Abstract
Background:
Patients with paroxysmal atrial fibrillation (AF) experience impaired quality of life (QoL) and psychological distress. Catheter ablation of AF can markedly improve QoL. However, the effect of catheter ablation of AF on psychological status is unknown.
Hypothesis:
Depression, anxiety, and QoL improve after catheter ablation in patients with paroxysmal AF.
Methods:
A total of 166 consecutive patients with symptomatic paroxysmal AF were examined. Eighty‐two patients (55 men, mean age 55.9 ± 6.1 y) underwent catheter ablation and 84 patients (58 men, mean age 57.2 ± 5.4 years) received antiarrhythmic drug (AAD) therapy. The Self‐Rating Depression Scale, Self‐Rating Anxiety Scale, and Medical Outcomes Survey 36‐item Short‐Form questionnaires were completed by these patients at baseline, and at 3, 6, 9, and 12 months of follow‐up. Results in the ablation group were compared with those of the AAD group.
Results:
In the ablation group, 42.7% of patients showed symptoms of depression and 37.8% showed symptoms of anxiety, which were similar to those in the AAD group. Both groups similarly displayed reduced physical and mental QoL. Catheter ablation was effective in reducing symptoms of depression and anxiety and improving QoL, and it was superior to AAD therapy (all P < 0.001). Multiple regression analysis demonstrated that catheter ablation, no AF recurrence, avoidance of warfarin use, higher baseline depression and anxiety scores, and lower baseline QoL scores contributed to improvement of depression, anxiety, and QoL, respectively.
Conclusions:
Catheter ablation is more effective for improving depression, anxiety, and QoL in patients with paroxysmal AF compared with AAD therapy. Clin. Cardiol. 2012 doi: 10.1002/clc.22039
This work was supported by grants from the National Science Foundation Council of China (No. 30971239 and No. 81070147) and the Beijing Natural Science Foundation (No. 7101004). The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Atrial fibrillation (AF) is one of the most common arrhythmias requiring treatment.1 It can considerably impair quality of life (QoL) and psychological status of patients.2., 3., 4. Catheter ablation is effective for restoring and maintaining sinus rhythm, and it improves QoL in paroxysmal AF patients.5., 6. However, little is known regarding the effect of catheter ablation on psychological distress, such as depression and anxiety. This study aimed to determine how treatment with catheter ablation or antiarrhythmic drug (AAD) leads to changes in depression, anxiety, and QoL in patients with symptomatic paroxysmal AF over a 12‐month period. We also aimed to identify what patient‐related factors may contribute to these changes.
Methods
Study Population
Between February 2009 and January 2010, a total of 175 consecutive patients with a primary diagnosis of symptomatic paroxysmal AF were prospectively enrolled in our study. Paroxysmal AF was defined as an episode of AF that spontaneously converted to sinus rhythm within 7 days. Exclusion criteria were age <18 years, contraindication to anticoagulation, previous nonpharmacological interventions for AF, New York Heart Association functional class III or IV, myocardial infarction, cardiac surgery or transient ischemic attack/stroke within the previous 6 months, malignancy of any type, and presence of an implanted cardioverter defibrillator. The study was approved by the institutional ethics review committee, and all patients provided written informed consent.
As the study was initiated, the decision to proceed with catheter ablation was based on: (1) the recommendation of a physician when patients had failed to respond to ≥2 AADs of different classes; and (2) patients' own preference. Consequently, 85 patients underwent ablation, constituting the ablation group. Ninety patients received continued treatment with an AAD, constituting the AAD group.
Study Design
Sociodemographic and clinical details were recorded for all study participants after enrollment. A set of research questionnaires examining depression, anxiety, and QoL were completed in the clinic and 12‐lead electrocardiography and 24‐hour ambulatory Holter recordings were employed to monitor rhythm status at baseline and during each scheduled clinical follow‐up (at the end of 3, 6, 9, and 12 months following initial treatment). All participants were followed up by the same physician. Figure 1 shows a flowchart and the treatment strategy of the study.
Figure 1.
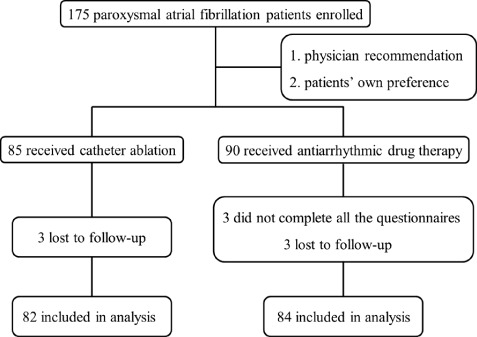
Flowchart of the study protocol.
Catheter Ablation
Prior to ablation, all AADs (except for amiodarone) were discontinued in patients for ≥5 half‐lives. After exclusion of left atrial thrombus by transesophageal echocardiography, low‐molecular‐weight heparin was administered subcutaneously until the ablation procedure day.
The ablation strategies in our center have been reported previously in detail.7., 8. Briefly, 1 or 2 8‐Fr‐long sheaths (SL1; St. Jude Medical, Minnetonka, MN) were introduced into the left atrium using a modified Brockenbrough technique. After transseptal catheterization, left atrial geometry was reconstructed with a 3.5‐mm cool saline‐irrigated ablation catheter (Navi‐Star Thermo‐Cool; Biosense‐Webster Inc., Diamond Bar, CA) guided by an electroanatomic mapping system (CARTO XP, Biosense‐Webster). Circumferential isolation of the right pulmonary vein (PV) antrum (PVA) was performed first, followed by isolation of the left PVA. Radiofrequency ablation was controlled by a temperature setting of 45°C with an energy output at 35 watts and saline irrigation speed at 17 mL/minute until the following criteria for complete lesion formation were met: local potential amplitude was significantly decreased (>80%), local potential duration was markedly widened, or accumulated ablation at the same site lasted for 20 seconds at the posterior wall or for 30 seconds at the anterior wall. The endpoint of circumferential PVA ablation was PV isolation, confirmed by either an ablation catheter or a circular mapping catheter (Lasso; Biosense‐Webster). If a typical atrial flutter had been documented before or during the procedure, the cavotricuspid isthmus was ablated with an endpoint of bidirectional conduction block. After PVA isolation and bidirectional block of the cavotricuspid isthmus, burst atrial pacing from the distal coronary sinus was initiated to determine if any sustained non‐PV foci initiating atrial tachyarrhythmia (>10 minutes) could be induced, and additional mapping‐guided ablations were performed as required. If there were neither contraindications nor intolerance, patients would receive AADs for 2 months after ablation and then discontinue them if no AF recurred. All patients were given warfarin after the ablation procedure, bridged with low‐molecular‐weight heparin. Warfarin was discontinued after 3 months if no AF was detected. After the postprocedural blanking period of 3 months, any symptomatic episodes of documented atrial tachyarrhythmias lasting ≥30 seconds, as well as any episode of asymptomatic atrial tachyarrhythmias lasting ≥10 minutes on 24‐hour ambulatory Holter recordings, were considered as AF recurrence.
Medical Treatment
All patients enrolled in the AAD group were given AADs for sinus‐rhythm control throughout the follow‐up period. The choice of drugs and dosage regimens was at the discretion of the physician based on published guidelines.9 The following AADs, either alone or in combination, were considered clinically appropriate: propafenone, sotalol, and amiodarone. Amiodarone was usually reserved for those who had failed therapy with other drugs or suffered from significant structural heart diseases. The strategy of early pharmacological or electrical cardioversion, followed by prophylactic AADs to maintain sinus rhythm, was employed if AF caused hypotension or worsening of heart failure. In patients with a CHADS2 (congestive heart failure, hypertension, age ≥75 years, diabetes, stroke or transient ischemia attack [doubled]) score ≥2, anticoagulation treatment with warfarin was administered with a target international normalized ratio between 2 and 3.
Assessment of Depression, Anxiety, and Quality of Life
The Self‐Rating Depression Scale (SDS)10 and the Self‐Rating Anxiety Scale (SAS)11 are self‐report questionnaires used to assess symptoms of depression and anxiety, respectively. Each scale consists of 20 items and every participant was asked to rate all the items regarding how they had felt during the past week by 4‐point Likert response formats. The total scores on each scale, ranging from 20 to 80, were further converted into score indices, with a higher score index indicating a greater level of depression or anxiety. A score ≥50 in the SDS or SAS index was considered to indicate symptoms of depression or anxiety. The Chinese versions of the SDS and the SAS have been widely validated and have demonstrated acceptable psychometric properties in previous studies.12., 13.
The Medical Outcomes Survey 36‐item Short‐Form (SF‐36)14 is a reliable generic instrument for general QoL. It measures 8 health domains, including physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health. Scores in each domain are standardized, ranging from 0 to 100. Additionally, a physical component summary (PCS) score and a mental component summary (MCS) score were derived from the 8 domains, with higher scores indicating better physical and mental QoL. The Chinese version of the SF‐36, which has had its reliability and validity established in the Chinese population, was used in the present study.15., 16. Furthermore, normative data for the general population have been previously published.15 Both PCS and MCS had acceptable internal consistency,17 with Cronbach's α coefficient being 0.85 and 0.87, respectively.
Statistical Analysis
All statistical analyses were performed with SPSS 13.0 (SPSS Inc., Chicago, IL). Continuous data are presented as mean ± SD and were compared by unpaired or paired Student t tests. Categorical data were compared by χ 2 tests or Fisher exact tests. Repeated‐measures analysis of variance was used to compare changes in SDS, SAS, PCS, and MCS scores with time between the 2 treatment groups. Univariate linear regression was used to assess how (1) baseline sociodemographic and clinical characteristics, (2) baseline SDS, SAS, and SF‐36 scores, (3) AF recurrence, and (4) use of warfarin during the study period related to changes in psychological status and QoL during 12 months. Multivariable linear stepwise regression models were employed to estimate contributors to changes in depression, anxiety, and QoL scores. Treatment strategy and all other variables with a P value ≤0.05 in univariate regression analyses were entered into the models. All probability values were 2‐sided, and a P value ≤0.05 was considered statistically significant.
Results
Patients
During the initial 6 months, 3 patients in the AAD group were lost to follow‐up and 6 patients, 3 in each group, did not complete all the questionnaires. These 9 patients were excluded from our data analysis. In total, 82 patients in the ablation group and 84 in the AAD group completed the 12‐month follow‐up. Their baseline characteristics are summarized in Table 1. The 2 treatment groups appeared well‐matched with respect to sociodemographic and clinical characteristics. There were no significant differences between patients who did and those who did not complete the study (P = 0.116 − 0.531).
Table 1.
Sociodemographic and Clinical Characteristics of the Patients in the 2 Treatment Groups
| Characteristics | Ablation Group (n = 82) | AAD Group (n = 84) | P Value |
|---|---|---|---|
| Age, y | 55.9 ± 6.1 | 57.2 ± 5.4 | 0.136 |
| Male sex | 55 (67.1) | 58 (69.1) | 0.785 |
| Partner status (married/partner) | 78 (95.1) | 78 (92.9) | 0.388 |
| Employment status (employed) | 38 (46.3) | 41 (48.8) | 0.750 |
| History of AF (mo) | 90.1 ± 90.1 | 86.9 ± 90.8 | 0.821 |
| Maximum AF duration (min) | 13.5 ± 19.3 | 13.1 ± 19.2 | 0.893 |
| Failed AAD (n) | 2.2 ± 0.9 | 2.0 ± 0.7 | 0.094 |
| LA diameter (mm) | 39.0 ± 5.9 | 38.6 ± 6.2 | 0.733 |
| LVEF (%) | 59.6 ± 7.5 | 58.9 ± 8.3 | 0.573 |
| Hypertension | 53 (64.6) | 51 (60.7) | 0.602 |
| DM | 11 (13.4) | 16 (19.1) | 0.326 |
| CAD | 8 (9.8) | 10 (11.9) | 0.656 |
| Prior stroke/TIA | 7 (8.5) | 7 (8.3) | 0.962 |
| Dilated cardiomyopathy | 2 (2.4) | 4 (4.8) | 0.352 |
| Valvular disease | 3 (3.7) | 5 (6.0) | 0.373 |
Abbreviations: AAD, antiarrhythmic drug; AF, atrial fibrillation; CAD, coronary artery disease; DM, diabetes mellitus; LA, left atrial; LVEF, left ventricular ejection fraction; TIA, transient ischemic attack. Values are presented as mean ± SD or n (%).
Treatment of Atrial Fibrillation
For the ablation group, the mean procedural time was 159 ± 31 minutes, and the mean fluoroscopy and ablation times were 20 ± 6 minutes and 56 ± 10 minutes, respectively. All PVAs were targeted, and the success rate of PVA isolation was 100%. At discharge, all patients were in sinus rhythm. In the 29 patients with recurrent AF, 10 patients received a repeated ablation procedure. No patients experienced severe complications, such as cardiac tamponade, PV stenosis, or thromboembolic events. In the AAD group, 51 patients (60.7%) were on amiodarone, 32 (38.1%) were on propafenone, 16 (19.0%) were on sotalol, and 14 (16.7%) were on ≥2 AADs.
At the end of the follow‐up, 59 (72.0%) patients treated with ablation were in sinus rhythm, whereas only 17 (20.2%) in the AAD group were in sinus rhythm (P < 0.001). None of the patients in the ablation group and 3 (3.6%) in the AAD group (P = 0.246) had thromboembolic events.
Depression, Anxiety, and Quality of Life at Baseline
Prior to treatment, patients in the ablation group and the AAD group displayed similar levels of depression, anxiety, and QoL (Table 2). Depression and anxiety were highly coexistent, with 71% of the patients in the ablation group and 72.6% in the AAD group reporting symptoms of anxiety and depression simultaneously. Lower PCS and MCS scores were observed in both groups compared with the age‐matched general population (all P < 0.05).
Table 2.
Baseline Psychological Characteristics of the Patients in the 2 Treatment Groups
| Variables | Ablation Group (n = 82) | AAD Group (n = 84) | P Value |
|---|---|---|---|
| Mean SDS score | 49.4 ± 10.6 | 48.7 ± 10.8 | 0.712 |
| SDS score ≥50 | 35 (42.7) | 32 (38.1) | 0.547 |
| Mean SAS score | 48.6 ± 11.1 | 47.7 ± 10.3 | 0.590 |
| SAS score ≥50 | 31 (37.8) | 29 (34.5) | 0.660 |
| Mean PCS score | 42.6 ± 6.4 | 43.0 ± 6.2 | 0.678 |
| Mean MCS score | 42.2 ± 7.2 | 43.2 ± 7.2 | 0.360 |
Abbreviations: AAD, antiarrhythmic drug; MSC, mental component summary; PCS, physical component summary; SAS, Self‐Rating Anxiety Scale; SDS, Self‐Rating Depression Scale.
Values are presented as mean ± SD or n (%).
Changes in Depression, Anxiety, and Quality of Life During Follow‐up
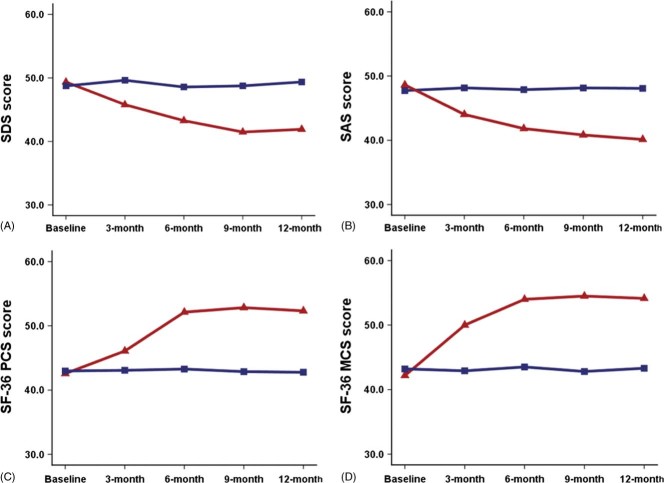
Twelve months after ablation, mean SDS and SAS scores were significantly decreased compared with baseline scores (both P < 0.001). On the other hand, changes in mean SDS (P = 0.389) and SAS scores (P = 0.395) were not significant in the AAD group after 12 months of treatment. This finding demonstrated that catheter ablation achieved a better intervention effect than AAD therapy (F = 31.56, P < 0.001 for SDS; F = 35.60, P < 0.001 for SAS) (Figures 2A and 2B).
Figure 2.
Changes in the SDS, SAS, and SF‐36 PCS and MCS scores over time in the ablation group (red) and AAD group (blue). Abbreviations: AAD, antiarrhythmic drug; MCS, mental component summary; PCS, physical component summary; SAS, Self‐Rating Anxiety Scale; SDS, Self‐Rating Depression Scale; SF‐36, Medical Outcomes Survey 36‐item Short‐Form.
In the ablation group, 26.8% of the patients reported a significant reduction in symptoms of depression (P < 0.001) and 21.4% had a significant reduction in symptoms of anxiety (P = 0.002) at the 12‐month follow‐up. The AAD group demonstrated no significant reduction in symptoms of depression or anxiety (P = 0.530 and P = 0.206, respectively).
Notably, the PCS and MCS scores were increased significantly more in the ablation group than those in the AAD group (PCS within‐subject increase: 10.3 vs 0.4; MCS within‐subject increase: 12.4 vs 0.7, respectively; both P < 0.001). This also demonstrated that catheter ablation achieved a better intervention effect than AAD therapy. The greatest improvement in the physical QoL (42.6 to 52.1, P < 0.001) and mental QoL (42.2 to 54.0, P < 0.001) was reported by patients in the ablation group between baseline and 6 months of follow‐up, with PCS (P = 0.191) and MCS (P = 0.569) scores reaching normal levels and remaining at these levels to the end of the follow‐up period. In contrast, no significant changes in PCS and MCS scores were observed in the AAD group throughout the follow‐up period (P = 0.850 and P = 0.942, respectively) (Figures 2C and 2D).
Contributors to Improvement of Depression, Anxiety, and Quality of Life
Among the 29 patients with recurrent AF in the ablation group, 16 patients (19.5%) were on warfarin. A total of 41 patients (48.8%) were administered warfarin in the AAD group. At the 12‐month follow‐up, those who remained on warfarin had less reduction in SDS (within‐subject reduction: −1.0 vs 7.7; P = 0.002) and SAS scores (within‐subject reduction: −1.1 vs 11.9; P < 0.001), and less increase in PCS (within‐subject increase: 0.5 vs 12.4; P < 0.001) and MCS scores (within‐subject increase: 1.2 vs 14.5; P < 0.001) than those who did not remain on warfarin. Furthermore, patients who did not suffer from AF recurrence had a greater reduction in SDS (within‐subject reduction: 7.47 vs −0.11; P < 0.001) and SAS scores (within‐subject reduction: 10.16 vs −2.1; P < 0.001), and a higher increase in PCS (within‐subject reduction: 11.37 vs −0.88; P < 0.001) and MCS scores (within‐subject reduction: 13.15 vs −0.12; P < 0.001) than those who did suffer from AF recurrence.
As expected, catheter ablation was associated with a reduction in depression and anxiety and improvement in physical and mental QoL, independent of baseline sociodemographic and clinical variables, with the same association for no AF recurrence and avoidance of warfarin use at follow‐up (Table 3). In addition, baseline SDS (β = 0.153), SAS (β = 0.236), PCS (β = −0.191), and MSC (β = −0.217) scores were related to improvement of depression, anxiety, and physical and mental QoL, respectively.
Table 3.
Multivariable Linear Regression Analyses for Changes in SDS, SAS, PCS, and MCS Scores
| SDS | SAS | PCS | MCS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | t | P Value | β | t | P Value | β | t | P | β | t | P Value | |
| Catheter ablation | −0.375 | −3.405 | 0.001 | −0.420 | −7.217 | <0.001 | 0.324 | 4.541 | <0.001 | 0.207 | 3.168 | 0.006 |
| No AF recurrence | −0.187 | −2.680 | 0.021 | −0.178 | −2.751 | 0.013 | 0.332 | 8.116 | <0.001 | 0.468 | 9.205 | <0.001 |
| Avoidance of warfarin use | −0.290 | −3.0 | 0.003 | −0.131 | −2.488 | 0.024 | 0.295 | 3.190 | 0.002 | 0.163 | 2.332 | 0.018 |
Abbreviations: AF, atrial fibrillation; MSC, mental component summary; PCS, physical component summary; SAS, Self‐Rating Anxiety Scale; SDS, Self‐Rating Depression Scale.
Discussion
Our study demonstrated that catheter ablation resulted in a sustainable reduction in psychological distress and an improvement in QoL for patients with symptomatic paroxysmal AF. Catheter ablation was a better treatment strategy than AAD therapy. Contributors to the improvement of depression, anxiety, and QoL included catheter ablation, avoidance of warfarin use, initial higher levels of depression and anxiety, and lower QoL.
Psychological distress in patients with coronary heart disease18., 19., 20. and heart failure21., 22. has been studied extensively. To date, however, little is known regarding the extent of psychological distress in patients with AF.3., 23. The present study revealed that high levels of self‐reported symptoms of depression and anxiety and compromised QoL were present in individuals with symptomatic paroxysmal AF at baseline, which is consistent with previous studies.3., 23. Patients with paroxysmal AF may often experience an impaired sense of well‐being accompanied by psychological distress due to arrhythmia symptoms.24 It is conceivable that arrhythmia symptoms could increase the perception of AF burden and the likelihood that patients will suffer from affective disorders and disengage from daily physical activities. This may further evoke low mood and perpetuate a vicious circle of psychological distress, leading to poor mental and physical QoL. The current study found that levels of depression and anxiety were continuously reduced after the ablation procedure, with approximately one‐fourth of the individuals in the ablation group reporting a dramatic relief in symptoms of depression or anxiety at the end of the study period. Moreover, PCS and MCS scores observed at the 6‐month follow‐up reached normal levels and remained at these levels during the next 6 months in the ablation group, consistent with observations reported by Pappone et al25 and Purerfellner et al.26 In contrast, no changes in the levels of depression and anxiety or QoL were observed in patients treated with AADs in our study. This outcome indicates that catheter ablation is an effective treatment strategy for AF patients in terms of reducing negative affectivity and enhancing QoL.
Many factors may have contributed to the better effectiveness of catheter ablation than AAD therapy. First, patients with paroxysmal AF could have suffered from arrhythmia symptoms that were so severe and disruptive that better rhythm control resulting from ablation was perceived as a more remarkable recovery than what had been achieved from the original medical treatment, with reduced levels of psychological distress and better QoL. Second, successful AF ablation enabled patients to experience such a great reduction in symptoms that they could become physically more active and enjoy their lives more fully, and subsequently regain a positive affective disposition. Although some asymptomatic recurrent atrial arrhythmias after the ablation procedure could not be monitored, adverse effects on depression and anxiety might be negligible. Third, successful AF ablation freed patients from AAD therapy and, therefore, helped avoid adverse effects from long‐term AAD use. In addition, successful ablation enabled discontinuation of oral anticoagulation therapy, although this remains controversial.27., 28., 29., 30. In general, patients treated medically need concomitant long‐term anticoagulation with warfarin, which requires frequent international normalized ratio measurements and causes concern regarding an increased risk of bleeding, both of which may lead to negative affectivity and limit QoL improvement in patients.31
There are a few limitations in our study. The major limitation is the nonrandomized assignment of patients to different treatment strategies. The results could be affected by placebo effects. It is important to note that this study was conducted in subjects meeting strict inclusion criteria from a single center; therefore, the findings of this study may not apply to all AF populations because of possible geographic bias and high patient selection. The second limitation is that our study had a relatively small sample size. However, our finding of better effectiveness of catheter ablation compared with AADs was consistent and significant. The third limitation is that the follow‐up period was relatively short. Longer follow‐up periods might reveal additional changes in psychological factors. Interestingly, Wokhlu et al31 reported that AF ablation produced sustained QoL improvement after 2 years, even in patients with recurrence.
Conclusion
Catheter ablation more effectively improves depression, anxiety, and QoL than AAD therapy. Therefore, patients with paroxysmal AF have broader health benefits than merely alleviation of clinical symptoms during a 12‐month follow‐up.
Acknowledgements
The authors sincerely thank Lin Li for revising the manuscript.
References
- 1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 2. Dorian P, Jung W, Newman D, et al. The impairment of health‐related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol. 2000;36:1303–1309. [DOI] [PubMed] [Google Scholar]
- 3. Thrall G, Lip GY, Carroll D, et al. Depression, anxiety, and quality of life in patients with atrial fibrillation. Chest. 2007;132:1259–1264. [DOI] [PubMed] [Google Scholar]
- 4. Gehi AK, Sears S, Goli N, et al. Psychopathology and symptoms of atrial fibrillation: implications for therapy. J Cardiovasc Electrophysiol. 2012;23:473–478. [DOI] [PubMed] [Google Scholar]
- 5. Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–340. [DOI] [PubMed] [Google Scholar]
- 6. Dorian P, Paquette M, Newman D, et al. Quality of life improves with treatment in the Canadian Trial of Atrial Fibrillation. Am Heart J. 2002;143:984–990. [DOI] [PubMed] [Google Scholar]
- 7. Sang C, Jiang C, Dong J, et al. A new method to evaluate linear block at the left atrial roof: is it reliable without pacing? J Cardiovasc Electrophysiol. 2010;21:741–746. [DOI] [PubMed] [Google Scholar]
- 8. Dong J, Liu X, Long D, et al. Single‐catheter technique for pulmonary vein antrum isolation: is it sufficient to identify and close the residual gaps without a circular mapping catheter? J Cardiovasc Electrophysiol. 2009;20:273–279. [DOI] [PubMed] [Google Scholar]
- 9. Fuster V, Rydén LE, Cannom DS, et al. 2011. ACCF/AHA/HRS Focused Updates Incorporated Into the ACC/AHA/ESC 2006 Guidelines for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e269–e367. [DOI] [PubMed] [Google Scholar]
- 10. Zung WW. A self‐rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. [DOI] [PubMed] [Google Scholar]
- 11. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371–379. [DOI] [PubMed] [Google Scholar]
- 12. Liu XC, Oda S, Peng X, et al. Life events and anxiety in Chinese medical students. Soc Psychiatry Psychiatr Epidemiol. 1997;32:63–67. [DOI] [PubMed] [Google Scholar]
- 13. Fang Y, Yuan C, Xu Y, et al. Comparisons of the efficacy and tolerability of extended‐release venlafaxine, mirtazapine, and paroxetine in treatment‐resistant depression: a double‐blind, randomized pilot study in a Chinese population. J Clin Psychopharmacol. 2010;30:357–364. [DOI] [PubMed] [Google Scholar]
- 14. McHorney CA Jr, Ware JE, Raczek AE. The MOS 36‐Item Short‐Form Health Survey (SF‐36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. [DOI] [PubMed] [Google Scholar]
- 15. Wang R, Wu C, Zhao Y, et al. Health related quality of life measured by SF‐36: a population‐based study in Shanghai, China. BMC Public Health. 2008;8:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li L, Wang HM, Shen Y. Chinese SF‐36 Health Survey: translation, cultural adaptation, validation, and normalisation. J Epidemiol Community Health. 2003;57:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lam CL, Tse EY, Gandek B, et al. The SF‐36 summary scales were valid, reliable, and equivalent in a Chinese population. J Clin Epidemiol. 2005;58:815–822. [DOI] [PubMed] [Google Scholar]
- 18. Huffman JC, Smith FA, Blais MA, et al. Recognition and treatment of depression and anxiety in patients with acute myocardial infarction. Am J Cardiol. 2006;98:319–324. [DOI] [PubMed] [Google Scholar]
- 19. Shen BJ, Avivi YE, Todaro JF, et al. Anxiety characteristics independently and prospectively predict myocardial infarction in men the unique contribution of anxiety among psychologic factors. J Am Coll Cardiol. 2008;51:113–119. [DOI] [PubMed] [Google Scholar]
- 20. Vaccarino V, Johnson BD, Sheps DS, et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute–sponsored WISE study. J Am Coll Cardiol. 2007;50:2044–2050. [DOI] [PubMed] [Google Scholar]
- 21. Jiang W, Kuchibhatla M, Cuffe MS, et al. Prognostic value of anxiety and depression in patients with chronic heart failure. Circulation. 2004;110:3452–3456. [DOI] [PubMed] [Google Scholar]
- 22. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure a meta‐analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. [DOI] [PubMed] [Google Scholar]
- 23. Lane DA, Langman CM, Lip GY, et al. Illness perceptions, affective response, and health‐related quality of life in patients with atrial fibrillation. J Psychosom Res. 2009;66:203–210. [DOI] [PubMed] [Google Scholar]
- 24. Guédon‐Moreau L, Capucci A, Denjoy I, et al. Impact of the control of symptomatic paroxysmal atrial fibrillation on health‐related quality of life. Europace. 2010;12:634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pappone C, Rosanio S, Augello G, et al. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long‐term study. J Am Coll Cardiol. 2003;42:185–197. [DOI] [PubMed] [Google Scholar]
- 26. Pürerfellner H, Martinek M, Aichinger J, et al. Quality of life restored to normal in patients with atrial fibrillation after pulmonary vein ostial isolation. Am Heart J. 2004;148:318–325. [DOI] [PubMed] [Google Scholar]
- 27. Themistoclakis S, Corrado A, Marchlinski FE, et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol. 2010;55: 735–743. [DOI] [PubMed] [Google Scholar]
- 28. Oral H, Chugh A, Ozaydin M, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation. 2006;114:759–765. [DOI] [PubMed] [Google Scholar]
- 29. Nademanee K, Schwab MC, Kosar EM, et al. Clinical outcomes of catheter substrate ablation for high‐risk patients with atrial fibrillation. J Am Coll Cardiol. 2008;51:843–849. [DOI] [PubMed] [Google Scholar]
- 30. Hussein AA, Saliba WI, Martin DO, et al. Natural History and long‐term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:271–278. [DOI] [PubMed] [Google Scholar]
- 31. Wokhlu A, Monahan KH, Hodge DO, et al. Long‐term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol. 2010;55:2308–2316. [DOI] [PubMed] [Google Scholar]