Abstract
Background
The effect of body fat distribution on left ventricular (LV) mass and geometry has been recently recognized. However, data regarding circulating inflammatory markers in relation to regional visceral fat deposits, which are metabolically active tissues that can impact cardiac structural remodeling, remain sparse.
Hypothesis
We hypothesized that obesity has adverse effects on cardiac function and structure.
Methods
We consecutively studied 1071 asymptomatic subjects (age 49.5 ± 10.5 years, 39.4% female) free from significant valvular disorders, chronic lung disease, or renal disease. Echocardiography‐defined cardiac structures and LV geometries including LV mass, mass‐to‐volume ratio, and fractional shortening were all determined. Body fat composition (Tanita‐305 Body‐Fat Analyzer; Tanita Corp., Tokyo, Japan) was obtained and calculated. Multivariate regression models from various models were used to represent the independent association between body fat and echo‐derived ventricular mass and geometries.
Results
In multivariable analysis, increasing body fat was significantly related to increase in left atrial (LA) and LV diameter, posterior wall thickness, relative wall thickness (RWT), LV mass, mass‐to‐volume ratio, and decreased midwall fractional shortening with or without stress correction (all P < 0.001). When LV mass and severity of mitral regurgitation was further added, the independent association between increased body fat composition and larger LA diameter remained significant (β coefficient = 0.37, P < 0.001). Elevated high‐sensitivity C‐reactive protein (Hs‐CRP) level was associated with larger LA diameter, increased RWT, and worsened midwall mechanics in the female gender (all interaction P < 0.05).
Conclusions
Accumulated body fat seemed to be related to worse ventricular midwall contractility and atrial remodeling, particularly in the female gender, with high systemic inflammation. These gender and Hs‐CRP–specific modification effects may potentiate the pathological mechanisms involved in heart failure with preserved ejection fraction.
Introduction
In recent years, there is increasing recognition of the impact of body fat composition and distribution on the relationship between obesity and cardiovascular disease.1 A large body of evidence documents that abdominal fat accumulation, as assessed by anthropometric indicators such as waist circumference or waist‐hip ratio (WHR), is positively associated with occurrence of cardiovascular events.2 Total body fat burden assessed by conventional bioelectrical impedance analysis (BIA) has the advantage of lower cost and clinical ease of use. However, the accuracy of such measures may not reflect real adipose tissue burden and could be confounded by several clinical variables.3 Furthermore, it provides no information on fat distribution at specific sites, therefore failing to describe the anatomy of visceral adipose depots.4
Through direct measurement of intra‐abdominal fat, specifically visceral adipose tissue surrounding vital organs, investigators have demonstrated the link between its activity and cardiovascular diseases.5, 6 Although visceral fat is biologically active and acts as a key mediator of various metabolic derangements and systemic inflammation7 with cytokine effects, the exact mechanisms that result in adverse cardiovascular events are not fully understood.8, 9
Methods
Subjects
In this retrospective analysis, we consecutively studied 1071 subjects who took part in a primary cardiovascular health survey in a tertiary medical center in Taipei, Taiwan, from 2006 to 2008. A detailed review of medical history through a structured questionnaire along with a physical examination was performed on all subjects. Patients with chronic lung disorders, atrial fibrillation, history of pacemaker implantation, significant valvular heart diseases, or end‐stage renal disease were excluded. The presence of coronary artery diseases (CAD) was defined as a history of previous myocardial infarction, previous angioplasty, or more than 50% luminal narrowing on coronary angiography. Hypertension history (HTN) was defined as a history of systolic blood pressure above 140 mm Hg, diastolic blood pressure above 90 mm Hg, or previously diagnosed HTN under medication control. Diabetes (DM) was defined as a fasting glucose level above 126 mg/dL or previously diagnosed DM under medication control. Hyperlipidemia was defined as a history and/or use of lipid‐lowering drugs such as statins or fibrates on a daily basis.
Anthropometric Measurements
All baseline characteristics and information regarding anthropometric measures were collected. Standardized sphygmomanometer cuff‐defined resting blood pressures were measured at rest by medical staff blinded to the other test results. Body fat percentage was assessed by utilizing BIA from foot‐to‐foot using the Tanita‐305 Body‐Fat Analyzer (Tanita Corp., Tokyo, Japan), which provides a printout estimate of impedance and calculated body fat percentage. Measurements were performed in the standing position, through 2 footpad electrodes in contact with soles and heels on both feet. A 0.8‐mA current with a 50‐kHz frequency was applied via source electrodes on both soles, and the voltage drop compared with heel electrodes.
Echocardiographic Assessment
Each subject underwent 2‐dimensional and M‐mode transthoracic echocardiogram using a Hewlett‐Packard Sonos 5500 series instrument (Hewlett‐Packard Co., Palo Alto, CA) equipped with a 2.5 to 4.5 MHz transducer. Left ventricular (LV) end‐diastolic and end‐systolic volumes were calculated by the z‐derived method,10 which allows accurate quantification of LV volume and mass even in subjects with dilated cardiac chambers. LV geometry, including concentric hypertrophy, defined as relative wall thickness (RWT) ≥0.42, and LV mass index ≥115 gm/m2 for males and ≥95 gm/m2 for females; eccentric hypertrophy, defined as the same LV mass index criteria with RWT <0.42, were assessed. For those subjects with RWT ≥0.42 and normal LV mass index were classified as concentric remodeling.
Endocardial and midwall fractional shortening was computed by a previously described formula.11 Circumferential wall stress was also derived from end‐systolic pressure representing mean arterial pressure as in the previously described equation.12 Both midwall fractional shortening and circumferential wall stress were used to estimate stress‐corrected LV afterload. Any patient with significant valvular heart disease or pulmonary hypertension (defined as estimated systolic pulmonary arterial pressure more than 50 mm Hg) was excluded. Mitral regurgitation (MR) was assessed by mapping jet expansion in the left atrium in 4‐chamber views during the systole phase. MR was considered mild when the regurgitant jet area occupied <20% of the left atrial area, moderate when it occupied 20% to 40%, and severe when it occupied more than 40% of the left atrial area.
Statistical Analysis
Continuous data were shown as mean and standard deviation, with categorical data expressed as the frequency and proportion of occurrence in all subjects. The association between LV structure, function, and body fat composition were presented with a univariable regression model with the standardized coefficient between 2 continuous variables reported. Multivariable regression models were used to determine the significance of clinical covariate‐adjusted relations between body fat composition in relation to various LV geometric parameters, stratified by gender, and systemic inflammation status in terms of circulating high‐sensitivity C‐reactive protein (Hs‐CRP) level (≥0.3 mg/dL). Because the LV geometric remodeling may be influenced by age, gender, body mass index (BMI), blood pressure, renal function, and hypertension, an analysis of covariance was also conducted to adjust these relevant covariates for comparison of mean values among the 3 groups.
All data were analyzed using a STATA 8.2 commercial software package (Stata Corp., College Station, TX). The significance of P level (α value) for all analysis was 2‐sided, with a value <0.05 considered statistically significant.
Results
Baseline Demographic and Anthropometric Data
Out of 1071 subjects, 1063 (age 49.5 ± 10.5, 39.4% female) were selected for the final analysis. Among those excluded, 4 had atrial fibrillation, 1 had history of pacemaker implantation, and 3 had significant valvular heart disease (severe mitral regurgitation: 2; severe aortic stenosis: 1). Baseline demographic and anthropometric characteristics and metabolic parameters stratified by fat mass are summarized in Table 1. We observed that both systolic and diastolic blood pressures were significantly related to increasing body fat mass (both trend P < 0.001). For anthropometric parameters, increasing body fat tertiles were significantly associated with graded increases in BMI, body weight, waist circumference, and WHR (all trend P < 0.001). With each increasing body fat tertile, there were stepwise increases in fasting glucose, HbA1c, all lipid profiles, and higher serum Hs‐CRP level (all trend P < 0.001). Only estimated glomerular filtration rate was not significantly related (P = 0.83). Also, subjects in larger body fat tertiles were more likely to have hypertension, hyperlipidemia (both trend P < 0.001) or CAD (P = 0.001).
Table 1.
Baseline Demographics, Anthropometrics, Metabolic Parameters, and Medical Histories of the Entire Cohort (n = 1063) Stratified by Body Fat Composition
| Fat mass (kg) | 1st Tertile | 2nd Tertile | 3rd Tertile | Trend P |
|---|---|---|---|---|
| 3.68–14.6 | 14.6–19.61 | 19.61–40.4 | ||
| Age (y) | 50.5 ± 12.7 | 51.6 ± 9.1 | 50.9 ± 11.3 | 0.49 |
| No. of females | 164 (39.1%) | 162 (38.7%) | 177 (42.2%) | 0.509 |
| SBP (mm Hg) | 116.3 ± 16.8 | 121.3 ± 16.5 | 125.7 ± 17 | <0.001 |
| DBP (mm Hg) | 71.5 ± 9.6 | 75.7 ± 10.1 | 78.2 ± 10.4 | <0.001 |
| HR (bpm) | 74.5 ± 10.2 | 74.8 ± 9.6 | 75.8 ± 11 | 0.03 |
| Height (cm) | 163.2 ± 8.2 | 163.7 ± 8.4 | 164.4 ± 9 | 0.04 |
| Weight (kg) | 56.7 ± 8.3 | 65 ± 8.5 | 75.4 ± 11.6 | <0.001 |
| BMI (kg/m2) | 21.2 ± 2.1 | 24.2 ± 1.8 | 27.8 ± 3.1 | <0.001 |
| WC (cm) | 74.8 ± 7.7 | 82.6 ± 7.3 | 90.8 ± 8.8 | <0.001 |
| WHR | 0.85 ± 0.07 | 0.89 ± 0.07 | 0.91 ± 0.07 | <0.001 |
| Fasting glucose (mg/dL) | 96.7 ± 23.3 | 100.2 ± 21.2 | 106.6 ± 29.9 | <0.001 |
| HbA1c (mg/dL) | 5.69 ± 0.94 | 5.8 ± 0.81 | 6.08 ± 1.18 | <0.001 |
| Cholesterol (mg/dL) | 188.2 ± 33.3 | 198.5 ± 33 | 202.4 ± 43.1 | <0.001 |
| TG (mg/dL) | 96 ± 51.8 | 136.7 ± 97.3 | 171 ± 102.5 | <0.001 |
| LDL (mg/dL) | 118.9 ± 30.1 | 131.5 ± 30.2 | 134.1 ± 33.7 | <0.001 |
| HDL (mg/dL) | 60.7 ± 16 | 54.1 ± 15 | 50.1 ± 14.1 | <0.001 |
| eGFR (mL/min/1.73m2) | 88 ± 17 | 87.2 ± 18.3 | 88.4 ± 18.5 | 0.83 |
| Hs‐CRP (mg/dL) | 0.19 ± 0.25 | 0.21 ± 0.23 | 0.29 ± 0.4 | <0.001 |
| Hypertension (%) | 58 (13.8%) | 77 (18.4%) | 114 (27.2%) | <0.001 |
| Diabetes (%) | 31 (7.4%) | 25 (6%) | 40 (9.6%) | 0.145 |
| Hyperlipidemia (%) | 11 (2.6%) | 20 (4.8%) | 41 (9.8%) | <0.001 |
| Smoking, current (%) | 87 (20.7%) | 77 (18.4%) | 83 (19.8%) | 0.691 |
| CAD history (%) | 11 (2.6%) | 22 (5.3%) | 35 (8.4%) | 0.001 |
Abbreviations: BMI, body‐mass index; CAD, coronary artery disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; HR, heart rate; Hs‐CRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; SBP, systolic blood pressure; TG, triglyceride; WC, waist circumference; WHR, waist‐hip ratio.
Echocardiographic Parameters
In Tables 2 and 3, we demonstrated the uni‐ and multivariable models between body fat composition and several cardiac structural and functional parameters by different genders in the whole cohort (n = 1063). Increasing body fat composition was associated with larger left atrial (LA) diameter, increased LV septal and posterior wall thickness, larger LV diameters, larger LV mass, increased RWT, and mass‐to‐volume ratio for both males and females (all P ≤ 0.001), along with significant decrease in LV midwall fractional shortening without stress correction (P = 0.015). After adjustment for baseline clinical variables and hypertension, the multivariable models showed that for each standardized increase in body fat composition, there was significant increase in LA and LV diameter, posterior wall thickness, RWT, LV mass, mass‐to‐volume ratio, and decrease in midwall fractional shortening with or without stress correction (all P < 0.001). When LV mass and MR severity was further added, the independent association between increased body fat composition and larger LA diameter remained significant (β coefficient = 0.37, P < 0.001).
Table 2.
Univariable Models Regarding the Association Between Body Fat Composition and Echocardiographic Parameters in the Whole Study Cohort by Different Genders (n = 1063)
| Females (n = 419) | Males (n = 644) | All (n = 1063) | ||||
|---|---|---|---|---|---|---|
| β Coefficient | P Value | β Coeffficient | P Value | β Coefficient | P Value | |
| LA (mm) | 0.46 | <0.001 | 0.33 | <0.001 | 0.36 | <0.001 |
| IVS (mm) | 0.4 | <0.001 | 0.21 | <0.001 | 0.25 | <0.001 |
| PWT (mm) | 0.44 | <0.001 | 0.23 | <0.001 | 0.3 | <0.001 |
| LVIDd (mm) | 0.34 | <0.001 | 0.14 | <0.001 | 0.25 | <0.001 |
| LVIDs (mm) | 0.26 | <0.001 | 0.13 | 0.001 | 0.20 | 0.001 |
| RWT | 0.28 | <0.001 | 0.14 | <0.001 | 0.16 | <0.001 |
| LVEF (%) | −0.05 | 0.306 | −0.02 | 0.661 | −0.02 | 0.462 |
| FSendo (%) | 0.007 | 0.887 | −0.02 | 0.569 | −0.01 | 0.569 |
| FSmw (%) | −0.14 | 0.004 | −0.09 | 0.021 | −0.07 | 0.015 |
| FScmw (%) | −0.1 | 0.033 | −0.08 | 0.04 | −0.058 | 0.059 |
| LV mass (gm) | 0.54 | <0.001 | 0.27 | <0.001 | 0.35 | <0.001 |
| MVR | 0.32 | <0.001 | 0.17 | <0.001 | 0.22 | <0.001 |
Abbreviations: FScmw, stress‐corrected midwall fractional shortening; FSendo, endocardial fractional shortening; FSmw, midwall fractional shortening; IVS, interventricular septum; LA, left atrium; LV, left ventricular; LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal diastolic dimension; LVIDs, left ventricular internal systolic dimension; MVR, mass‐volume ratio; PWT, posterior wall thickness; RWT, relative wall thickness.
Table 3.
Multivariable Models Regarding the Association Between Body Fat Composition and Echocardiographic Parameters in the Whole Study Cohort (n = 1063)
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| β Coefficient | P Value | β Coefficient | P Value | β Coefficient | P Value | |
| LA (mm)a | 0.35 | <0.001 | 0.38 | <0.001 | 0.37 | <0.001 |
| IVS (mm) | 0.25 | 0.001 | 0.28 | <0.001 | 0.24 | 0.011 |
| PWT (mm) | 0.29 | <0.001 | 0.32 | <0.001 | 0.28 | 0.003 |
| LVIDd (mm) | 0.25 | <0.001 | 0.29 | <0.001 | 0.28 | <0.001 |
| LVIDs (mm) | 0.20 | <0.001 | 0.23 | <0.001 | 0.24 | 0.029 |
| RWT | 0.16 | <0.001 | 0.17 | <0.001 | 0.13 | <0.001 |
| LVEF (%) | −0.02 | 0.509 | −0.03 | 0.278 | −0.05 | 0.073 |
| FSendo (%) | 0.01 | 0.698 | −0.02 | 0.421 | −0.02 | 0.076 |
| FSmw (%) | −0.07 | <0.001 | −0.08 | <0.001 | −0.07 | <0.001 |
| FScmw (%) | −0.06 | <0.001 | −0.06 | <0.001 | −0.06 | <0.001 |
| LV mass (gm) | 0.35 | <0.001 | 0.39 | <0.001 | 0.35 | <0.001 |
| MVR | 0.22 | <0.001 | 0.24 | <0.001 | 0.19 | <0.001 |
Abbreviations: FScmw, stress‐corrected midwall fractional shortening; FSendo, endocardial fractional shortening; FSmw, midwall fractional shortening; IVS, interventricular septum; LA, left atrium; LV, left ventricular; LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal diastolic dimension; LVIDs, left ventricular internal systolic dimension; MVR, mass‐volume ratio; PWT, posterior wall thickness; RWT, relative wall thickness.
Model 1: adjusting for age; model 2: adjusting for age and gender; model 3: adjusting for age, gender, body mass index, blood pressure, renal function in terms of estimated glomerular filtration rate and hypertension history.
LV mass and severity of mitral regurgitation added in the model.
Figure 1 demonstrated the linear associations between LA diameter and different fat depots by genders. The association between LA diameter and body fat mass was more significant in the female gender (R = 0.4, P < 0.001). In Figure 2, the associations between LA diameter, LV relative wall thickness, and LV midwall function based on different genders and categorized by Hs‐CRP interaction are illustrated. Higher Hs‐CRP level was associated with larger LA diameter, increased RWT, and worsened midwall mechanics in the female gender (all interaction P < 0.05).
Figure 1.
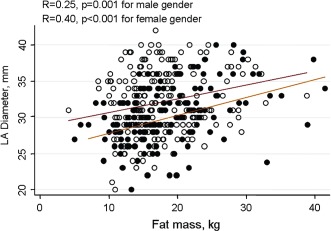
The positive linear association between body fat mass and left atrial (LA) diameter by different genders. The association between LA diameter and fat mass was more obvious in the female gender than male.
Figure 2.

The distribution of left atrial (LA) diameter, relative wall thickness (RWT), and stress‐corrected midwall mechanics (FScmw) by different genders, further categorized by high‐sensitivity C‐reactive protein (Hs‐CRP) levels, with interaction presented (gender vs abnormally high Hs‐CRP: ≥0.3 mg/dL), after accounting for clinical covariates, renal function, and hypertension history. Higher Hs‐CRP level was associated with larger LA diameter, increased RWT, and worsened midwall mechanics in the female gender.
Discussion
Heart failure (HF) has emerged as a major public health issue in recent years.13 The effect of body mass composition and fat distribution on LV mass and geometry has also been well recognized.14 Accumulating evidence15, 16 points to increased LV mass is an independent risk factor for cardiovascular morbidity and mortality, which actually rises continuously with increasing LV mass,17, 18 indicating that these is no clear boundary between normal or abnormal LV mass values.
Obesity, defined as increased BMI, and the metabolic syndrome encompass a cluster of major and emerging risk factors for cardiovascular disease and HF. Central or visceral obesity can indirectly exert its negative cardiovascular effects through deleterious metabolic changes and systemic inflammation. In addition, visceral obesity can negatively impact ventricular mass and geometry, both potential precursors in HF development.19, 20
In the present study of over 1000 participants who underwent cardiovascular health evaluation, we found that body fat mass was independently associated with adverse remodeling in LV geometry in terms of RWT and midwall function, which still holds true even after multivariable adjustment, especially in females. Although there seemed to be a paradoxical decrease in RWT among males (Figure 2), this variation was relatively negligible based on real clinical RWT data (only ranging between 40% and 41%, which can be regarded as clinically insignificant).
In recent years, exaggerated systemic inflammation and its strong link to metabolic disorders has been widely studied21; however, an established hypothesis bridging these observation has not been well proposed. Although metabolic syndrome accompanied by systemic inflammation has recently emerged as a clinical risk factor for HF incidence,22 the exact pathophysiologies linking it to cardiac structural and functional changes remained largely unexplored.
In previous literature, it has been well documented that endocardial fractional shortening may appear supranormal in hypertensive patients compared with normal adults, especially in the presence of left ventricular hypertrophy.23, 24 On the other hand, midwall fractional shortening may be impaired in hypertensive patients with normal left ventricular ejection fraction.25, 26 Recent findings of the LIFE (Losartan Intervention For Endpoint reduction) study suggested that reduced midwall function can predict reduced benefit of blood pressure control on cardiovascular morbidity and mortality.27 As demonstrated in our data, it is worth noting that midwall function was more adversely associated with body fat mass in females. This may imply that females with higher body fat are more prone to develop unfavorable cardiac structural remodeling than males. This further supports the epidemiological findings of the preponderance of females and obesity in heart failure with preserved ejection fraction.28
Limitations
There are several limitations in our study. First, our study has a male gender predominance, which may be somewhat biased. Second, this survey is retrospective and cross‐sectional, without longitudinal follow‐up or validation with clinical outcomes. Third, our data come from asymptomatic Asian participants who to part in a primary cardiovascular health survey, and who therefore may not be fully representative of the broader general population in daily outpatient clinics. We also acknowledge that our data regarding LA structural remodeling were mainly focused on LA diameter rather than 2‐dimensional echo‐based volume measurements. Therefore, further research based on LA volume measures may be warranted to confirm our findings.
Conclusion
The current study showed that although body fat mass is negatively related to LV excursion in terms of reduced midwall contractile function, it can also exert independent effects on LV remodeling process, leading to subsequent LV mass increase and LA dilation. Worse ventricular midwall contractility seems to be more prevalent in females with high systemic inflammation. These gender‐ and Hs‐CRP–specific modification effects may potentiate the pathological mechanisms involved in heart failure with preserved ejection fraction. Because BIA and heart echo are relatively low‐cost and effective tools, more screening tests based on these methods may be encouraged in obese or overweight individuals for cardiovascular risk stratification.
This work was supported in part by a research grant from Mackay Memorial Hospital and NSC 101‐2314‐B‐195‐020.
The authors have no conflicts of interest to disclose.
References
- 1. Baumgartner RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic disease. Obes Res. 1995;3:73–95. [DOI] [PubMed] [Google Scholar]
- 2. Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848. [DOI] [PubMed] [Google Scholar]
- 3. Dehghan M, Merchant AT. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr J. 2008;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cornier MA, Despres JP, Davis N, et al. Assessing adiposity: a scientific statement from the american heart association. Circulation. 2011;124:1996–2019. [DOI] [PubMed] [Google Scholar]
- 5. de Feyter PJ. Epicardial adipose tissue: an emerging role for the development of coronary atherosclerosis. Clin Cardiol. 2011;34:143–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iwasaki K, Matsumoto T, Aono H, et al. Relationship between epicardial fat measured by 64‐multidetector computed tomography and coronary artery disease. Clin Cardiol. 2011;34:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Langenberg C, Bergstrom J, Scheidt‐Nave C, et al. Cardiovascular death and the metabolic syndrome: role of adiposity‐signaling hormones and inflammatory markers. Diabetes Care. 2006;29:1363–1369. [DOI] [PubMed] [Google Scholar]
- 8. Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. [DOI] [PubMed] [Google Scholar]
- 9. Kardys I, Knetsch AM, Bleumink GS, et al. C‐reactive protein and risk of heart failure. The Rotterdam Study. Am Heart J. 2006;152:514–520. [DOI] [PubMed] [Google Scholar]
- 10. de Simone G, Devereux RB, Ganau A, et al. Estimation of left ventricular chamber and stroke volume by limited M‐mode echocardiography and validation by two‐dimensional and Doppler echocardiography. Am J Cardiol. 1996;78:801–807. [DOI] [PubMed] [Google Scholar]
- 11. Shimizu G, Hirota Y, Kita Y, et al. Left ventricular midwall mechanics in systemic arterial hypertension: myocardial function is depressed in pressure‐overload hypertrophy. Circulation. 1991;83:1676–1684. [DOI] [PubMed] [Google Scholar]
- 12. Aurigemma GP, Silver KH, Priest MA, et al. Geometric changes allow normal ejection fraction despite depressed myocardial shortening in hypertensive left ventricular hypertrophy. J Am Coll Cardiol. 1995;26:195–202. [DOI] [PubMed] [Google Scholar]
- 13. Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. [DOI] [PubMed] [Google Scholar]
- 14. Lauer MS, Anderson KM, Kannel WB, et al. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA. 1991;266:231–236. [PubMed] [Google Scholar]
- 15. Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 16. Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol. 1995;25:879–844. [DOI] [PubMed] [Google Scholar]
- 17. Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi‐Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corradi D, Maestri R, Callegari S, et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol. 2004;13:313–316. [DOI] [PubMed] [Google Scholar]
- 19. Karason K, Sjostrom L, Wallentin I, et al. Impact of blood pressure and insulin on the relationship between body fat and left ventricular structure. Eur Heart J. 2003;24:1500–1505. [DOI] [PubMed] [Google Scholar]
- 20. Gates PE, Gentile CL, Seals DR, et al. Adiposity contributes to differences in left ventricular structure and diastolic function with age in healthy men. J Clin Endocrinol Metab. 2003;88:4884–4890. [DOI] [PubMed] [Google Scholar]
- 21. Wang J, Sarnola K, Ruotsalainen S, et al. The metabolic syndrome predicts incident congestive heart failure: a 20‐year follow‐up study of elderly Finns. Atherosclerosis. 2010;210:237–242. [DOI] [PubMed] [Google Scholar]
- 22. Horwich TB, Fonarow GC. Glucose, obesity, metabolic syndrome, and diabetes relevance to incidence of heart failure. J Am Coll Cardiol. 2010;55:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Simone G, Di Lorenzo L, Costantino G, et al. Supernormal contractility in primary hypertension without left ventricular hypertrophy. Hypertension. 1988;11:457–463. [DOI] [PubMed] [Google Scholar]
- 24. Hartford M, Wikstrand JC, Wallentin I, et al. Left ventricular wall stress and systolic function in untreated primary hypertension. Hypertension. 1985;7:97–104. [DOI] [PubMed] [Google Scholar]
- 25. de Simone G, Devereux RB, Koren MJ, et al. Midwall left ventricular mechanics. An independent predictor of cardiovascular risk in arterial hypertension. Circulation. 1996;93:259–265. [DOI] [PubMed] [Google Scholar]
- 26. Verdecchia P, Schillaci G, Reboldi G, et al. Prognostic value at midwall shortening fraction and its relation with left ventricular mass in systemic hypertension. Am J Cardiol. 2001;87:479–482. [DOI] [PubMed] [Google Scholar]
- 27. Wachtell K, Gerdts E, Palmieri V, et al. In‐treatment midwall and endocardial fractional shortening predict cardiovascular outcome in hypertensive patients with preserved baseline systolic ventricular function: the Losartan Intervention For Endpoint reduction study. J Hypertens. 2010;28:1541–1546. [DOI] [PubMed] [Google Scholar]
- 28. Lam CSP, Donal E, Kraigher‐Krainer E, et al. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]


