ABSTRACT
Elucidating the effects of obstructive sleep apnea (OSA) on cardiovascular outcomes is crucial in risk assessments and therapeutic recommendations for affected individuals. The Sleep and Stent Study is a multicenter observational study investigating the relationships between OSA and cardiovascular outcomes in patients treated with percutaneous coronary intervention (PCI). Eight centers in 5 countries (Singapore, China and Hong Kong, India, Myanmar, and Brazil) are participating in the study, and the recruitment target is 1600 patients. Adult patients age 18 to 80 years who have undergone successful PCI are eligible. Recruited patients will undergo an overnight sleep study using a level‐3 portable diagnostic device before hospital discharge. The sleep tracings will be analyzed by a certified sleep technologist and audited by a sleep physician, both of whom will be blinded to other study data. The patients will be divided into 2 groups based on apnea‐hypopnea index (AHI): OSA (AHI ≥15) and non‐OSA (AHI <15) groups. The primary study endpoint of cardiovascular death, myocardial infarction, stroke, and unplanned revascularization will be compared between the OSA and non‐OSA groups at a median follow‐up of 2 years. Secondary endpoints include all‐cause mortality, target‐vessel revascularization, stent thrombosis, and hospitalization for heart failure. As of December 31, 2013, a total of 1358 patients have been recruited. Based on the complete preliminary results of the first 785 recruited patients, the prevalence of OSA was 48.3%. We expect the follow‐up for primary endpoint to be completed in late 2015; study results will be presented in 2016.
Introduction
Coronary artery disease (CAD) is the leading cause of morbidity and mortality in developed countries. In the United States, CAD affects >15 million people and constitutes up to 17% of deaths in the adult population.1 Patients presenting with symptomatic CAD are frequently treated with percutaneous coronary intervention (PCI).2 Despite significant advancements in PCI technology and devices in the past few decades, studies of real‐world practice have revealed that the long‐term clinical outcomes of patients treated with PCI remain suboptimal.3, 4 In the Swedish Coronary Angiography and Angioplasty Registry (SCAAR), which included 28 953 Swedish patients who underwent coronary stent implantation, 19.2% of the patients suffered death or myocardial infarction (MI) at a median follow‐up time of 2.7 years.3 There remains an unmet need to further improve the clinical outcomes of patients who undergo PCI for CAD.
Obstructive sleep apnea (OSA) is an emerging cardiovascular risk factor that is present in 2% to 7% of the general population.5 OSA is characterized by recurrent episodes of complete or partial upper‐airway obstruction due to the collapse of upper‐pharyngeal soft tissue during sleep, resulting in intermittent oxygen deprivation. The pathophysiological mechanisms of OSA, including sympathetic activation, production of vasoactive substances, and activation of inflammatory and procoagulant pathways, have been implicated in cardiovascular pathogenesis.6 This is associated with the development of cardiovascular disease, including hypertension, CAD, stroke, and heart failure. In the healthy general population, untreated OSA is independently and strongly associated with adverse long‐term cardiovascular outcomes.7, 8 It was previously observed that patients with untreated severe OSA had an approximately 3× higher incidence of both fatal and nonfatal cardiovascular events compared with healthy individuals.8
Current data indicate that the prevalence of OSA is greater in patients with CAD compared with the general population.9, 10 The estimated prevalence of OSA ranges between 26% and 69% in CAD patients.11 However, whether OSA exerts a deleterious effect on the evolution of patients who have undergone PCI for CAD is yet to be established. Although multiple studies have shown a strong association between OSA and cardiovascular events in the general population, corresponding evidence in patients treated with PCI has been limited. In 105 patients with acute MI treated with PCI, the incidence of major adverse cardiovascular events was significantly higher in OSA patients after 18‐month follow‐up (16% vs 3%; adjusted hazard ratio: 5.02, P = 0.04).12 Similar results were also obtained in another study on 89 acute coronary syndrome (ACS) patients treated with PCI.13 The incidence of cardiac death, reinfarction, and target‐vessel revascularization at 8‐month follow‐up was higher in the OSA group than in the non‐OSA group (23.5% vs 5.3%, P = 0.022). Restudy angiography at 6‐month follow‐up showed that the incidence of restenosis was significantly higher in the OSA group than in the non‐OSA group (36.5% vs 15.4%, P = 0.026). However, drug‐eluting stents, the state‐of‐the‐art device in PCI, were not used in this study. Finally, it has been proposed that apnea‐induced sympathetic activation and hypoxemia could be trigger factors for stent thrombosis.14
Taken together, the aforementioned studies provide preliminary evidence that OSA may lead to adverse cardiovascular outcomes after PCI. However, existing data were generated from small‐scale, single‐center studies. Therefore, in the Sleep and Stent Study, we aim to conduct a large‐scale, multinational cohort study to determine the association between OSA and the incidence of adverse cardiovascular outcomes over a long‐term follow‐up period. We hypothesize that OSA is an independent risk factor for the development of major adverse cardiovascular and cerebrovascular events (MACCE) in CAD patients treated with PCI. Results from the Sleep and Stent Study will advance the fundamental understanding of the burden and prognostic implications of OSA in patients undergoing PCI for CAD.
Study Objectives
The main objective of the Sleep and Stent Study is to determine the association between OSA and MACCE after a median of 2 years' follow‐up in patients undergoing clinically indicated PCI. MACCE is defined as a composite endpoint of cardiovascular mortality, MI, stroke, and unplanned revascularization.
The secondary objectives are to investigate the prevalence of OSA in patients undergoing PCI and to determine if OSA is associated with a higher incidence of the secondary endpoints of all‐cause mortality, target‐vessel revascularization, stent thrombosis, and hospitalization for heart failure after long‐term follow‐up.
Methods
Study Population and Design
The Sleep and Stent Study is a prospective, observational, multinational cohort study of patients undergoing PCI for CAD. A total of 1600 adult patients undergoing clinically indicated PCI for CAD will be enrolled. The study involves 8 medical centers in 5 countries (Singapore, China and Hong Kong, Brazil, Myanmar, India), all with on‐site cardiac catheterization facilities and cardiac care units. The study is led by the coordinating center (National University Heart Centre, Singapore), which is responsible for the overall study design and study conduct. The estimated duration of the Sleep and Stent Study is 8 years (2011–2019).
Eligible patients (Table 1) who have undergone successful PCI in any of the participating centers during the study period will be approached for recruitment. All subjects and/or their legally authorized representatives must provide written informed consent before any study‐related procedure can be conducted. As a standard clinical practice in all participating centers, patients who have undergone PCI will be admitted to the hospital for monitoring. As such, all recruited patients will undergo an overnight sleep study prior to hospital discharge during the index PCI admission. The patients will then be separated into OSA and non‐OSA groups based on apnea‐hypopnea index (AHI) ≥15. This cutoff is based on epidemiologic studies showing that the risk of adverse cardiovascular events increases particularly in patients with an AHI ≥15.5 The study design is shown in Figure 1.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion criteria |
|---|
| Age ≥18 and <80 years |
| Successful PCI to ≥1 of the epicardial coronary arteries |
| Exclusion criteria |
| Known OSA on CPAP treatment |
| Intubation for mechanical ventilation |
| IABP or other hemodynamic support device |
| Sedation or other muscle relaxant given before overnight sleep study |
| Perceived high risk of malignant ventricular arrhythmia |
| Cardiogenic shock with SBP <90 mm Hg |
| Clinical HF requiring oxygen supplementation |
| Pregnancy |
| History of malignancy (except nonmelanoma skin cancer, cervical in situ carcinoma, breast DCIS, or stage 1 prostate carcinoma) |
| Inability to provide informed consent |
Abbreviations: CPAP, continuous positive airway pressure; DCIS, ductal carcinoma in situ; HF, heart failure; IABP, intra‐aortic balloon pump; OSA, obstructive sleep apnea; PCI, percutaneous coronary intervention; SBP, systolic blood pressure.
Figure 1.
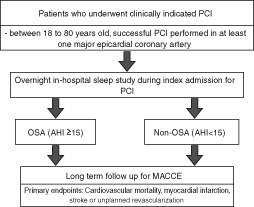
Study flowchart. Abbreviations: AHI, apnea‐hypopnea index; MACCE, major adverse cardiac and cerebrovascular event; OSA, obstructive sleep apnea; PCI, percutaneous coronary intervention.
There will be no financial incentive offered to patients who participate in the Sleep and Stent Study. All recruited patients will be treated according to the standard clinical practices of the local institutions, and no clinical privilege will be gained from participation in the Sleep and Stent Study.
Percutaneous Coronary Intervention
As the Sleep and Stent Study aims to evaluate the prognostic implications of OSA in the clinical setting, there are no restrictions on the presenting indications of patients for PCI (ie, ST‐segment elevation myocardial infarction, non–ST‐segment elevation myocardial infarction, unstable angina, stable CAD silent ischemia, staged PCI) or the usage of pharmacologic and device strategies during PCI. PCI is defined as any intervention to a major epicardial coronary artery with a significant stenosis based on ≥70%‐diameter narrowing on visual estimation (≥50% for left main) and/or fulfilling physiological criteria for revascularization (ie, fractional flow reserve ≤0.80). All PCIs will be conducted in accordance to the most up‐to‐date guidelines.15
Overnight Sleep Study
All sleep studies will be conducted using the Embletta Gold standardized level‐3 portable diagnostic device (Natus Medical Inc., Ontario, Canada; Figure 2). In comparison with full in‐laboratory polysomnography, the level‐3 portable diagnostic device is limited by an inability to examine sleep staging and the lack of an objective measurement of sleep duration. Nevertheless, it has been validated for use against in‐laboratory polysomnography16 and possesses a sensitivity and specificity ranging from 92% to 97% and 64% to 96%, respectively.17, 18, 19 Outputs recorded from the portable diagnostic device include nasal airflow (nasal pressure transducer), thoraco‐abdominal movements (inductive respiratory bands), arterial oxygen saturation (pulse oximetry), snoring episodes (derived from the integrated pressure transducer), limb movement, electrocardiogram, and body position (continuous actigraphy). All studies will be manually scored by an independent sleep technologist with registered polysomnographic technologist credentials (RPSGT) who is blinded to the patients' clinical characteristics. To ensure the accuracy and consistency of scorings, 50% of all sleep studies will be audited by a blinded investigator with expertise in sleep medicine.
Figure 2.
The level‐3 portable diagnostic sleep device.
The primary measure of the sleep study will be the AHI, quantified as the total number of apneas and hypopneas per hour of sleep. An apnea is defined as a ≥90% decrease in airflow from the baseline value for ≥10 seconds. Apneas are further classified as obstructive or central based on the presence or absence, respectively, of respiratory‐related chest‐wall movement. Hypopnea is defined as a 30% to 90% reduction in airflow from the baseline value lasting ≥10 seconds, in conjunction with ≥3% oxygen desaturation. An example of an apneic event is shown in Supplementary Figure 1. The respiratory event scoring will be performed according to the American Academy of Sleep Medicine 2007 (alternative) guidelines.20
To ensure consistency in the quality of the overnight sleep studies conducted, all sleep studies will be graded for quality based on the criteria in Supplementary Table 1, developed after a review of published scoring guidelines.21 All tracings graded as poor would be considered failed sleep studies and excluded from the final analysis of the study. As the main objective of the Sleep and Stent Study is to investigate the relationship between OSA and MACCE, patients with predominantly central sleep apnea (CSA) will be excluded from the primary study analysis.
Obstructive Sleep Apnea Questionnaire Administration
During the index hospitalization period for PCI, recruited patients will be approached to complete the Berlin Questionnaire (BQ) and Epworth Sleepiness Scale (ESS) questionnaire (Supplementary Figures 2 and 3). The BQ is a validated screening tool to identify individuals at high risk for OSA.22 The questionnaire consists of 10 items in 3 categories, assessing the presence and intensity of snoring, the severity of daytime somnolence, and the presence of hypertension and obesity (body mass index ≥30 kg/m2). A positive score in ≥2 categories determines high risk for OSA. The ESS is a validated questionnaire that identifies the perceived likelihood of falling asleep during 8 everyday situations.23, 24 Patients rate their perceived likelihood of falling asleep during each scenario on a scale of 0 to 3, with a total score between 0 and 24. Excessive daytime sleepiness is defined as an ESS score >10. Both questionnaires will be administered through face‐to‐face interviews by an investigator. However, an inability or failure to complete the questionnaires would not result in exclusion from the Sleep and Stent Study.
Referral to Sleep Clinic
The general recommendation for the Sleep and Stent Study is to refer patients diagnosed with OSA, particularly those with excessive daytime sleepiness, to the sleep clinic for further evaluation and consideration of continuous positive airway pressure (CPAP) therapy, the gold‐standard treatment for OSA. However, the final decision is left to the discretion of the individual site principal investigators, in accordance with the standard clinic practice in their county/center. Based on the preliminary data from the first 550 Sleep and Stent Study patients, it is estimated that the number of willing patients who will visit the sleep clinic is low (3.5%; 8 out of 227 referred OSA patients). Given the small number of OSA patients expected to take up regular CPAP therapy, it is anticipated that the number of study patients on CPAP therapy will not significantly affect the sample size requirement of our study.
Baseline Data Collection
The following baseline patient information will be collected prospectively after patient discharge from the index PCI: demographic information, cardiovascular risk factors, medical conditions, current medications, indications for PCI, PCI procedure characteristics, relevant laboratory blood test values, left ventricular ejection fraction, and the BQ, ESS, and sleep study results.
Endpoints Definitions
The primary and secondary endpoints, as well as their concise definitions, are included in Table 2. All endpoints are defined in accordance with the proposed definitions by the Standardized Data Collection for Cardiovascular Trials Initiative.25
Table 2.
Definitions of Primary and Secondary Study Endpoints
| Primary endpoints |
|---|
| Cardiovascular mortality: Death due to AMI, SCD, HF or cardiogenic shock, stroke, or other cardiovascular causes, including arrhythmias unrelated to SCD, aortic aneurysm rupture, PE, PAD, or complications of cardiovascular intervention, including cardiac surgery and nonsurgical revascularization. |
| MI: Based on an evidence of myocardial necrosis (cardiac biomarkers) in combination of a clinical setting consistent with MI (ECG, symptoms). |
| Unplanned revascularization: An unforeseen repeat PCI or bypass graft placement in any coronary vessel (ie, either at the lesion treated during the index PCI, in another segment of the vessel treated at the index PCI, or in another vessel other than the vessel treated at index PCI). |
| Stroke/TIA: Stroke is defined as global or focal cerebral, spinal cord, or retinal injury resulting in acute neurological dysfunction and is further classified into ischemic, hemorrhagic, or undetermined stroke. TIA is defined as a transient episode of neurological dysfunction due to focal temporary cerebral, spinal cord, or retinal ischemia without acute infarction. |
| Secondary endpoints |
| All‐cause mortality: Death due to any cause. |
| TVR (all and ischemia‐driven): An unforeseen repeat PCI or bypass graft placement for any segment of the vessel treated during the index PCI. |
| ST: Based on the level of evidence (definite, probable, or possible ST) and the elapsed time since stent implantation (acute, subacute, late, or very late ST). |
| Hospitalization for HF: The presence of CHF being the primary disease process accounting for clinical and physical signs of HF, with a need for additional or increased HF therapy, requiring at least a 24‐hour stay in an inpatient unit or ED. |
Abbreviations: AMI, acute myocardial infarction; CHF, congestive heart failure; ECG, electrocardiogram; ED, emergency department; HF, heart failure; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; PE, pulmonary embolism; SCD, sudden cardiac death; ST, stent thrombosis; TIA, transient ischemic attack; TVR, target‐vessel revascularization.
In an effort to reduce data variability and minimize bias, all the clinical events reported by site investigators will be adjudicated by a clinical event committee. Members of the clinical event committee, who are not involved in the collection of outcomes data, will blindly review all the pertinent clinical and diagnostic source documentation and adjudicate cardiovascular outcomes.
Follow‐up
Clinical follow‐up will occur at 1, 6, and 12 months, and every year thereafter, beginning from the date of index PCI. There will be no restriction of relevant treatment during the follow‐up period, and optimum pharmacologic management will be provided to all patients enrolled in the study.
Follow‐up will be conducted in the form of an office visit, but telephone contact will be allowed in the event of a defaulted visit. Information obtained during all follow‐up contacts/visits will include any MACCE, rehospitalizations, and recatheterizations, and original source documents will be submitted for any clinical events recorded. Every attempt will be made to collect follow‐up information, except for those patients who specifically withdraw consent for release of such information.
Patients who consecutively miss 2 scheduled follow‐up visits, are unreachable after multiple attempts at communication via telephone, and have not officially withdrawn from the study will be considered lost to follow‐up. OSA patients who have taken up CPAP therapy (>3 months of compliance) during the duration of the study period would be excluded from the final outcome analysis.
Patient Discontinuation
Once enrolled, each patient should remain in the study until the required follow‐up period is complete. However, follow‐up will be terminated in the presence of the following events: voluntary withdrawal, withdrawal by an investigator as clinically indicated, death, or loss to follow‐up.
Study Duration
The maximum duration of study participation for each patient will be approximately 5 years from the date of index PCI. The end of the study is defined as the date on which the last recruited patient completes the 5‐year follow‐up. The primary completion date is defined as the date when the study cohort has reached a median follow‐up of 2 years.
Sample‐Size Calculation
According to our pilot data, 38.4% of the patients have OSA, based on an AHI ≥15. The expected adverse‐event rate for OSA patients who underwent clinically indicated PCI is 18% and that for non‐OSA patients is 12% at a median follow‐up duration of 2 years. Based on 80% power and a significance level of 5%, the required sample size is 1250 (OSA, n = 500; non‐OSA, n = 750). Given that an estimated 20% of the sleep studies will not be successful (due to premature device removal by the patients or technical errors) and that 3% of the OSA patients will take up regular CPAP therapy and be excluded from outcomes analysis, a minimum of 1580 recruited patients is needed. The projected sample size of 1600 participants will provide sufficient power to test the primary hypothesis at the median follow‐up period of 2 years, but further follow‐up may be needed to obtain sufficient power to test all primary and secondary hypotheses, both overall and within subgroups of a priori interest.
Ethical Considerations
Institutional review board/ethics committee approval for the protocol and informed‐consent form has been obtained by all investigators prior to study participation. The Sleep and Stent Study will be carried out in compliance with the following principles and regulations: (1) the International Conference on Harmonisation (ICH) Harmonised Tripartite Guideline for Good Clinical Practice, 1996 (the Principles of Good Clinical Practice, Institutional Review Board, and Informed Consent sections); (2) the Declaration of Helsinki and amendments, World Medical Association Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects; and (3) local, country‐specific regulations where applicable.
Data Management
The Sleep and Stent Study is led by the coordinating center, and both baseline and follow‐up data collection will be conducted based on standardized procedures designed by the coordinating center. All recorded study variables will be stored in a database managed by the Data Management Core Laboratory at the National University Heart Centre, Singapore. The study variables will be accessible only to 2 study investigators responsible for the accuracy and analysis of the information. Legal requirements for data protection will be enforced, and all relevant information collected will be processed using properly validated methods.
Statistical Analysis
Categorical variables will be presented as frequencies and percentages, and continuous variables will be summarized using descriptive statistics of central tendency and dispersion. A Cox proportional hazards model will be used to compare the MACCE rates between OSA and non‐OSA groups. Similarly, the Kaplan‐Meier curves and the MACCE‐free survival rates will be compared using the log‐rank test. A multivariate analysis will be conducted using a Poisson regression model to account for the interactions between variables and possible confounding variables between OSA and non‐OSA groups. A similar secondary analysis will be performed comparing the secondary endpoints rates between OSA and non‐OSA groups using a Cox proportional hazards model. All statistical analyses will be conducted using Stata software, release 13 (StataCorp LP; College Station, TX), assuming a 2‐sided test with a 5% level of significance.
Current Progress and Preliminary Results
As of December 31, 2013, a total of 1358 patients have been recruited into the study. Complete baseline demographic and clinical characteristics, as well as the sleep‐study data, are available for the first 785 recruited patients and shown in Table 3. The prevalence of OSA was 48.3%. Compared with the non‐OSA group, patients in the OSA group were more likely to be male and of Chinese and Malay ethnicity, and had a significantly greater body mass index, neck circumference, and waist circumference. When looking at cardiovascular risk factors, OSA patients had a significantly higher prevalence of hypertension. There were no significant differences in the left ventricular ejection fraction or baseline laboratory markers, including cardiac markers, renal markers, blood markers, lipid markers, and fasting glucose levels (results not shown). However, OSA patients had a significantly higher glycated hemoglobin level compared with non‐OSA patients (median, 6.6%; range, 4.6%–15.6%; and median, 6.3%; range, 4.3%–14.0%, respectively). The clinical indications for PCI were similar for both OSA and non‐OSA patients. Compared with non‐OSA patients, OSA patients were more likely to undergo multivessel PCI, had a lower number of drug‐eluting stents implanted during PCI and a higher number of bare‐metal stents implanted during PCI. Apart from an increased usage of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers in OSA patients (80.7% vs 74.1%, P = 0.027), medications prescribed upon hospital discharge were similar in both OSA and non‐OSA groups (data not shown).
Table 3.
Baseline Characteristics of the First 785 Sleep and Stent Study Patients
| Characteristics | Overall, N = 785 | OSA, AHI ≥15, n = 379 | Non‐OSA, AHI <15, n = 406 | P Value |
|---|---|---|---|---|
| Male sex, n (%) | 670 (85.4) | 335 (88.4) | 335 (82.5) | 0.020 |
| Ethnicity, n (%) | ||||
| Chinese | 515 (65.6) | 253 (66.8) | 262 (64.5) | 0.001 |
| Malay | 129 (16.4) | 72 (19.0) | 57 (14.0) | |
| Indian | 115 (14.6) | 38 (10.0) | 77 (19.0) | |
| Others | 26 (3.3) | 16 (4.2) | 10 (2.5) | |
| Age, y, mean (SD) | 57.6 (10.0) | 58.1 (9.8) | 57 (10.1) | 0.129 |
| BMI, kg/m2, mean (SD)a | 25.7 (3.7) | 26.5 (3.0) | 25 (3.3) | <0.001 |
| Neck circumference, cm, mean (SD)b | 39 (4.8) | 39.8 (4.6) | 38.4 (4.9) | 0.001 |
| Waist circumference, cm, mean (SD)b | 94.9 (13.0) | 97.2 (12.9) | 93 (12.8) | <0.001 |
| Cardiovascular risk factors, n (%) | ||||
| Smoking | 303 (38.6) | 151 (39.8) | 152 (37.4) | 0.489 |
| Hyperlipidemiac | 531 (69.2) | 252 (67.7) | 279 (70.6) | 0.386 |
| Hypertension | 491 (62.5) | 259 (68.3) | 232 (57.1) | 0.001 |
| DM | 359 (45.7) | 182 (48.0) | 177 (43.6) | 0.214 |
| Family history of premature CAD | 69 (8.8) | 29 (7.7) | 40 (9.9) | 0.277 |
| Concomitant conditions, n (%) | ||||
| Previous AMI | 149 (19.0) | 68 (17.9) | 81 (20.0) | 0.473 |
| Previous PCI | 164 (20.9) | 73 (19.3) | 91 (22.4) | 0.278 |
| Previous CABG | 26 (3.3) | 15 (4.0) | 11 (2.7) | 0.329 |
| Previous stroke/TIA | 48 (6.1) | 25 (6.6) | 23 (5.7) | 0.586 |
| CKD | 42 (5.4) | 22 (5.8) | 20 (4.9) | 0.585 |
| Indications for PCI, n (%) | 0.532 | |||
| STEMI | 240 (30.6) | 114 (30.1) | 126 (31.0) | |
| NSTEMI | 156 (19.9) | 83 (21.9) | 73 (18.0) | |
| UA | 140 (17.8) | 68 (17.9) | 72 (17.7) | |
| Stable CAD/others | 249 (31.7) | 114 (30.1) | 135 (33.3) | |
| Multivessel PCI, n (%) | 119 (15.2) | 72 (19.0) | 47 (11.6) | 0.004 |
| Revascularization devices, n (%)d | 0.008 | |||
| DES | 840 (78.1) | 403 (75.2) | 437 (80.9) | |
| BMS | 70 (6.5) | 48 (9.0) | 22 (4.1) | |
| Bioresorbable vascular scaffolds | 68 (6.3) | 38 (7.1) | 30 (5.6) | |
| Bioengineered stents | 6 (0.5) | 5 (0.9) | 1 (0.2) | |
| DEB | 36 (3.4) | 16 (3.0) | 20 (3.7) | |
| Plain balloons | 53 (4.9) | 25 (4.7) | 28 (5.2) | |
| Sleep study characteristics | ||||
| AHI, median (range) | 14.5 (0.1–89.3) | 30.0 (15.0–89.3) | 6.2 (0.1–14.9) | <0.001 |
| Baseline SpO2, median (range) | 94.3 (79.5–98.9) | 94.1 (79.5–98.4) | 94.6 (81.6–98.9) | <0.001 |
| Lowest SpO2, median (range) | 86.0 (50.0–95.0) | 83.0 (50.0–95.0) | 88.0 (57.0–95.0) | <0.001 |
| Total % of time SpO2 <90%, median (range)e | 3.45 (0–49.4) | 8.4 (0–49.1) | 1.1 (0–49.4) | <0.001 |
| ESS >10, n (%)f | 116 (19.0) | 67 (23.2) | 49 (15.3) | 0.014 |
| High risk based on BQ, n (%)g | 262 (44.7) | 157 (56.7) | 105 (34.0) | <0.001 |
Abbreviations: AHI, apnea‐hypopnea index; AMI, acute myocardial infarction; BMI, body mass index; BMS, bare‐metal stents; BQ, Berlin Questionnaire; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; DEB, drug‐eluting balloons; DES, drug‐eluting stents; DM, diabetes mellitus; ESS, Epworth Sleepiness Scale; FFR, fractional flow reserve; IVUS, intravascular ultrasound; NSTEMI, non–ST‐segment elevation myocardial infarction; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; SD, standard deviation; SpO2, arterial oxygen saturation; STEMI, ST‐segment elevation myocardial infarction; TIA, transient ischemic attack; UA, unstable angina.
Data were not obtained for certain parameters in some patients.
n = 716. b n = 550. c n = 767. d n = 1076 (number of lesions intervened were greater than number of study subjects). en = 664. fn = 609. gn = 586.
The median AHI of the first 785 Sleep and Stent Study patients was 14.5. Among the 785 patients, 167 patients (21.3%) had an AHI <5, 239 patients (30.4%) had an AHI of 5 to <15, 189 patients (24.1%) had an AHI of 15 to <30, and 190 patients (24.2%) had an AHI ≥30. A significantly greater number of OSA patients had an ESS score >10 or were high risk for OSA based on the BQ.
Discussion
The Sleep and Stent Study is the first large‐scale, observational, multinational study to investigate the association between OSA and the incidence of MACCE in CAD patients undergoing PCI over a long‐term follow‐up period. The results of this study would provide an up‐to‐date paradigm of the prognostic implications of OSA in patients with existing CAD.
The preliminary data of the first 785 Sleep and Stent Study patients show that the prevalence of OSA in our cohort of CAD patients undergoing PCI is similar to that reported in existing literature.11 The demographic and clinical profile of our patients is also similar to current literature.26 The Randomized Intervention With CPAP in Coronary Artery Disease and Sleep Apnea (RICCADSA) trial included >600 CAD patients treated with PCI or coronary artery bypass grafting (NCT00519597). The preliminary results of the Sleep and Stent Study are consistent with published data on the occurrence and predictors of OSA in patients enrolled in the RICCADSA trial,27 suggesting that age, male sex, body mass index, and ESS score were predictors of OSA. Of note, it is interesting to observe in our preliminary study results that a significantly greater number of OSA patients had undergone multivessel PCI in comparison with non‐OSA patients. In addition, OSA patients had a significantly lower number of drug‐eluting stents and a greater number of bare‐metal stents implanted during PCI. This result is similar to a study on 81 patients who had undergone computed tomography scanning, reporting a greater number of OSA patients having multivessel stenotic plaque involvement compared with non‐OSA patients.28 Further in‐depth analysis upon the completion of the Sleep and Stent Study to elucidate possible confounders for this relationship is required.
The strength of this study lies in the recruitment of study participants from various medical centers in different countries. This would allow the results to be generalizable to a wider cohort of patients and allow a study of the impact of OSA in the real‐world scenario. In addition, the long follow‐up duration would provide an in‐depth understanding of the cardiovascular effects of OSA. As the Sleep and Stent Study employs the use of in‐hospital sleep study using a standardized level‐3 portable diagnostic device, based on the long‐term prognostic effects of OSA, inference regarding the feasibility of this method for OSA diagnosis in the clinical setting can also be made. Identification of the predictors of OSA in our study cohort of patients would also guide future clinical suspicion of OSA in at‐risk CAD patients and facilitate future diagnosis of OSA.
There are some limitations in the Sleep and Stent Study. First, recent evidence has suggested that the timing of sleep studies may influence OSA diagnosis in patients with ACS.29 Therefore, it has been proposed that some of the OSA diagnosed during the acute phase of ACS may be transient. Yet, the prognostic implication of in‐hospital vs postdischarge sleep study remains unclear, and in‐hospital sleep studies are still commonly used in clinical research. In fact, a default rate of 18% for postdischarge sleep study among patients who gave informed consent was recently reported.30 Second, given the short hospitalization period in patients after PCI, it may not be possible to conduct a repeat sleep study should the initial sleep study fail. Third, as patients with habitual snoring and obesity may be more willing to participate in this study, the prevalence of OSA in these patients may be higher than the actual prevalence among all post‐PCI patients. Lastly, as most of the study patients will be managed by various primary‐care physicians at various time intervals after PCI, it would be difficult to accurately obtain data on medication compliance and physical activity on subsequent follow‐up, and these data will not be available for analysis.
CSA is a consequence of heart failure and is commonly observed in patients with heart failure.31 CSA is independently associated with an increased mortality. In patients with OSA, episodes of CSA can also be present.32 However, the pathophysiological mechanism of OSA and CSA are markedly different, and no strong relationship exists between the 2 types of sleep‐disordered breathing. As such, patients with central‐predominant apneas are excluded from the primary analysis of our study.
Several large‐scale randomized controlled trials evaluating the effects of CPAP therapy on the outcomes of OSA patients are currently underway. The RICCADSA trial (NCT00519597), including >600 CAD patients, seeks to identify the effects of CPAP therapy in OSA patients on the composite outcomes of cardiovascular mortality, stroke, MI, and unplanned revascularization after 3‐year follow‐up. In this trial, patients are separated into 4 groups for comparison: nonsleepy (ESS score <10) OSA patients (AHI ≥15) on CPAP, nonsleepy OSA patients without CPAP, sleepy (ESS score ≥10) OSA patients on CPAP, and non‐OSA patients (AHI <5). The Continuous Positive Airway Pressure in Patients with Acute Coronary Syndrome and Obstructive Sleep Apnea (ISAACC) trial is currently underway to evaluate CPAP therapy on the incidence of long‐term cardiovascular events in nonsleepy (ESS score ≤10) OSA patients (AHI ≥15) with ACS (NCT01335087). The Sleep Apnea Cardiovascular Endpoints (SAVE) study (NCT00738170) aims to determine the effects of CPAP therapy on the reduction of cardiovascular events in 5000 patients with established cardiovascular disease and moderate to severe OSA (AHI >30) over a 3‐ to 5‐year follow‐up period.
Each of the aforementioned randomized controlled trials evaluating the effects of CPAP treatment on cardiovascular outcome is focused on a particular population of cardiovascular disease patients, with different definitions being used to define the OSA group: for the RICCADSA trial, patients with CAD treated with PCI or coronary artery bypass grafting, with OSA based on AHI ≥15; for the ISAACC trial, nonsleepy OSA patients (AHI ≥15) with ACS33; and for the SAVE trial, patients with established coronary or cerebrovascular disease and OSA (AHI >30).
Conclusion
Compared with the aforementioned randomized controlled trials, the Sleep and Stent Study is focused on determining the effects of OSA on future MACCE in an all‐inclusive group of CAD patients treated with PCI. This would address whether OSA is an independent risk factor for future MACCE in CAD patients treated with PCI. As such, the results will be important in the understanding of the burden and cardiovascular effects of OSA in CAD patients. This will facilitate future resource allocation and the planning of preventive strategies to address the yet‐unmet and growing clinical needs of patients with cardiovascular disease. Together with the results from randomized controlled trials evaluating the usage of CPAP therapy in CAD patients, this will guide future treatment and management of patients with CAD.
Supporting information
Supporting Figure 1. Sleep study tracing showing 2 consecutive apnea cycles. A: cessation of nasal airflow; B: respiratory effort; C: oxygen desaturation following the apnea.
Supporting Figure 2. Berlin Questionnaire.
Supporting Figure 3. Epworth Sleepiness Scale.
Criteria for Overall Sleep Study Quality Grading
Acknowledgments
The authors thank Mr. Anand Kailasam for his help in the preparation of this manuscript.
This study is funded by the Investigator‐Sponsored Research Program of the Boston Scientific Corporation. The study was designed solely by the investigators. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association [published correction appears in Circulation. 2013;127:e841]. Circulation. 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Park SJ, Kim YH. Current status of percutaneous coronary intervention with drug‐eluting stents in Asia. Circulation. 2008;118:2730–2737. [DOI] [PubMed] [Google Scholar]
- 3. Lagerqvist B, James SK, Stenestrand U, et al; SCAAR Study Group . Long‐term outcomes with drug‐eluting stents versus bare‐metal stents in Sweden. N Engl J Med. 2007;356:1009–1019. [DOI] [PubMed] [Google Scholar]
- 4. Park K, Park KW, Rha SW, et al. Comparison of 5‐year clinical outcomes between sirolimus‐ versus paclitaxel‐eluting stent: Korean multicenter network analysis of 9000‐patient cohort. Circ Cardiovasc Interv. 2012;5:174–184. [DOI] [PubMed] [Google Scholar]
- 5. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jaffe LM, Kjekshus J, Gottlieb SS. Importance and management of chronic sleep apnoea in cardiology. Eur Heart J. 2013;34:809–815. [DOI] [PubMed] [Google Scholar]
- 7. Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep‐disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marin JM, Carrizo SJ, Vicente E, et al. Long‐term cardiovascular outcomes in men with obstructive sleep apnea‐hypopnea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. [DOI] [PubMed] [Google Scholar]
- 9. Shahar E, Whitney CW, Redline S, et al. Sleep‐disordered breathing and cardiovascular disease: cross‐sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. [DOI] [PubMed] [Google Scholar]
- 10. Mooe T, Rabben T, Wiklund U, et al. Sleep‐disordered breathing in men with coronary artery disease. Chest. 1996;109:659–663. [DOI] [PubMed] [Google Scholar]
- 11. De Torres‐Alba F, Gemma D, Armada‐Romero E, et al. Obstructive sleep apnea and coronary artery disease: from pathophysiology to clinical implications. Pulm Med. 2013;2013:768064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee CH, Khoo SM, Chan MY, et al. Severe obstructive sleep apnea and outcomes following myocardial infarction. J Clin Sleep Med. 2011;7:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yumino D, Tsurumi Y, Takagi A, et al. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol. 2007;99:26–30. [DOI] [PubMed] [Google Scholar]
- 14. Hrynkiewicz‐Szymanska A, Szymanski FM, Filipiak KJ, et al. Can obstructive sleep apnea be a cause of in‐stent thrombosis? Sleep Breath. 2011;15:607–609. [DOI] [PubMed] [Google Scholar]
- 15. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–e651. [DOI] [PubMed] [Google Scholar]
- 16. Tiihonen P, Hukkanen T, Tuomilehto H, et al. Evaluation of a novel ambulatory device for screening of sleep apnea. Telemed J E Health. 2009;15:283–289. [DOI] [PubMed] [Google Scholar]
- 17. Verse T, Prisig W, Junge‐Hulsing B, et al. Validation of the POLY‐MESAM seven‐channel ambulatory recording unit. Chest. 2000;117:1613–1618. [DOI] [PubMed] [Google Scholar]
- 18.Tonelli de Oliveria AC, Martinez D, Vasconcelos LF, et al. Diagnosis of obstructive sleep apnea syndrome and its outcomes with home portable monitoring. Chest. 2009;135:330–336. [DOI] [PubMed]
- 19. Calleja JM, Esnaola S, Rubio R, et al. Comparison of a cardiorespiratory device versus polysomnography for diagnosis of sleep apnoea. Eur Respir J. 2002;20:1505–1510. [DOI] [PubMed] [Google Scholar]
- 20. Iber C, Ancoli‐Israel S, Chesson AL, et al, eds. AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 21. Redline S, Sanders MH, Lind BK, et al; Sleep Heart Health Research Group. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep. 1998;21:759–767. [PubMed] [Google Scholar]
- 22. Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. [DOI] [PubMed] [Google Scholar]
- 23. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 24. Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea: the Epworth Sleepiness Scale. Chest. 1993;103:30–36. [DOI] [PubMed] [Google Scholar]
- 25. Hicks KA, Hung HMJ, Mahaffey KW, et al; for the Standardized Data Collection for Cardiovascular Trials Initiative. Standardized Definitions for End Point Events in Cardiovascular Trials http://www.cdisc.org/stuff/contentmgr/files/0/2356ae38ac190ab8ca4ae0b222392b37/misc/cdisc_november_16__2010.pdf. Published October 20, 2010. Accessed October 2013.
- 26. Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118:1080–1111. [DOI] [PubMed] [Google Scholar]
- 27. Glantz H, Thunström E, Herlitz J, et al. Occurrence and predictors of obstructive sleep apnea in a revascularized coronary artery disease cohort. Ann Am Thorac Soc. 2013;10:350–356. [DOI] [PubMed] [Google Scholar]
- 28. Sharma S, Gebregziabher M, Parker AT, et al. Independent association between obstructive sleep apnea and noncalcified coronary plaque demonstrated by noninvasive coronary computed tomography angiography. Clin Cardiol. 2012;35:641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Low TT, Hong WZ, Tai BC, et al. The influence of timing of polysomnography on diagnosis of obstructive sleep apnea in patients presenting with acute myocardial infarction and stable coronary artery disease. Sleep Med. 2013;14:985–990. [DOI] [PubMed] [Google Scholar]
- 30. Tan A, Hau W, Ho HH, et al. Obstructive sleep apnea and coronary plaque characteristics; Chest. 2013; doi: 10.1378/chest.13-1163. [DOI] [Google Scholar]
- 31. Bradely TD, Floras JS. Sleep apnea and heart failure: Part II: central sleep apnea. Circulation. 2003;107:1822–1826. [DOI] [PubMed] [Google Scholar]
- 32. Moruzzi P, Sarzi‐Braga S, Rossi M, et al. Sleep apnoea in ischaemic heart disease: differences between acute and chronic coronary syndromes. Heart. 1999;82:343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Esquinas C, Sánchez‐de‐la Torre M, Aldomá A, et al. Rationale and methodology of the impact of continuous positive airway pressure on patients with ACS and nonsleepy OSA: the ISAACC Trial. Clin Cardiol. 2013;36:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure 1. Sleep study tracing showing 2 consecutive apnea cycles. A: cessation of nasal airflow; B: respiratory effort; C: oxygen desaturation following the apnea.
Supporting Figure 2. Berlin Questionnaire.
Supporting Figure 3. Epworth Sleepiness Scale.
Criteria for Overall Sleep Study Quality Grading


