Abstract
Background
The incremental predictive value of red cell distribution width (RDW) for major adverse cardiac events (MACEs) has not been fully investigated in patients with acute myocardial infarction (AMI).
Hypothesis
The aim of this study was to determine the incremental value of RDW to the established risk factors in predicting clinical outcomes after AMI.
Methods
Between November 2005 and January 2010, 1596 patients with AMI (1070 male; mean age, 64.5 ± 11.9 years) were analyzed in this study. Baseline levels of RDW were measured at the time of admission. The 12‐month MACEs were defined as death and nonfatal MI.
Results
The RDW levels were significantly higher in patients with 12‐month MACEs (13.8 ± 1.3% vs 13.3 ± 1.2%, P < 0.001). In a Cox proportional hazards model, RDW (hazard ratio [HR]: 1.19, P = 0.016) was an independent predictor for 12‐month MACEs. Adding RDW to established risk factors and hemoglobin levels significantly improved prediction for 12‐month MACEs, as shown by the net reclassification improvement (0.297; P = 0.012) and integrated discrimination improvement (0.0143; P = 0.042). The likelihood ratio test showed that RDW added incremental predictive value to the combination of hemoglobin and established risk factors (P = 0.005). Patients were categorized into 4 groups according to quartiles of RDW at baseline. Adjusted HRs for 12‐month MACEs were 1 (RDW ≤12.6%, reference), 4.24 (RDW 12.7%–13.1%, P = 0.01), 4.36 (RDW 13.2%–13.9%, P = 0.008), and 6.18 (RDW 13.2%–13.9%, P = 0.001), respectively.
Conclusions
In post‐myocardial infarction patients, baseline RDW levels at admission could provide incremental predictive value to established risk factors for predicting 12‐month MACEs.
Introduction
It has been known that low hemoglobin levels are independent cardiovascular risk factors in patients with cardiovascular disease.1, 2, 3 Red cell distribution width (RDW) is a standard part of the complete blood count laboratory test. A recent article reported that RDW was a strong independent predictor of cardiovascular outcomes in patients with heart failure, even after adjustment for hematocrit.4, 5 However, association of RDW with clinical outcome after acute myocardial infarction (AMI) has not been fully investigated.6, 7, 8 Moreover, the incremental value of RDW to established risk factors, including hemoglobin, in patients with AMI has not been elucidated.
Therefore, the aims of this study were to determine the association between RDW and clinical outcomes and to determine the incremental value of RDW to the established risk factors in predicting clinical outcomes after AMI.
Methods
Study Population
This observational study included 1596 consecutive patients with AMI who were enrolled in the Korea AMI Registry (KAMIR) from the authors' single center between November 2005 and January 2010. Since November 2005, KAMIR has been a Korean, prospective, open, observational, multicenter online registry of AMI with support from the Korean Society of Cardiology. Details of KAMIR have been published.9 The RDW levels were retrospectively collected, because they had not been entered into the KAMIR database. The AMI was diagnosed by characteristic clinical presentation, serial changes on the electrocardiogram suggesting infarction, and increase in cardiac enzymes.10
We analyzed baseline demographic characteristics, initial presentation, initial vital signs, electrocardiogram findings, results of laboratory tests, procedural data, and medications. Blood samplings for baseline laboratory tests, except for the lipid measurement, were collected at admission. Blood specimens were collected in 3.6% ethylenediaminetetraacetic acid tubes to examine the hematologic parameters including RDW. Baseline levels of RDW were measured by Advia 2120 hematology analyzer (Bayer Diagnostics, Dublin, Ireland) within 2 hours of admission. Overnight fasting blood was also sampled for lipid levels. Left ventricular ejection fraction (LVEF) was determined by 2‐dimensional echocardiography.
Mean follow‐up duration was 1634 ± 342 days. The 12‐month major adverse cardiac events (MACEs) were defined as death and nonfatal myocardial infarction (MI). During the follow‐up period, follow‐up data were obtained by reviewing medical records and telephone interviews with patients. All data were recorded on an electronic Web‐based case report form.
Statistical Analyses
Data are expressed as mean ± standard deviation for continuous variables and percentages for categorical variables. All comparisons between baseline variables were assessed with the Student t test for continuous variables and the Pearson χ2 test for categorical variables. Univariate analyses were performed to determine the predictors for 12‐month MACEs. A Cox proportional hazards model was used to determine independent predictors of 12‐month MACEs. Variables with P values of <0.05 on univariate analysis were entered into the Cox proportional hazards model. The RDW was entered as a linear term. To evaluate model calibration, we calculated the Hosmer‐Lemeshow χ2, a measure of deviation between observed and predicted outcomes in deciles of predicted risk.
The likelihood ratio test was performed to examine the incremental predictive value of the parameters in the Cox proportional hazards model. The factors added to the model at each step were considered significant when the test statistic, twice the difference in the log‐likelihood associated with each model, corresponded to P < 0.05. Further, the study subjects were divided into 4 categories based on the baseline RDW levels. Cox proportional hazards model analyses were used to compute the hazard ratios (HRs) and 95% confidence intervals (CIs) of 12‐month MACEs for increasing RDW quartiles, with the lowest quartile as the reference.
The increased discriminative value after the addition of hemoglobin and/or RDW to the established risk factors was estimated using 3 measures (the Harrell's C index, net reclassification improvement [NRI], and integrated discrimination improvement [IDI]). The Harrell's C index (C‐statistic) is defined as the proportion of usable patient pairs, in which the predictions and outcomes are concordant.11 We estimated receiver operating characteristic curves and compared the areas under the receiver operating characteristic curves (C‐statistic with 95% CI) in corresponding logistic models.12 The NRI and IDI were calculated by analyzing the differences in individual estimated probability for 12‐month MACEs after the addition of hemoglobin and/or RDW to a model containing the established risk factors.13 Because no prior risk categories exist for 12‐month MACEs, we calculated the category‐free NRI.13 For all analyses, a 2‐sided P < 0.05 was considered statistically significant. Statistical analysis was performed using SAS version 9.2 software (SAS Institute Inc., Cary, NC).
Results
The baseline characteristics of the study subjects are shown in Table 1. The mean age was 64.5 ± 11.9 years, and 1070 (67.0%) were male. The RDW ranged from 11.0% to 26.0% (median, 13.1%; mean, 13.3 ± 1.2%), and 35 (7.5%) had RDW levels outside the normal range of 11.5% to 14.5% with RDW <11.5% (n = 12, 0.8%) and >14.5% (n = 179, 11.2%).
Table 1.
Clinical Characteristics in Patients With or Without Major Adverse Cardiac Events
| Variable | Overall (n = 1,596) | MACE | P Value | |
|---|---|---|---|---|
| No (n = 1384) | Yes (n = 212) | |||
| Demographics | ||||
| Age (y) | 64.5 ± 11.9 | 63.3 ± 11.5 | 72.3 ± 10.9 | <0.001 |
| Male | 1070 (67.0%) | 950 (68.6%) | 120 (56.6%) | 0.001 |
| Body mass index (kg/m2) | 23.7 ± 3.06 | 23.8 ± 3.05 | 22.6 ± 2.91 | <0.001 |
| Initial presentation | ||||
| Systolic blood pressure (mmHg) | 136.5 ± 29.8 | 137.3 ± 28.4 | 131.2 ± 37.1 | 0.043 |
| Heart rate (beats/min) | 79.6 ± 20.1 | 78.2 ± 19.0 | 89.1 ± 24.3 | <0.001 |
| ST‐elevation myocardial infarction | 688 (43.4%) | 608 (44.1%) | 80 (38.6%) | 0.141 |
| Killip class >1 | 408 (25.6%) | 279 (20.2%) | 129 (61.1%) | <0.001 |
| Past history | ||||
| Previous coronary heart disease | 295 (18.7%) | 236 (17.3%) | 59 (28.5%) | <0.001 |
| Hypertension | 725 (45.9%) | 613 (44.6%) | 112 (54.4%) | 0.009 |
| Diabetes mellitus | 454 (28.8%) | 374 (27.3%) | 80 (38.6%) | 0.001 |
| Hyperlipidemia | 416 (28.9%) | 391 (31.1%) | 25 (13.7%) | <0.001 |
| Current smoking | 651 (43.1%) | 578 (43.9%) | 73 (37.6%) | 0.099 |
| Left ventricular ejection fraction (%) | 51.2 ± 11.2 | 52.2 ± 10.6 | 42.5 ± 12.7 | <0.001 |
| Laboratory findings | ||||
| Hemoglobin (g/dL) | 13.3 ± 2.10 | 13.5 ± 1.96 | 11.9 ± 2.40 | <0.001 |
| Red cell distribution width (%) | 13.3 ± 1.24 | 13.3 ± 1.22 | 13.8 ± 1.27 | <0.001 |
| Serum creatinine (mg/dL) | 1.17 ± 1.16 | 1.06 ± 0.98 | 1.85 ± 1.82 | <0.001 |
| Peak creatine‐kinase MB (ng/mL) | 63.8 ± 157.0 | 61.2 ± 149.3 | 81.3 ± 201.6 | 0.172 |
| Peak cardiac troponin I (ng/mL) | 48.5 ± 91.3 | 47.0 ± 83.5 | 58.8 ± 132.4 | 0.219 |
| Total cholesterol (mg/dL) | 179.2 ± 42.5 | 180.4 ± 41.7 | 169.9 ± 47.8 | 0.002 |
| Triglycerides (mg/dL) | 142.6 ± 120.6 | 145.5 ± 125.9 | 119.6 ± 61.1 | <0.001 |
| High‐density lipoprotein cholesterol (mg/dL) | 44.4 ± 12.5 | 44.6 ± 12.4 | 43.1 ± 12.9 | 0.147 |
| Low‐density lipoprotein cholesterol (mg/dL) | 118.5 ± 38.9 | 119.4 ± 38.1 | 111.5 ± 43.9 | 0.026 |
| Percutaneous coronary intervention at index hospitalization | 1,201 (75.7%) | 1117 (80.9%) | 84 (40.6%) | <0.001 |
| Discharge medication | ||||
| Antiplatelet agents | 1510 (94.6%) | 1335 (96.5%) | 175 (82.5%) | <0.001 |
| β‐blockers | 1349 (84.5%) | 1207 (87.2%) | 142 (67.0%) | <0.001 |
| ACE‐I/ARBs | 1356 (85.0%) | 1216 (87.9%) | 140 (66.0%) | <0.001 |
| Lipid‐lowering drugs | 1126 (70.6%) | 999 (72.2%) | 127 (59.9%) | <0.001 |
Abbreviations: ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II type 1 receptor blocker; MACE, major adverse cardiac event.
Data are mean ± standard deviation for continuous variables and percentages for categorical variables.
During the 12‐month follow‐up, 212 (13.3%) MACEs including 174 (10.9%) deaths and 38 (2.4%) nonfatal MIs occurred. The RDW levels were significantly higher in patients with 12‐month MACEs (13.8 ± 1.3% vs 13.3 ± 1.2%, P < 0.001) (Table 1). In multivariate analysis, RDW (HR: 1.19, 95% CI: 1.03‐1.37, P = 0.016) in addition to body mass index (BMI) (HR: 0.91, 95% CI: 0.84‐0.99), previous coronary heart disease (CHD) (HR: 2.25, 95% CI: 1.38‐3.67), serum creatinine (HR: 1.23, 95% CI: 1.09‐1.39), and percutaneous coronary intervention (PCI) (HR: 0.27, 95% CI: 0.16‐0.45) was an independent prognostic factor for 12‐month MACEs after adjustment for confounding variables in the Cox proportional hazards model (Table 2).
Table 2.
Cox Proportional Hazards Model for 12‐Month Major Adverse Cardiac Events
| Variable | Hazard Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age >65 years | 1.56 | 0.89‐2.72 | 0.118 |
| Male | 0.72 | 0.43‐1.20 | 0.204 |
| Body mass index | 0.91 | 0.84‐0.99 | 0.025 |
| Previous coronary heart disease | 2.25 | 1.38‐3.67 | 0.001 |
| Hypertension | 1.22 | 0.75‐1.99 | 0.428 |
| Diabetes mellitus | 1.17 | 0.71‐1.92 | 0.535 |
| Serum creatinine | 1.23 | 1.09‐1.39 | 0.001 |
| Total cholesterol | 1.00 | 0.999‐1.01 | 0.171 |
| Percutaneous coronary intervention | 0.27 | 0.16‐0.45 | <0.001 |
| Antiplatelet agent use | 0.62 | 0.26‐1.50 | 0.288 |
| β‐blockers use | 0.79 | 0.43‐1.42 | 0.425 |
| ACE‐I/ARBs use | 0.82 | 0.47‐1.41 | 0.471 |
| Hemoglobin | 0.96 | 0.84‐1.11 | 0.603 |
| Red cell distribution width | 1.19 | 1.03‐1.37 | 0.016 |
Abbreviations: ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II type 1 receptor blocker.
The incremental predictive values of the established risk factors, hemoglobin, and RDW in the Cox proportional hazards model are shown in the Figure 1. The RDW added incremental value to the combination of hemoglobin and established risk factors in predicting 12‐month MACEs.
Figure 1.
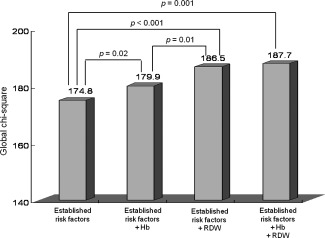
Incremental predictive value of the established risk factors, hemoglobin (Hb), and red cell distribution width (RDW) levels by Cox proportional hazards model. The RDW levels have predictive value incremental to the combination of hemoglobin and established risk factors including sex, age, body mass index, prior coronary heart disease, hypertension, diabetes mellitus, serum creatinine, total cholesterol, percutaneous coronary intervention, antiplatelet agents, β‐blockers, and angiotensin‐converting enzyme inhibitors/angiotensin II type 1 receptor blockers.
The study subjects were divided into 4 groups based on baseline RDW levels, as follows: quartile 1 (<12.6%, n = 445); quartile 2 (12.7%–13.1%, n = 364); quartile 3 (13.2%–13.9%, n = 400), and quartile 4 (>13.9%, n = 387). Age, heart rate, and Killip class > significantly increased as the RDW levels increased, whereas male, BMI, ST‐segment elevation MI, current smoking, LVEF, hemoglobin, serum levels of total cholesterol, triglyceride, low‐density lipoprotein cholesterol, PCI at index hospitalization, and prescription rate of each discharge medication significantly decreased as the RDW levels increased. Patients with previous CHD and hypertension were more frequently observed in patients with the highest RDW quartiles. A graded relationship between RDW levels and 12‐month MACEs was observed—9.9% in quartile 1, 23.6% in quartile 2, 25.9% in quartile 3, and 40.6% in quartile 4, respectively (P for trend < 0.001).
The 12‐months MACEs increased as the quartiles of RDW increased (Table 3). The HRs of 12‐month MACEs from the lowest (referent) to the highest tertile were as follows: 1, 3.03 (95% CI: 1.82‐5.05), 3.07 (95% CI: 1.86‐5.08), and 5.22 (95% CI: 3.24‐8.41), respectively, in crude analysis; 1, 2.82 (95% CI: 1.69‐4.69), 2.86 (95% CI: 1.73‐4.74), and 4.31 (95% CI: 2.66‐6.96), respectively, in age‐ and sex‐adjusted models; and 1, 2.58 (95% CI: 1.55‐4.30), 2.49 (95% CI: 1.51‐4.14), and 2.88 (95% CI: 1.74‐4.75), respectively, in an age‐, sex‐, and hemoglobin‐adjusted models. In fully adjusted models, the HRs of 12‐month MACEs from the lowest (referent) to the highest quartile were as follows: 1, 4.24 (95% CI: 1.41‐12.75), 4.36 (95% CI: 1.47‐12.91), and 6.18 (95% CI: 2.10‐18.21), respectively.
Table 3.
Multivariate Analysis for 12‐Month Major Adverse Cardiac Events According to Red Cell Distribution Width Quartiles
| Quartile 1 [n = 445] | Quartile 2 [n = 364] | Quartile 3 [n = 400] | Quartile 4 [n = 387] | |
|---|---|---|---|---|
| Crude | 1 (reference) | 3.03 (1.82‐5.05)a | 3.07 (1.86‐5.08)a | 5.22 (3.24‐8.41)a |
| Model 1b | 1 (reference) | 2.82 (1.69‐4.69)a | 2.86 (1.73‐4.74)a | 4.31 (2.66‐6.96)a |
| Model 2c | 1 (reference) | 2.58 (1.55‐4.30)a | 2.49 (1.51‐4.14)a | 2.88 (1.74‐4.75)a |
| Model 3d | 1 (reference) | 4.24 (1.41‐12.75)a | 4.36 (1.47‐12.91)a | 6.18 (2.10‐18.21)a |
Data are presented as hazard ratios (95% confidence intervals).
P < 0.05.
Adjusted for sex and age.
Adjusted for sex, age, and hemoglobin.
Adjusted for sex, age, hemoglobin, body mass index, prior coronary heart disease, hypertension, diabetes mellitus, serum creatinine, total cholesterol, percutaneous coronary intervention, antiplatelet agents, β‐blockers, and angiotensin‐converting enzyme inhibitors/angiotensin II type 1 receptor blockers.
Reclassification of patients with or without 12‐month MACEs at the time of follow‐up is presented in Table 4. The addition of hemoglobin to established risk factors yielded an NRI of 0.167 (P = 0.158) and 0.0080 of IDI (P = 0.200), but these were not statistically significant compared to established risk factors. The addition of RDW to established risk factors significantly improved the reclassification (0.321; P = 0.007) and the integrated discrimination (0.0126; P = 0.045) of subjects compared to established risk factors. The addition of RDW to established risk factors and hemoglobin also significantly improved the reclassification (0.297; P = 0.012) and the integrated discrimination (0.0143; P = 0.042) of subjects compared to established risk factors.
Table 4.
Discrimination of Multivariate Logistic Regression Models in Predicting 12‐Month Major Adverse Cardiac Events
| Models | Discrimination | ||||
|---|---|---|---|---|---|
| Harrell's C Index | Net Reclassification Improvement | P Value | Integrated Discrimination Improvement | P Value | |
| Established risk factors | 0.820 | Reference | Reference | ||
| Established risk factors + Hb | 0.824 | 0.167 | 0.158 | 0.0080 | 0.200 |
| Established risk factors + RDW | 0.830 | 0.321 | 0.007 | 0.0126 | 0.045 |
| Established risk factors + Hb + RDW | 0.831 | 0.297 | 0.012 | 0.0143 | 0.042 |
Abbreviations: Hb, hemoglobin; RDW, red cell distribution width. The net reclassification improvement was defined as (Pimproved_prediction_among_patients with MACE + Pimproved_prediction_among_patients without MACE) − (Pworsened_prediction_among_patients with MACE + Pworsened_prediction_among_patients without MACE), where P = proportion of patients. The integrated discrimination improvement was defined as (Σi MACE (Pnew(i) ‐ Pold(i))/n [patients with MACE]) ‐ (Σj non‐MACE (Pnew(j) ‐Pold(j))/n [patients without MACE]), where P = predicted probability of major adverse cardiac events. Established risk factors defined as sex, age >65 years, body mass index, prior coronary heart disease, hypertension, diabetes mellitus, serum creatinine, total cholesterol, percutaneous coronary intervention, antiplatelet agents, β‐blockers, and angiotensin‐converting enzyme inhibitors/angiotensin II type 1 receptor blockers use.
Discussion
In the present study, the main findings were that baseline RDW level at admission was an independent prognostic factor for 12‐month MACEs in patients with AMI. Moreover, RDW had a predictive value incremental to the combination of hemoglobin and established risk factors. There was a graded positive independent association between RDW level and the risk of 12‐month MACEs when RDW levels were stratified into 4 groups.
Increased levels of RDW have been associated with age as well as pathologic conditions including ineffective red cell production, increased red cell destruction, and during or after blood transfusion.14, 15, 16 Recently, higher levels of RDW have been shown to be a strong independent predictor of cardiovascular mortality in patients with heart failure.5 Higher levels of RDW also had a graded independent association with the risk of all‐cause death, the development of new heart failure, and coronary events in patients with AMI or prior MI who had an absence of clinical evidence of heart failure at baseline.6, 7, 8
Although higher RDW is independently associated with adverse cardiovascular outcomes after AMI, the mechanism is not clearly understood. Possible mechanisms for the modest association of RDW and cardiovascular outcome after AMI are suggested. First, increased RDW may reflect some common conditions such as nutritional deficiencies and influence of comorbidities. In part, RDW may be a simple marker for poor health, reflecting comorbid conditions and/or malnutrition, although we did not directly assess nutritional status.17, 18 Measurements of nutritional status are complex and unreliable for some micronutrients. Because of the association between RDW and nutritional factors, one might consider RDW as a biomarker for nutritional status of vitamin B12, folic acid, and iron, which play an important role in oxidative stress defense mechanisms. It has been known that oxidative stress is associated with atherosclerosis and the risk of cardiovascular outcomes.19, 20, 21 In the present study, patients with 12‐month MACEs were more likely to be older and had much lower BMI, hemoglobin, serum levels of total cholesterol, triglycerides, and low‐density lipoprotein cholesterol. Interestingly, RDW increases with age, and BMI, hemoglobin, serum levels of total cholesterol, triglycerides, and low‐density lipoprotein cholesterol decreased as the RDW levels increased. RDW might have a clinical relevance as an easy and inexpensive surrogate marker for nutritional status associated with poor prognosis.
Second, RDW also may be related to other known markers of prognosis in CHD such as underlying inflammatory stress. Inflammatory stress has been shown to influence bone marrow function, which leads to inadequate production of erythropoietin through inhibition of erythropoietin‐induced erythrocyte maturation.22, 23 The infarction‐related inflammatory response with excess cytokine production may suppress erythropoiesis and impair iron metabolism.24, 25 A low hemoglobin level is a risk factor for a worse outcome in patients with CHD after AMI and PCI, and is associated with an increase in RDW.26, 27 In the present study, however, higher levels of RDW were associated with the risk of death and nonfatal MI even after adjustment for hemoglobin in patients with AMI. Moreover, RDW levels provided increment predictive value to the combination of hemoglobin and established risk factors for predicting 12‐month MACEs. These findings are noteworthy in that RDW incurs no additional costs as part of the complete blood count in contrast to other expensive novel markers.28
Our study has some limitations that should be considered. First, the retrospective analysis of data collected prospectively is major limitation of this study. Therefore, we cannot completely exclude the possibility of residual confounding factors, and our results should only be regarded as hypothesis generating. Second, we adjusted the RDW for all known relevant factors but not for the nutrients (such as iron, folate, and vitamin B12), because these data were unavailable. Given these limitations, the present findings are consistent with previous studies.
Conclusion
We identified that RDW was an independent predictor of death and nonfatal MI even after adjustment for hemoglobin, and had a predictive value incremental to the combination of hemoglobin and established risk factors in patients with AMI. Further studies are required to confirm the association between RDW and cardiovascular outcomes in patients with AMI.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Wu WC, Rathore SS, Wang Y, et al. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230–1236. [DOI] [PubMed] [Google Scholar]
- 2. Lee PC, Kini AS, Ahsan C, et al. Anemia is an independent predictor of mortality after percutaneous coronary intervention. J Am Coll Cardiol. 2004;44:541–546. [DOI] [PubMed] [Google Scholar]
- 3. Reinecke H, Trey T, Wellmann J, et al. Haemoglobin‐related mortality in patients undergoing percutaneous coronary interventions. Eur Heart J. 2003;24:2142–2150. [DOI] [PubMed] [Google Scholar]
- 4. Choi DJ, Han S, Jeon ES, et al.; KorHF Registry. Characteristics, outcomes and predictors of long‐term mortality for patients hospitalized for acute heart failure: a report from the Korean Heart Failure Registry. Korean Circ J. 2011;41:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Felker GM, Allen LA, Pocock SJ, et al.; CHARM Investigators. Red cell distribution width as a novel prognostic marker in heart failure: data from CHARM Program and the Duke Databank for Cardiovascular Disease. J Am Coll Cardiol. 2007;50:40–47. [DOI] [PubMed] [Google Scholar]
- 6. Dabbah S, Hammerman H, Markiewicz W, et al. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am J Cardiol. 2010;105:312–317. [DOI] [PubMed] [Google Scholar]
- 7. Nabais S, Losa N, Gaspar A, et al. Association between red blood cell distribution width and outcomes at six months in patients with acute coronary syndromes. Rev Port Cardiol. 2009;28:905–924. [PubMed] [Google Scholar]
- 8. Tonelli M, Sacks F, Arnold M, et al.; for the Cholesterol and Recurrent Events (CARE) Trial Investigators. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117:163–168. [DOI] [PubMed] [Google Scholar]
- 9. Lee JH, Park HS, Chae SC, et al.; Korea Acute Myocardial Infarction Registry Investigators. Predictors of six‐month major adverse cardiac events in 30‐day survivors after acute myocardial infarction (from the Korea Acute Myocardial Infarction Registry). Am J Cardiol. 2009;104:182–189. [DOI] [PubMed] [Google Scholar]
- 10. The Joint European Society of Cardiology/American College of Cardiology Committee . Myocardial infarction redefined—a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J. 2000;21:1502–1513. [DOI] [PubMed] [Google Scholar]
- 11. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 12. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated ROC‐curves: a non‐parametric approach. Biometrics. 1988;443:837–845. [PubMed] [Google Scholar]
- 13. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. [DOI] [PubMed] [Google Scholar]
- 14. Brightwell RF, Crawford GP, Cale JB, et al. Ageing and the haematological profiles of an Australian community. Ann Hum Biol. 1998;25:1–10. [DOI] [PubMed] [Google Scholar]
- 15. Roberts GT, El Badawi SB. Red blood cell distribution width index in some hematologic diseases. Am J Clin Pathol. 1985;83:222–226. [DOI] [PubMed] [Google Scholar]
- 16. Fossat C, David M, Harle JR, et al. New parameters in erythrocyte counting. Value of histograms. Arch Pathol Lab Med. 1987;111:1150–1154. [PubMed] [Google Scholar]
- 17. Ozkalemkas F, Ali R, Ozkocaman V, et al. The bone marrow aspirate and biopsy in the diagnosis of unsuspected nonhematologic malignancy: a clinical study of 19 cases. BMC Cancer. 2005;5:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spell DW, Jones DV Jr, Harper WF, et al. The value of a complete blood count in predicting cancer of the colon. Cancer Detect Prevent. 2004;28:37–42. [DOI] [PubMed] [Google Scholar]
- 19. McCord JM. Iron, free radicals, and oxidative injury. Semin Hematol. 1998;35:5–12. [PubMed] [Google Scholar]
- 20. Yeun JY, Kaysen GA. C‐reactive protein, oxidative stress, homocysteine, and troponin as inflammatory and metabolic predictors of atherosclerosis in ESRD. Curr Opin Nephrol Hypertens. 2000;9:621–630. [DOI] [PubMed] [Google Scholar]
- 21. Emdin M, Passino C, Michelassi C, et al. Prognostic value of serum gamma‐glutamyl transferase activity after myocardial infarction. Eur Heart J. 2001;22:1802–1807. [DOI] [PubMed] [Google Scholar]
- 22. Chiari MM, Bagnoli R, De Luca PD, et al. Influence of acute inflammation on iron and nutritional status indexes in older inpatients. J Am Geriatr Soc. 1995;43:767–771. [DOI] [PubMed] [Google Scholar]
- 23. Pierce CN, Larson DF. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion. 2005;20:83–90. [DOI] [PubMed] [Google Scholar]
- 24. Tracey KJ, Wei H, Manogue KR, et al. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988;167:1211–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nemeth E, Rivera S, Gabayan V, et al. IL‐6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mahu JL, Leclercq C, Suquet JP. Usefulness of red cell distribution width in association with biological parameters in an epidemiological survey of iron deficiency in children. Int J Epidemiol. 1990;19:646–654. [DOI] [PubMed] [Google Scholar]
- 27. Felker GM, Adams KF Jr, Gattis WA, et al. Anemia as a risk factor and therapeutic target in heart failure. J Am Coll Cardiol. 2004;44:959–966. [DOI] [PubMed] [Google Scholar]
- 28. Kim HC. Clinical utility of novel biomarkers in the prediction of coronary heart disease. Korean Circ J. 2012;42:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]


