Abstract
Background
The use of left ventricular assist devices (LVADs) has become a state‐of‐the‐art therapy for advanced cardiac heart failure; however, multiple reports in the literature describe an increased risk for gastrointestinal (GI) bleeding in these patients. We characterized this association by reviewing recent studies on this topic.
Hypothesis
GI bleeding occurs frequently in patients with LVADs, especially with devices with nonpulsatile flow patterns.
Methods
We performed a comprehensive literature review to identify articles that reported GI bleeding in patients with LVADs. Databases used included PubMed, EMBASE, Scopus, Web of Knowledge, and Ovid. Baseline and outcome data were then ed from these reports.
Results
We identified 10 case reports and 22 case series with 1543 patients. The mean age was 54.2 years. Most patients had nonpulsatile LVADs (1316, 85.3%). Three hundred and seventeen patients (20.5%) developed GI bleeding; this occurred more frequently in patients with nonpulsatile LVADs. Multiple procedures were performed without complications but often did not identify a definite bleeding site. Suspect lesions occurred throughout the GI tract but were more frequent in the upper GI tract. Many patients had arteriovenous malformations. All patients received medical therapy. None of the patients had their LVAD replaced. The use of anticoagulation did not appear to predispose these patients to more GI bleeding episodes.
Conclusions:
Patients with LVADs have frequent GI bleeds, especially from arteriovenous malformations, which can occur throughout the GI tract. Most diagnostic and therapeutic interventions can be used safely in these patients. The pathogenesis of the GI bleeding in these patients may involve the use of anticoagulant medications, the formation of arteriovenous malformations, loss of von Willebrand factor activity, and mucosal ischemia.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Approximately 5 million people have congestive heart failure (CHF), and 1 out of every 1000 patients age >65 years will eventually develop CHF.1, 2 A common treatment modality for end‐stage CHF patients is cardiac transplantation. However, the difference between the number of patients who need a heart transplant and the number of available donors demonstrates the need for alternative methods for treating severe CHF. Additionally, growing numbers of patients are not eligible for heart transplant or have maximized their medical therapy but still need cardiac support.3 The use of left ventricular assist devices (LVADs) has become a state‐of‐the‐art therapy for advanced heart failure and provides options for patients with severe CHF for possible transplantation (bridge to transplantation), for bridge to myocardial recovery, or for destination therapy (end‐stage therapy). In the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial, patients treated with LVADs had a 1‐and 2‐year survival rate that was twice as high as the survival in patients treated with traditional medications alone.4
The 2 main types of LVADs have different flow patterns: pulsatile and nonpulsatile. The earliest LVAD, approved by the US Food and Drug Administration in 1994, was a pulsatile device (Thoratec HeartMate IP; Thoratec, Pleasanton, CA) that mimicked the natural physiology of the heart. However, this type of device had important drawbacks, including limitation on its durability, necessitating replacement within 15 to 18 months, and excess size and weight (570 g), restricting its use in children and adults with small body size.3 Newer nonpulsatile devices are easier to use and have better durability: HeartMate II and HeartMate III (Thoratec), Jarvik 2000 (Jarvik Heart Inc., New York, NY), VentrAssist (Ventracor, Sydney, Australia), DuraHeart (Terumo Corp., Tokyo, Japan), HVAD (HeartWare International, Framingham, MA), MTI Heart (MiTi Heart Corp., Albany, NY), and MicroMed DeBakey (MicroMed, Houston, TX). When comparing outcomes and risk profiles, initial studies have suggested similar results with pulsatile and nonpulsatile devices.5 Despite the advances in technology, there are significant complications and risks involved when using newer LVADs. These include renal dysfunction,6, 7 neurological complications,8 and infection.9 Multiple case reports and case series of patients with LVADs have noted an increased risk for developing gastrointestinal (GI) bleeding. This complication has been more pronounced in patients with nonpulsatile LVADs, who may develop arteriovenous malformations (AVMs) in their intestines secondary to low pulse pressures. We investigated this complication by reviewing recent studies of LVAD‐associated GI bleeding.
Methods
We performed a comprehensive literature review for any case reports, abstracts, randomized controlled trials, or case series that discussed LVAD use and GI bleeding. We used several databases, including PubMed, EMBASE, Scopus, Web of Knowledge, and Ovid. The following keywords were used: left ventricular assist devices, GI bleeding, GI hemorrhage, AVM, and heart‐assist devices. This was done by 2 independent authors. No language limits or date limits were imposed. We included both human and animal models. We used backward “snowballing” (ie, scanning of references of retrieved articles and pertinent reviews) to retrieve more studies. Baseline and outcome data were then abstracted, including the study design; the type of LVAD; the total number, age, and sex of patients; the etiology of the heart disease; the number of patients who developed GI bleeding; the type and site of bleeding; management decisions; and outcomes for those patients. These studies did not use a uniform definition of GI bleeding and did not consistently report all the information we wanted to collect. Consequently, the numbers used in the denominator for various calculations could vary. This search was completed on December 10, 2012.
Results
Patient Characteristics
We identified 10 case reports and 22 case series that documented GI bleeding events in 1543 patients with LVADs (Table 1). The average age was 54.2 years (range, 16–77 y) and 65% were men (n = 1003). Most patients had either a dilated cardiomyopathy (72.6%) or an ischemic cardiomyopathy (24.4%). Most of the devices (1316, 85.3%) were nonpulsatile LVADs (Jarvik, Thoratec, HeartMate II, and Incor [Berlin Heart AG, Germany]). Three hundred and seventeen patients (20.5%; 95% confidence interval: 18.9%‐22.3%) developed GI bleeding during management with a LVAD. Gastrointestinal bleeding occurred in 14 patients with pulsatile LVADs (10.4%) and 265 patients with nonpulsatile LVADs (20.8%, P < 0.01 by χ 2 test).
Table 1.
Case Reports and Case Series Identified
| Case (Reference) | LVAD (No. of Patients) | Pulse (Y/N) | Average Age, y | Heart Disease (No. of Patients) | GIB | Source of GIB | Type GIB |
|---|---|---|---|---|---|---|---|
| 142 | Incor | N | 45 | DCM | 1 | Small intestine | Pousse bleeding |
| 27 | HeartMate II | N | 73 | ICM | 1 | Gastric fundus | Trauma from LVAD device |
| 348 | ThermoCardiosystems, Inc. HeartMate | Y | 52 | ICM | 1 | Gastric ulcer | Ulcer |
| 411 | Incor | N | 65 | DCM | 1 | Distal small intestine | Angiodysplasia |
| 512 | Jarvik | N | 59 | ICM | 1 | Distal duodenal and proximal jejunum | Unk |
| 646 | Thoratec | N | 59 | ICM | 1 | Sigmoid | Unk |
| 713 | HeartMate II | N | 70 | ICM | 1 | Cecum | Angiodysplasia |
| 814 | Incor | N | 42 | DCM | 1 | Unk | Unk |
| 949 | HeartMate II | N | 59 | ICM | 1 | Stomach | Hemorrhagic gastritis |
| 1050 | ThermoCardiosystems, Inc. HeartMate | Y | 44 | ICM | 1 | Small intestine | Aortoenteric fistula |
| 1119 | Jarvik 2000 | N | 60, 51, 66 | ICM | 3 | Unk | Unk |
| 1216 | VentrAssist, HeartMate II, Jarvik | N | 52 | ICM (9), AS (1) | 2 | Unk | AVM (1) |
| Novacor, HeartMate XVE | Y | 54 | 6 | ||||
| 1333 | HeartMate (38), MicroMed (8), VentrAssist (9) | N | 55.1 | NR | 12 | Unk | Unk |
| HeartMate XVE (8) | Y | 55.7 | 3 | ||||
| 1410 | HeartMate II | N | 54.8 | ICM (24) | 8 only on HeartMate II | No sources (11), stomach polyp (2), esophageal erosion (1), GE junction (1), Dieulafoy lesions (1), Dieulafoy lesions vs angiodysplasia (1). Seventeen total bleeding episodes noted, with no source identified in 11 patients. | No sources (11), stomach polyp (2), esophageal erosion (1), GE junction (1), Dieulafoy lesions (1), Dieulafoy lesions vs angiodysplasia (1). Seventeen total bleeding episodes noted, with no source identified in 11 patients. |
| VentrAssist | N | 53.2 | |||||
| HeartMate XVE | Y | 53.2 | |||||
| 1515 | HeartMate II | N | 77 | ICM | 1 | Pylorus (1), mid–small bowel (1), fundus of stomach (1) | Ulcer (1), angiodysplasia (2) |
| HeartMate II | N | 71 | ICM | 1 | |||
| HeartMate II | N | 48 | ICM | 1 | |||
| 1640 | HeartMate II | N | 50.75 | ICM (18), DCM (13), CHD (1) | 5 | Unk | Unk |
| 1751 | Incor | N | NR | DCM (16), ICM (7), RCM (1) | 1 | Unk | Unk |
| 1843 | HeartMate | N | 51 | DCM (10), ICM (23) | 2 | Gastric (1), rest not mentioned | Unk |
| 1923 | HeartMate II | N | 42 | DCM (30), ICM (13) | 1 | Small bowel | AVM |
| 2044 | Thoratec (46) | Y | 62 | NA | 38 | Gastric and small bowel (8), mild gastritis (9), bleeding rectal ulcers (4), diverticulosis (8), pseudomembranous colitis (1), internal hemorrhoids (8) | Unk |
| HeartMate XVE (46) | Y | ||||||
| HeartMate II (40) | N | ||||||
| 2152 | HeartMate II | N | NR | NA | 13 | Unk | Unk |
| VentrAssist | N | ||||||
| 2232 | HeartMate II | N | 56.3 | ICM (33), DCM (40) | 24 | Unk | Unk |
| 233 | HeartMate II | N | 53 | ICM (17), idiopathic CM (17), DCM (3) | 5 | Unk | Unk |
| 2434 | HeartMate II | N | 40.4 | DCM (7), ICM (2) | 2 (HeartMate II) | Unk | |
| Thoratec PVAD | Y | Unk | |||||
| VentrAssist | N | ||||||
| 2553 | HeartMate II | N | 43 | ICM (13), DCM (13) | 3 | Unk | Unk |
| 2654 | HeartMate II | N | NR | NA | 23 | Upper GI tract (54%), lower GI tract (35%) | Gastric erosions (54%), ulcers/angiodysplasia (37%) |
| 2755 | VentrAssist | N | NR | NA | 24 | Unk | Unk |
| 2856 | Jarvik 2000 | N | 69 | DCM | 1 | Upper GI tract (1) | Gastric erosion (1) |
| 293 | VentrAssist | N | NR | DCM | 12 | Unk | Unk |
| Y | 3 | ||||||
| 3057 | HeartMate II | N | NR | DCM | 32 | Upper GI tract (17), lower GI tract (16) | Hemorrhagic gastritis (10), gastric AVMs (10), Mallory‐Weiss syndrome (2), diverticulosis (6), driveline erosion of the colon (1), sigmoid polyp (1), ischemic colitis (1), colocutaneous fistula (1), gastrocutaneous fistula (1) |
| 3157 | VentrAssist | N | 55 | DCM | 44 | Upper GI tract (22), lower GI tract (7) | Gastric erosions/ulcers (14), AVMs (8) |
| 3259 | HeartMate II | N | NR | DCM | 19 | Upper GI tract (1), lower GI tract (8), no definite source (4) | Unk |
| 3360 | HeartMate II | N | 52 | DCM (36), ICM (58), other (8) | 18 | Unk | Unk |
Abbreviations: AS, aortic stenosis; AVM, arteriovenous malformation; CHD, coronary heart disease; CM cardiomyopathy; DCM, dilated cardiomyopathy; GE, gastroesophageal; GI, gastrointestinal; GIB, gastrointestinal bleeding; ICM, ischemic cardiomyopathy; N, no; NA, not available; NR, not reported; PVAD, paracorporeal ventricular assist device; RCM, restrictive cardiomyopathy; Unk, unknown; Y, yes.
Gastrointestinal Bleeding Diagnosis
Multiple diagnostic tests were used in these patients to determine the cause of the GI bleeding. Some patients had esophagogastroduodenoscopy and/or colonoscopy, and others underwent exploratory laparotomy. For example, Stern et al evaluated 8 patients with nonpulsatile HeartMate II devices who developed ≥1 episode of bleeding. Thirty‐six diagnostic studies were performed in these patients, but only 10 studies had definite results and identified a bleeding site.10 Many studies reviewed for this article did not report the diagnostic testing used; a subset of the articles listed 188 procedures. These included 103 endoscopies, 49 colonoscopies, and 11 tagged red blood cell imaging studies. Seven patients had capsule endoscopy, which did not interfere with the LVAD function.10, 11, 12, 13, 14, 15 Pretransplant LVAD colonoscopies did not correlate with posttransplant bleeding findings. Grasso et al analyzed 52 patients with LVADs. Ten patients had preimplant colonoscopies with 7 pathological findings (4 polyps, 2 diverticulosis, 1 colitis).16 Three developed postimplant GI bleeding, but it was unclear if these occurred from the sites identified prior to LVAD placement. Deep double‐balloon enteroscopy had been performed without complications.17, 18 Other diagnostic strategies are listed in the Figure 1. The studies suggest that these patients may need extensive testing to determine the site of the GI bleeding, but that this can be done safely without interfering with the LVADs.
Figure 1.
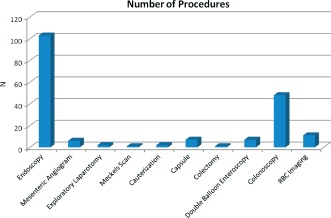
Diagnosis and therapy. All the procedures completed did not appear to interfere with LVAD use. Abbreviations: LVAD, left ventricular assist device; RBC, red blood cell.
Gastrointestinal Bleeding Sites and Pathology
Bleeding can occur throughout the GI tract. For example, Letsou et al reported 3 patients supported by Jarvik 2000 axial‐flow LVADs who had GI bleeding from the small intestine during follow‐up.19 Daas et al had 3 patients who had GI bleeds in the pylorus, in the mid–small bowel, and in the fundus of the stomach.15 Overall, our review demonstrates that the bleeding sites can occur in the esophagus (n = 2), in the stomach (n = 27), in the small intestine (n = 14), and in the colon/rectum (n = 23). Four studies reported the bleeding site in the upper GI tract (n = 95) or the lower GI tract (n = 66) (Table 1). However, in many patients no bleeding site was identified, or the necessary information was not reported (Table 1). For example, Stern et al did a retrospective review of bleeding complications in patients with HeartMate II LVAD and had a difficult time finding the source of the GI bleed. During 17 bleeding episodes, 36 diagnostic studies were performed, but only 10 tests identified a definite bleeding site. No definite bleeding sources could be identified in 65% of these episodes.10 These studies suggest that finding a bleeding site may be difficult and that bleeding appears to occur more frequently in the upper GI tract.
Many GI bleeds reported in the literature were from AVMs or angiodysplastic vessels (Table 1). Letsou et al noted that 3 of his patients developed AVMs.19 Grasso et al retrospectively analyzed 52 patients with LVADs. Gastrointestinal bleeding from AVMs occurred in 4% (1/25) of the patients with nonpulsatile LVADs and 7% (2/27) of the patients with pulsatile LVADs. The higher number of bleeds seen in the pulsatile groups was possibly explained by a time bias from a longer time with the device. Age was the only independent predictor of GI bleeding in patients in the Grasso series (multivariate analysis, P = 0.001). Other lesions identified in our literature review included polyps, Dieulafoy lesions, and ulcers (Table 1). Two patients even had physical trauma developing from the LVAD itself.17
Gastrointestinal Bleeding Therapy and Outcomes
All patients identified in our literature review received medical therapy, usually with acid‐suppression treatment and fluid resuscitation (Table 2). Five patients had cardiac transplants for their end‐stage CHF, and none developed GI rebleeding after transplant. Three patients eventually needed surgical therapy. No patients had their LVAD replaced, despite some GI bleeding directly related to the device itself.17 Crow et al retrospectively reviewed 101 LVAD recipients and determined their event rate for GI bleeding was 63/100 patient‐years for nonpulsatile devices after 15 days post–LVAD placement and 6.8/100 patient‐years bleeding events after 15 days for pulsatile devices (P < 0.0004). This difference persisted for bleeding occurring ≥31 days after device implantation, with 46.5 events/100 patient‐years for nonpulsatile devices and 4.7 events/100 patient‐years for pulsatile devices (P = 0.0028). Mortality rates were similar between the 2 groups (15% for nonpulsatile vs 17% for pulsatile, P = 0.69).3 Overall, Crow reported that the nonpulsatile LVADs were associated with higher rates of GI bleeding than pulsatile LVADs.3 Our literature review confirmed this observation (see first paragraph in Results). These results suggest that blood‐flow characteristics of LVADs contribute to the pathogenesis of GI bleeding (Table 2). Mortality attributed to GI bleeding was infrequent in these patients, but 2 articles did report a high GI‐bleeding recurrence rate.20, 21
Table 2.
Outcomes
| Case (Reference) | Author(s) | Outcomes |
|---|---|---|
| 142 | Garatti et al | After cardiac transplantation, no further bleeding. |
| 27 | Miller et al | No further bleeding found. |
| 348 | Hou et al | No further GIB, but later on found that LVAD had perforated through gastric wall. Patient had a closure of the defect without any complications. |
| 411 | Fenkel et al | No further bleeding found. |
| 512 | Seow and Zimmerman | No further bleeding found. |
| 646 | Tulchinsky | Embolized and no further bleeding. Used Tc‐99m RBC scan to identify the GIB. No problems noted. |
| 713 | Bechtel et al | A wireless capsule (PillCam SB) was used successfully to determine bleeding. No further bleeding. |
| 814 | Girelli et al | No interference was found between the capsule and LVAD. No bleeding noted. |
| 949 | Gogas et al | No further bleeding noted. |
| 1050 | Aleksic et al | Not mentioned. |
| 1119 | Letsou et al | (1) After cardiac transplantation, no further GIB. (2) Another patient had an ex‐lap; adhesions were lysed and burned, but continued bleeding. The patient died from multiorgan failure. (3) Received transplant and no further GIB. |
| 1216 | Grasso et al | Final outcome not known for each set of patients. When considering GIB from AVMs, there was 1 (4%) event in the nonpulsatile group and 2 (7%) in the pulsatile group (P = 0.607). For GIB from all sources, there were 2 (8%) in the nonpulsatile group and 6 (22%) in the pulsatile group (P = 0.16). Of the 10 subjects who received preimplant colonoscopies, 7 had pathologic findings (4 polyps, 2 diverticulosis, 1 colitis) and 3 went on to develop postimplant GIB (P = 1.000). On multivariate analysis, only age was found to be an independent predictor of GIB (P = 0.001). In conclusion, the authors noted that the nonpulsatile LVADs were not associated with an increase in AVMs or GIB. |
| 133 | Crow et al | Event rate was 63/100 patient‐years for nonpulsatile devices after 15 days; 6.8/100 patient‐years. All bleeding events after 15 d for pulsatile devices (P < 0.0004). This difference persisted for bleeding occurring ≥31 d after device implantation with 46.5 events/100 patient‐years for nonpulsatile devices vs 4.7 events/100 patient‐years for pulsatile events (P = 0.0028). Mortalities were similar between the 2 groups (15% for nonpulsatile vs 17% for pulsatile, P = 0.69). In conclusion, the authors noted that nonpulsatile LVADs have a higher rate of GIB than pulsatile LVADs. |
| 1410 | Stern et al | No significant changes in P value. HeartMate II patients who bled were significantly older and more likely to have been taking ASA preoperatively than those without GIB. The 2 HeartMate II patients who had a prior hx of GIB did not bleed postoperatively, whereas all GIB had no previous hx of GIB. Of the 8 HeartMate II patients who bled, 2 had heart transplants. Neither patient has had a recurrent episode of GIB since transplantation. Three HeartMate II patients who had implantation for destination therapy developed GIB within 3 mo of implantation. Three patients had recurrent episodes of GIB. |
| 1515 | Daas et al | No further bleeding after treatment of GIB. |
| 1641 | John et al | Stopped anticoagulation and reduced the pump speed. No further recurrence of GIB. |
| 1751 | Hetzer et al | Had consistent GIB, had to be hospitalized, and needed transfusion therapy. |
| 1843 | McCarthy et al | One resolution with endoscopic therapy. Another had massive upper GIB; taken to OR to oversew a bleeding ulcer. |
| 1923 | Frazier et al | One patient had GIB that was seen; had to stop anticoagulation and all antiplatelet therapy. The patient eventually died from cardiac thrombus. No other patients had GIB. |
| 2044 | Siddiqui et al | Not mentioned. |
| 2152 | Wang et al | Postoperative anticoagulation/antiplatelet regiments same for both GIB and non‐GIB. GIB patients were older (age 60.5 vs 49 y, P = 0.051) and had to be intubated preoperatively (38% vs 12%, P = 0.038). Perioperative morbidity and mortality showed no difference except for longer median ventilator support (184 vs 50 h, P = 0.001) and longer median hospital stay (37 vs 19 d, P < 0.001) for GIB. No bleeding in VentrAssist. |
| 2232 | Uriel et al | Patients with the HeartMate II had a high incidence of bleeding events during device support and at heart transplantation. All HeartMate II patients had reduced HMW vWF multimers. Eighteen of these (58%) had bleeding. Anticoagulation was similar in both groups. |
| 2333 | Crow et al | All CF‐LVAD recipients had AVWS after LVAD placement, demonstrated by reduced or absent HMW vWF multimers levels. However, not all recipients had bleeding complications. |
| 2434 | Heilmann et al | Nine patients underwent placement of an LVAD, whereas 3 underwent placement of a TAH, which is connected directly to the heart and large cardiac vessels without cannulas. Within 1 d of LVAD implantation, 4 of 5 patients evaluated demonstrated loss of HMW multimers and impaired vWF function. AVWS was present within 2 wks of implantation in 8 of 9 patients, and in all 7 tested patients after ≥3 mo. Patients with different LVAD types developed varying severities of AVWS. After LVAD explanation, HMW multimers were detectable and vWF function normalized in all patients. AVWS was not observed in the TAH patients studied. These results suggest that shear stress associated with exposure of blood to VAD cannulas and tubes may contribute to the development of AVWS. |
| 2553 | Meyer et al | Twenty‐six outpatients received an axial‐flow LVAD (HeartMate II; Thoratec) for a median support time of 4.5 mo. In all patients on devices, severe impairment of platelet aggregation as well as a loss of large vWF multimers were found. In 10 patients, a decreased vWF:CB/vWF:Ag ratio was observed. Bleeding episodes occurred with an incidence of 0.17 per patient‐year. After removal of the device, normal patterns of platelet aggregation, multimers analysis, and vWF:CB/vWF:Ag ratio were recorded. The authors concluded that a diagnosis of vWF syndrome type 2 was established in all patients after LVAD implantation, and bleeding events confirmed this finding. Reversibility of this condition was found after removal of the device. |
| 2654 | Aggarwal et al | There was high recurrence rate of bleeding after device implantation, the majority from the same site. Management strategies for bleeding included temporarily withholding anticoagulation, decreasing the speed of LVADs, and using octreotide. One patient died as direct consequence of GIB. |
| 2755 | Schaffer et al | Management strategies for bleeding included blood‐product administration. |
| 2856 | Tarzia et al | Early postoperative course was uneventful. Management strategies for bleeding included argon plasma coagulation during endoscopy. |
| 293 | Crow et al | There was high recurrence rate of bleeding after device implantation. Mortalities were similar between nonpulsatile (15%) and pulsatile (17%) groups (P = 0.69). |
| 3057 | Demirozu et al | All GIB episodes were successfully managed medically, without the need for surgical intervention. |
| 3158 | Kushnir et al | Overall mortality was 35%, none directly from GIB. |
| 3259 | Morgan et al | There were no deaths referable to GIB. Recurrent bleeding occurred in 4 patients. Hx of GIB prior to LVAD implantation was the only variable significantly different between patients with and without postimplant GIB, indicating the need of better understanding the pathophysiology and management of GIB prior to device implantation. |
| 3360 | John et al | Overall, 30‐d, 6‐mo, and 1‐yr survival for the BTT patients was 95.1%, 83.5%, and 78.8%, respectively. Major adverse events among BTT patients included RV failure (5%), LVAD driveline infections (24.5%), neurologic events (9.8%), and GIB (17.6%). |
Abbreviations: ASA, aspirin; AVM, arteriovenous malformation; AVWF, acquired von Willebrand syndrome; BTT, bridge to transplant; CF, continuous‐flow; ex‐lap, exploratory laparotomy; GIB, gastrointestinal bleeding; HMW, high molecular weight; hx, history; LVAD, left ventricular assist device; OR, operating room; RBC, red blood cell; RV, right ventricular; TAH, total artificial heart; Tc‐99m, technetium 99m; VAD, ventricular assist device; vWF, von Willebrand factor; vWF:Ag, plasma von Willebrand factor antigen; vWF:CB, von Willebrand factor collagen binding assay.
Discussion
Gastrointestinal bleeding in patients with an LVAD is a common occurrence. Our review indicates that almost 20% of patients with an LVAD developed a bleeding episode and that most of these patients had a nonpulsatile LVAD (HeartMate II, Jarvik, Thoratec, and Incor). When investigating the source of the GI bleeding, routine evaluation methods can be used without any concern for interference with the LVAD. Esophagogastroduodenoscopy, colonoscopy, bleeding scans, tagged red blood cell imaging scans, and angiograms were all used, and there was no reported interference or other problems. Even capsule endoscopy could be safely used to evaluate GI‐bleeding episodes in these patients. Bleeding was not localized to a particular region of the GI tract in patients with an LVAD. However, most authors did not report where the bleeding sites were located. Of those identified, many were AVMs or angiodysplastic vessels, which might be expected in older populations. Therapies included routine treatment of GI bleeding with volume resuscitation and acid suppression. Patients who bled did not have recurrent bleeding episodes after cardiac transplantation. The use of anticoagulation did not predispose these patients to more bleeding episodes.
The potential explanations for GI bleeding in these patients include anticoagulation, formation of AVMs, Heyde syndrome, and mucosal ischemia. Anticoagulation is needed postoperatively in patients with a continuous‐flow LVAD due to the risk of thrombosis with a foreign device. Nonpulsatile device recipients receive anticoagulation with warfarin (target range international normalized ratio [INR], 1.5–3), whereas pulsatile‐device recipients do not receive anticoagulation. Both nonpulsatile and pulsatile devices recipients take aspirin daily.3 Crow et al noted that when comparing GI bleeding rates in patients with LVADs with bleeding complications in patients on anticoagulation for other indications, the propensity for GI bleeding in LVAD patients could not be attributed to anticoagulation alone. In their series, the GI bleeding rates in the nonpulsatile LVAD group were much higher than the rates of all types of bleeding in patients receiving anticoagulation after placement of a mechanical valve. These patients had a GI‐bleeding rate of 63 events/100 patient‐years with nonpulsatile devices. Patients with mechanical valve replacement treated with combined antiplatelet and warfarin therapy had all‐cause bleeding rates of 2.7–4.6 events/100 patient‐years.3 Levine et al has reported that patients receiving anticoagulation for any reason had bleeding rates of 5.7/100 patient‐years (3/53 patients).22 Additionally, if the GI bleeding were due to anticoagulation alone, we would expect higher rates of all‐cause bleeding in patients with nonpulsatile devices. However, the all‐cause bleeding rates in patients with nonpulsatile and pulsatile devices are similar.3 In the REMATCH trial, bleeding occurred in 42% of 68 pulsatile LVAD recipients at 6 months' follow‐up.3, 4 In the Texas Heart Institute study of 280 HeartMate pulsatile LVAD recipients, all‐cause bleeding occurred in 48%.23 All‐cause bleeding requiring 2 units of blood in the HeartMate II LVAD nonpulsatile devices occurred in 53% of the 133 patients followed up for 180 days after device implantation.7 These studies and our literature review demonstrate increased GI bleeding in patients with nonpulsatile devices, despite similar overall bleeding rates in nonpulsatile and pulsatile LVAD recipients.
Recent studies on Heyde syndrome offers another explanation for bleeding in LVAD recipients. Heyde reported 10 patients who had both aortic stenosis and GI bleeding.24 The proposed theory in Heyde syndrome is that flow across a stenotic aortic valve induces a conformational change specific for the high‐molecular‐weight (HMW) multimers of von Willebrand factor (vWF), leading to its proteolysis by the vWF protease ADAMTS 13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13).25, 26 This reduces the number of the HMW multimers, impairs platelet‐mediated hemostasis in angiodysplastic vessels, and predisposes the patient to bleeding.21 The continuous impeller action of the nonpulsatile LVAD pump may cause vWF deformation, proteolysis, and ultimately a deficiency of the HMW multimers.3 All investigations of blood flow in different types of LVADs have reported increased shear stress, turbulence, and high velocity in the inflow and outflow cannulas of the LVADs.27, 28, 29, 30 Geisen et al recently analyzed 12 patients with LVADs and compared them with a control population to test this hypothesis. They found that the large vWF multimers were missing in the LVAD patients, but 5 out of the 6 controls displayed normal multimer patterns.31 Uriel et al reported that 31 patients, all randomly selected with HeartMate II, had reduced HMW vWF; 31% of these patients had bleeding episodes, whereas none of the patients with normal vWF levels had a bleeding event (P < 0.001).32 Crow et al discovered that all patients in that study had significant loss of vWF after continuous‐flow LVAD placement, even though they all had normal levels measured before implantation.33, 34, 35 In our data, an increased number of bleeding events occurred with the HeartMate II devices.
Additionally, LVAD devices may predispose patients to develop AVMs. The narrow pulse pressure seen in aortic stenosis and nonpulsatile LVADs may decrease intraluminal pressure and dilate the mucosal veins, leading to the formation of AVMs.3, 19 These AVMs are predisposed to bleeding due to anticoagulation and stress. Cappell and Lebwohl have demonstrated that narrow pulse pressures may trigger an increase in sympathetic tone, causing smooth‐muscle relaxation, arteriovenous dilation, and AVM formation.3, 36 In sheep implanted with nonpulsatile LVADs, thinning of the medial layer of the ascending aorta occurs; in porcine models, the use of LVADs can result in intestinal ischemia due to poor perfusion, which could possibly be seen throughout the entire arterial circulation.3, 37, 38, 39
If a patient with an LVAD develops GI bleeding, there are several approaches to reduce bleeding by manipulation of the pump. John et al temporarily discontinued anticoagulation and reduced the pump flow speed.40, 41 This was thought to increase the pulse pressure and reduce the potential risks of GI bleeding. In their cohort, the GI bleeding did not recur, and none of the patients developed thromboembolism. Additionally, if the bleeding is severe, proceeding to transplantation is another option. No patients identified in our review had recurrent GI bleeding after cardiac transplantation.10, 19, 42
However, there are clinical circumstances in which it is not possible to manipulate the LVAD or proceed to transplantation. The most important management step is stabilization of the patient with fluids and resuscitation. After that, endoscopy has been successfully used in patients with an LVAD10, 15, 19, 43, 44 without any interference with the device. If additional studies are needed, cauterization, capsule endoscopy,10, 11, 12, 13, 14, 15, 42, 45 mesenteric angiography,10, 14, 15, 19, 46 and push enteroscopy12, 15, 19, 44 all have been successfully used. If small‐bowel bleeding seems likely, a deep double‐balloon enteroscopy can be done with care to avoid upper‐abdominal pressure on or near the LVAD.17, 18 Decker et al outline strategies for performing this procedure with the assistance of a cardiologist, cardiothoracic surgeon, perfusionist, and cardiac anesthesiologist.18 Lastly, a change in anticoagulation medication may be necessary, but there is little information available to make this decision. Sponga and coworkers reported a patient with HeartMate II and severe and refractory upper GI bleeding induced by acquired vWF deficiency.47 This 57‐year‐old man required 60 transfusions over 23 weeks. His bleeding eventually stopped following cessation of aspirin and maintaining low‐dose warfarin therapy (INR, 1.5–2). This approach needs validation in a prospective trial but can be considered in selected patients. Finally, we think that whether the contribution of anticoagulation plays a role in the development of GI bleeding in patients with LVADs remains unclear, as long‐term follow‐up of these patients is not available. Clinical trials are required to address the question of whether it is safe to discontinue anticoagulation in LVAD patients or to aim for a lower INR, at least in patients with acquired coagulation abnormalities. Other options for acute treatment include Contact F, hormonal therapy, and blood transfusions.25
Our review clearly depends on the available literature. First, we did not find any trials comparing LVAD type and GI bleeding. The information came from individual case reports, abstracts, and case reviews on a series of patients who developed GI bleeds, and relevant information was not consistently reported in these various sources. Most studies did not investigate GI bleeding as a specific endpoint. Second, the individual studies were not powered to provide precise estimates of the event rate for GI bleeding with LVADs, but we tried to provide numerical estimates from combined data when possible.
Conclusion
Use of LVADs is becoming more frequent in the treatment of CHF, and these patients have significant complications. They have an increased risk for GI bleeding, especially after the implantation of nonpulsatile devices. Identifying the site of bleeding may be difficult. Treatment options include changes in the device parameters, cardiac transplantation, and local control of the GI bleeding sites. The pathogenesis likely involves changes in vWF activity and stimulation of new‐vessel formation in the GI tract. The management of these cases will require coordinated efforts by various subspecialists.
References
- 1. American Heart Association. Heart failure. http://www.heart.org/HEARTORG/Conditions/HeartFailure/Heart‐Failure_UCM_002019_SubHomePage.jsp. Accessed January 18, 2011..
- 2. Lloyd‐Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham heart study. Circulation. 2002;106:3068–3072. [DOI] [PubMed] [Google Scholar]
- 3. Crow S, John R, Boyle A, et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;137: 208–215. [DOI] [PubMed] [Google Scholar]
- 4. Rose EA, Gelijns AC, Moskowitz AJ, et al. Long‐term mechanical left ventricular assistance for end‐stage heart failure. N Engl J Med. 2001;345:1435–1443. [DOI] [PubMed] [Google Scholar]
- 5. Feller ED, Sorensen EN, Haddad M, et al. Clinical outcomes are similar in pulsatile and nonpulsatile left ventricular assist device recipients. Ann Thorac Surg. 2007;83:1082–1088. [DOI] [PubMed] [Google Scholar]
- 6. Kamdar F, Boyle A, Liao K, et al. Effects of centrifugal, axial, and pulsatile left ventricular assist device support on end‐organ function in heart failure patients. J Heart Lung Transplant. 2009;28:352–359. [DOI] [PubMed] [Google Scholar]
- 7. Miller LW, Pagani FD, Russell SD, et al. Use of a continuous‐flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. [DOI] [PubMed] [Google Scholar]
- 8. Hagan K. LVADs help mend a broken heart. Nurse Pract. 2010;35:28–37. [DOI] [PubMed] [Google Scholar]
- 9. Holman WL, Rayburn BK, McGiffin DC, et al. Infection in ventricular assist devices: prevention and treatment. Ann Thorac Surg. 2003;75(suppl):S48–S57. [DOI] [PubMed] [Google Scholar]
- 10. Stern DR, Kazam J, Edwards P, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg. 2010;25:352–356. [DOI] [PubMed] [Google Scholar]
- 11. Fenkel JM, Grasso M, Goldberg EM, et al. Capsule endoscopy is safe in patients with pulsatile Novacor PC left ventricular assist device. Gastrointest Endosc. 2007;65:559–560. [DOI] [PubMed] [Google Scholar]
- 12. Seow CH, Zimmerman MJ. Capsule endoscopy in the detection of small‐intestinal bleeding in patients supported by a nonpulsatile axial‐flow Jarvik 2000 left ventricular assist device. Gastrointest Endosc. 2006;63:1087. [DOI] [PubMed] [Google Scholar]
- 13. Bechtel JF, Wellhoner P, Charitos EI, et al. Localizing an occult gastrointestinal bleeding by wireless PillCam SB capsule videoendoscopy in a patient with the HeartMate II left ventricular assist device. J Thorac Cardiovasc Surg. 2010;139:e73–e74. [DOI] [PubMed] [Google Scholar]
- 14. Girelli CM, Tartara P, Vitali E. Lack of reciprocal interference between capsule endoscope and left ventricular assist device. Endoscopy. 2006;38:94–95. [DOI] [PubMed] [Google Scholar]
- 15. Daas AY, Small MB, Pinkas H, et al. Safety of conventional and wireless capsule endoscopy in patients supported with nonpulsatile axial flow heart‐mate II left ventricular assist device. Gastrointest Endosc. 2008;68:379–382. [DOI] [PubMed] [Google Scholar]
- 16. Grasso M, Fenkel J, Sorensen E, et al. Gastrointestinal bleeding from arteriovenous malformations in recipients of left ventricular assist devices. J Cardiac Fail. 2007;13:S115. [Google Scholar]
- 17. Miller ED, Steidley DE, Arabia FA, et al. Gastric erosion associated with left ventricular assist device: new technology, new complication. Gastrointest Endosc. 2009;70:181–183. [DOI] [PubMed] [Google Scholar]
- 18. Decker GA, Miller ED, Pasha SF, et al. Deep enteroscopy in patients with left ventricular assist devices: practical and technical considerations. Endoscopy. 2010;42(suppl 2):E194. [DOI] [PubMed] [Google Scholar]
- 19. Letsou GV, Shah N, Gregoric ID, et al. Gastrointestinal bleeding from arteriovenous malformations in patients supported by the Jarvik 2000 axial‐flow left ventricular assist device. J Heart Lung Transplant. 2005;24:105–109. [DOI] [PubMed] [Google Scholar]
- 20. Cody MC, O'Donovan TP, Hughes RW Jr. Idiopathic gastrointestinal bleeding and aortic stenosis. Am J Dig Dis. 1974;19:393–398. [DOI] [PubMed] [Google Scholar]
- 21. Vincentelli A, Susen S, Le Tourneau T. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349: 343–349. [DOI] [PubMed] [Google Scholar]
- 22. Levine MN, Raskob G, Beyth RJ, et al. Hemorrhagic complications of anticoagulant treatment: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:287S–310S. [DOI] [PubMed] [Google Scholar]
- 23. Frazier OH, Gemmato C, Myers TJ, et al. Initial clinical experience with the HeartMate II axial‐flow left ventricular assist device. Tex Heart Inst J. 2007;34:275–281. [PMC free article] [PubMed] [Google Scholar]
- 24. Heyde EC. Gastrointestinal bleeding in aortic stenosis. N Engl J Med. 1958;259:196. [Google Scholar]
- 25. Morishima A, Marui A, Shimamoto T, et al. Successful aortic valve replacement for Heyde syndrome with confirmed hematologic recovery. Ann Thorac Surg. 2007;83:287–288. [DOI] [PubMed] [Google Scholar]
- 26. Olsson M, Hultcrantz R, Schulman S, et al. Acquired platelet dysfunction may be an aetiologic factor in Heyde's syndrome—normalization of bleeding time after aortic valve replacement. J Intern Med. 2002;252:516–523. [DOI] [PubMed] [Google Scholar]
- 27. Markl M, Benk C, Klausmann D, et al. Three‐dimensional magnetic resonance flow analysis in a ventricular assist device. J Thorac Cardiovasc Surg. 2007;134:1471–1476. [DOI] [PubMed] [Google Scholar]
- 28. Konig CS, Clark C, Mokhtarzadeh‐Dehghan MR. Investigation of unsteady flow in a model of a ventricular assist device by numerical modelling and comparison with experiment. Med Eng Phys. 1999;21:53–64. [DOI] [PubMed] [Google Scholar]
- 29. Legendre D, Antunes P, Bock E, et al. Computational fluid dynamics investigation of a centrifugal blood pump. Artif Organs. 2008;32:342–348. [DOI] [PubMed] [Google Scholar]
- 30. Moosavi MH, Fatouraee N. Flow simulation of a diaphragm‐type ventricular assist device with structural interactions. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:1027–1030. [DOI] [PubMed] [Google Scholar]
- 31. Geisen U, Heilmann C, Beyersdorf F, et al. Non‐surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. Eur J Cardiothorac Surg. 2008;33:679–684. [DOI] [PubMed] [Google Scholar]
- 32. Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous‐flow mechanical device support contributes to a high prevalence of bleeding during long‐term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–1213. [DOI] [PubMed] [Google Scholar]
- 33. Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous‐flow ventricular assist device recipients. Ann Thorac Surg. 2010;90:1263–1269. [DOI] [PubMed] [Google Scholar]
- 34. Heilmann C, Geisen U, Benk C, et al. Haemolysis in patients with ventricular assist devices: major differences between systems. Eur J Cardiothorac Surg. 2009;36:580–584. [DOI] [PubMed] [Google Scholar]
- 35. Malehsa D, Meyer AL, Bara C, et al. Acquired von Willebrand syndrome after exchange of the HeartMate XVE to the HeartMate II ventricular assist device. Eur J Cardiothorac Surg. 2009;35:1091–1093. [DOI] [PubMed] [Google Scholar]
- 36. Cappell MS, Lebwohl O. Cessation of recurrent bleeding from gastrointestinal angiodysplasias after aortic valve replacement. Ann Intern Med. 1986;105:54–57. [DOI] [PubMed] [Google Scholar]
- 37. Saito S, Westaby S, Piggot D, et al. End‐organ function during chronic nonpulsatile circulation. Ann Thorac Surg. 2002;74:1080–1085. [DOI] [PubMed] [Google Scholar]
- 38. Miyama M. Renal, intestinal and whole body metabolic changes in pig LVAD model [article in Japanese]. Hokkaido Igaku Zasshi. 1999;74:331–337. [PubMed] [Google Scholar]
- 39. Miyama M, Dihmis WC, Deleuze PH, et al. The gastrointestinal tract: an underestimated organ as demonstrated in an experimental LVAD pig model. Ann Thorac Surg. 1996;61: 817–822. [DOI] [PubMed] [Google Scholar]
- 40. John R. Current axial‐flow devices—the HeartMate II and Jarvik 2000 left ventricular assist devices. Semin Thorac Cardiovasc Surg. 2008;20:264–272. [DOI] [PubMed] [Google Scholar]
- 41. John R, Kamdar F, Liao K, et al. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge‐to‐transplant therapy. Ann Thorac Surg. 2008;86:1227–1235. [DOI] [PubMed] [Google Scholar]
- 42. Garatti A, Bruschi G, Colombo T, et al. Noncardiac surgical procedures in patient supported with long‐term implantable left ventricular assist device. Am J Surg. 2009;197:710–714. [DOI] [PubMed] [Google Scholar]
- 43. McCarthy PM, Smedira NO, Vargo RL, et al. One hundred patients with the HeartMate left ventricular assist device: evolving concepts and technology. J Thorac Cardiovasc Surg. 1998;115:904–912. [DOI] [PubMed] [Google Scholar]
- 44. Siddiqui MA, Slaughter MS, Silva RG. Gastrointestinal complications in patients supported with ventricular assist devices. Gastroenterology. 2009;136:A213–A214. [Google Scholar]
- 45. Garatti A, Bruschi G, Girelli C, et al. Small intestine capsule endoscopy in magnetic suspended axial left ventricular assist device patient. Interact Cardiovasc Thorac Surg. 2006;5: 1–4. [DOI] [PubMed] [Google Scholar]
- 46. Tulchinsky M. Lower gastrointestinal bleeding diagnosed by red blood cell scintigraphy in a patient with a left ventricular assist device. Clin Nucl Med. 2008;33:856–858. [DOI] [PubMed] [Google Scholar]
- 47. Sponga S, Nalli C, Casonato A, et al. Severe upper gastrointestinal bleeding in HeartMate II induced by acquired von Willebrand deficiency: anticoagulation management. Ann Thorac Surg. 2012;94:e41–e43. [DOI] [PubMed] [Google Scholar]
- 48. Hou JK, Hampel H, Lukens FJ. Gastric ulceration and perforation as a complication of a left ventricular assist device. Gastrointest Endosc. 2005;61:629–631. [DOI] [PubMed] [Google Scholar]
- 49. Gogas BD, Parissis JT, Filippatos GS, et al. Severe anaemia and subcapital femur fracture in a patient with left ventricular assist device HeartMate II: the cardiologist's management of this rare patient. Eur J Heart Fail. 2009;11:806–808. [DOI] [PubMed] [Google Scholar]
- 50. Aleksic I, Baryalei MM, Schorn B, et al. Resection for CMV ileitis in a patient supported by a left‐ventricular assist device. Thorac Cardiovasc Surg. 1998;46:105–106. [DOI] [PubMed] [Google Scholar]
- 51. Hetzer R, Weng Y, Potapov EV, et al. First experiences with a novel magnetically suspended axial flow left ventricular assist device. Eur J Cardiothorac Surg. 2004;25:964–970. [DOI] [PubMed] [Google Scholar]
- 52. Wang IW, Guthrie T, Ewald G, et al. Gastrointestinal bleeding complications in continuous flow LVAD patients—is it device specific?. J Heart Lung Transplant. 2010;29:S8. [Google Scholar]
- 53. Meyer AL, Malehsa D, Bara C, et al. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circ Heart Fail. 2010;3:675–681. [DOI] [PubMed] [Google Scholar]
- 54. Aggarwal A, Pant R, Kumara S, et al. Incidence and management of gastrointestinal bleeding with continuous flow assist devices. Ann Thorac Surg. 2012;93:1534–1540. [DOI] [PubMed] [Google Scholar]
- 55. Schaffer JM, Arnaoutakis JG, Allen JG, et al. Bleeding complications and blood product utilization with left ventricular assist device implantation. Ann Thorac Surg. 2011;91:740–749. [DOI] [PubMed] [Google Scholar]
- 56. Tarzia V, Dal Lin C, Bottio T, et al. Occult gastrointestinal bleeding in patients with a left ventricular assist device axial flow pump: diagnostic tools and therapeutic algorithm. J Thorac Cardiovasc Surg. 2012;143:e28–e31. [DOI] [PubMed] [Google Scholar]
- 57. Demirozu ZT, Radovancevic R, Hochman LF, et al. Arteriovenous malformation and gastrointestinal bleeding in patients with the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2011;30:849–853. [DOI] [PubMed] [Google Scholar]
- 58. Kushnir VM, Sharma S, Ewald GA, et al. Evaluation of GI bleeding after implantation of left ventricular assist device. Gastrointest Endosc. 2012;75:973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Morgan JA, Paone G, Nemeh HW, et al. Gastrointestinal bleeding with the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2012;31:715–718. [DOI] [PubMed] [Google Scholar]
- 60. John R, Kamdar F, Eckman P, et al. Lessons learned from experience with over 100 consecutive HeartMate II left ventricular assist devices. Ann Thorac Surg. 2011;92:1593–1599. [DOI] [PubMed] [Google Scholar]


