whose hypertension is well controlled with angiotensin‐converting enzyme inhibitors. J Hum Hypertens. 2002;16:5–11. [DOI] [PubMed] [Google Scholar]
- 44. Heisler LK, Cowley MA, Tecott LH, et al. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. [DOI] [PubMed] [Google Scholar]
- 45. Thomsen WJ, Grottick AJ, Menzaghi F, et al. Lorcaserin, a novel selective human 5‐hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther. 2008;325:577–587. [DOI] [PubMed] [Google Scholar]
- 46. Zhou L, Sutton GM, Rochford JJ, et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin‐4 receptor signaling pathways. Cell Metab. 2007;6:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roth BL. Drugs and valvular heart disease. N Engl J Med. 2007;356:6–9. [DOI] [PubMed] [Google Scholar]
- 48. Launay JM, Hervé P, Peoc'h K, et al. Function of the serotonin 5‐hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med. 2002;8:1129–1135. [DOI] [PubMed] [Google Scholar]
- 49. Weissman NJ, Sanchez M, Koch GG, et al. Echocardiographic assessment of cardiac valvular regurgitation with lorcaserin from analysis of 3 phase 3 clinical trials. Circ Cardiovasc Imaging. 2013;6:560–567. [DOI] [PubMed] [Google Scholar]
- 50. Food US and Drug Administration , FDA briefing document, lorcaserin hydrochloride tablets, 10 mg. Presented at: Endocrinologic and Metabolic Drugs Advisory Committee Meeting: Silver Spring, MD; May 10, 2012.
- 51. Food US and Drug Administration , Addendum to the FDA briefing document. Presented at: Endocrinologic and Metabolic Drugs Advisory Committee Meeting: Silver Spring, MD; May 10, 2012.
- 52. Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol. 1999;83:897–902. [DOI] [PubMed] [Google Scholar]
- 53. Food US and Drug Administration . FDA briefing document, Contrave. Presented at: Endocrinologic and Metabolic Drugs Advisory Committee Meeting: Silver Spring, MD; December 7, 2010.
- 54. Jensen MD, Ryan DH, Apovian CM, et al. 2013. AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2013; doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [Google Scholar]
- 55. Food US and Drug Administration . Guidance for Industry, Diabetes Mellitus—Evaluating Cardiovscular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. Published December 17, 2008. [Google Scholar]
- 56. Hiatt WR, Goldfine AB, Kaul S. Cardiovascular risk assessment in the development of new drugs for obesity. JAMA. 2012;308:1099–1100. [DOI] [PubMed] [Google Scholar]
- 57. Colman E, Golden J, Roberts M, et al. The FDA's assessment of two drugs for chronic weight management. N Engl J Med. 2012;367:1577–1579. [DOI] [PubMed] [Google Scholar]
- 58. European Medicines Agency. Concept paper on the need for revision of the guidelines of medical products used in weight control . http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/10/WC500133166.pdf. Published Septem-ber 20, 2012.


