Abstract
Background
Many military veterans in the United States with coronary artery disease continue to smoke despite undergoing percutaneous coronary intervention (PCI). Previous studies have described improved cardiovascular outcomes in smokers, the so‐called “smokers' paradox.” In this study, we examined the effects of smoking on cardiovascular outcomes following PCI.
Hypothesis
Do patients who smoke have different post‐PCI outcomes than nonsmokers?
Methods
All patients who underwent PCI at a single US Veterans Administration hospital from 2004 to 2009 were followed. Outcomes of interest included myocardial infarction, unplanned coronary intervention, unplanned cardiac hospitalization, death, and a composite of events for 6 months after PCI. Changes in traditional risk factors were also assessed.
Results
Unadjusted analysis revealed that in almost all categories, smokers had lower incidence of adverse events than nonsmokers. However, after adjusting for the older age of the nonsmokers, no favorable statistical trend toward smokers was seen. Significant improvement in blood pressure and lipid levels were seen in both groups.
Conclusions
After adjusting for differences in age, there did not appear to be any protective effect of smoking on cardiovascular outcomes following PCI. Smokers achieved similar degrees of risk factor optimization during the follow‐up period as their nonsmoker counterparts. Aggressive efforts to decrease the prevalence of smoking must be maintained.
Introduction
The United States Surgeon General has called smoking “the leading preventable cause of disease and deaths in the US.”1 More than 18% of the 2.4 million annual deaths in the United States can be attributed to smoking.2, 3 The US Veterans Administration (VA), which is responsible for medical care for over 23 million US military personnel, spends in excess of $44 billion per year on healthcare costs for veterans.4 However, the prevalence of smoking in the VA population is significantly higher than the general population (31% vs 23%).5, 6
Overall, smoking is believed to be associated with adverse long‐term prognosis post‐PCI.7 These adverse outcomes are linked to the ability of tobacco to induce coronary vasoconstriction and reduce prostacyclin production.8, 9 This biochemical derangement leads to endothelial dysfunction9 and abnormal platelet activation, all of which can enhance restenosis following percutaneous coronary intervention (PCI).10 Although multiple studies in smokers have yielded conflicting results on coronary angiographic outcomes post‐PCI,7, 11, 12, 13, 14, 15 a phenomenon known as the “smoker's paradox” has often been observed, where smokers appear to have lower rates of adverse outcomes compared to nonsmokers.7
In this single‐center, retrospective cohort, VA study, we analyzed the relationship between smoking and clinical outcomes including mortality, myocardial infarction (MI), unplanned cardiac hospitalization, unplanned cardiac intervention, and overall composite events at 6 months post‐PCI.
Methods
Patient Population
The study population was derived from 772 unique veteran subjects who underwent PCI for various clinical indications at Jesse Brown VA Hospital (JBVA) from 2004 to 2009. Of the 772 veterans, information about smoking was available for 759 patients. (Figure 1)
Figure 1.
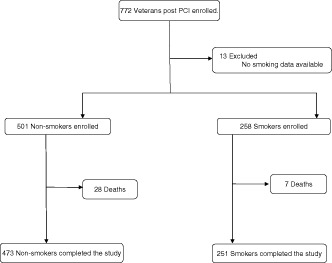
Study selection. Abbreviations: PCI, percutaneous coronary intervention.
Clinical Data
All patients who underwent percutaneous revascularization at JBVA were enrolled in the study according to a protocol developed in compliance with regulations of the Health Insurance Portability and Accountability Act, and approved by the JBVA institutional review board (IRB) and the University of Illinois at Chicago (UIC) IRB. Data were obtained by reviewing patients' information using the computerized patient record system, which can access patient information from any VA hospital in the country. In addition to collecting information pertaining to smoking, demographic information including gender, race, height, and weight were obtained through retrospective chart review. Baseline medical information during the time of index PCI was tabulated. These data included indication for PCI, cardiac risk factors (such as smoking, diabetes mellitus [DM], hypertension [HTN]), prior cardiac history, medications, and laboratory values on admission, all of which were stratified by smoking status. Six month follow‐up data after the index PCI were collected. Outcomes analyzed were mortality, MI, unplanned coronary intervention, unplanned cardiac rehospitalization, and composite major adverse cardiac events (MACE). After 6 months, cardiac risk factors were once again assessed by smoking status.
Smoking Status
Smoking status of patients at the beginning of the study and at 6‐month follow‐up was obtained from retrospective chart review. Because self‐reported truthful disclosure of smoking behavior had been well validated in previous studies (false reporting rate of about 4%–7%),16, 17, 18 patients reporting any cigarette smoking, regardless of amount and type of tobacco use, were considered smokers.
The study population was divided into 2 groups (Figure 1) on the basis of smoking status at baseline. The first group comprised of nonsmokers, classified as patients who had never smoked or had quit smoking at any point prior to index PCI. The second group were labeled smokers and consisted of patients who were smoking up to the time of their index PCI. Owing to the short‐term follow‐up period of only 6 months, patients who quit after their index PCI were still grouped in the smoker group.
End Points
The primary end points of the study were death from any cause, MI, unplanned cardiac hospitalization, unplanned coronary intervention, and MACE. Secondary end points of the study were to determine adequacy of treatment of secondary preventative strategies through cardiac risk factor modification.
Statistical Analysis
Data Was analyzed using SAS statistical software package (SAS Institute Inc., Cary, NC). Continuous variables were summarized as mean ± standard deviation (SD). Student t test was used to assess differences in continuous variables between the nonsmoking and smoking groups. Categorical variables were displayed as percentages and were compared using χ2 test. For statistical purposes, patients with multiple events during the follow‐up period were only counted once with respect to the MACE end point regardless of the number of individual events. To study the relationship between outcomes and multiple categorical and continuous variables by smoking status, univariate, multivariate, and paired t test analysis were used. To control for age, logistic regression model was used for categorical variables, whereas generalized linear model was used for continuous variables. Probability values of P < 0.05 were considered significant.
Results
Baseline Patient Demographic and Clinical Characteristics
Of the total 772 unique patients during the enrollment period, 759 (98.3%) had available smoking data. At the time of the index PCI, 258 patients were smokers, whereas 501 were nonsmokers. Nonsmokers and smokers undergoing percutaneous intervention had marked differences in baseline clinical characteristics as outlined in Table 1. Both groups were overwhelmingly male, reflective of the overall veteran population. Smokers were on average 7 years younger than nonsmokers (61 years old vs 68 years old, P ≤ 0.001). The nonsmoker cohort had a higher incidence of DM and increased medication usage, particularly statins and angiotensin‐converting enzyme inhibitors/angiotensin receptor blocker (ACEI/R) ACEI/ARB, than smokers.
Table 1.
Baseline Characteristics of Nonsmokers and Smokers
| Nonsmokers, n = 501 | Smokers, n = 258 | P Value | Age‐Adjusted P Value | |
|---|---|---|---|---|
| Age, y (±SD) | 68 (10.4) | 61 (8.9) | <0.001 | |
| Sex | ||||
| Male, n (%) | 493 (98.4) | 256 (99.2) | <0.001 | 0.46 |
| Female, n (%) | 8 (1.6) | 2 (0.8) | ||
| Race, n (%) | ||||
| Caucasian | 202 (40.3) | 105 (40.7) | ||
| African American | 255 (50.9) | 144 (55.8) | ||
| Hispanic | 30 (6) | 3 (1.2) | 0.03 | 0.76 |
| Asian | 7 (1.4) | 1 (0.4) | ||
| Other | 7(1.4) | 5 (1.9) | ||
| PCI indications, n (%) | ||||
| Planned | 33 (7) | 12 (5) | 0.16 | 0.58 |
| Positive stress test | 162 (32) | 89 (34) | ||
| Stable angina | 81 (16) | 32 (12) | ||
| Unstable angina | 96 (19) | 40 (15) | ||
| NSTEMI | 66 (13) | 47 (18) | ||
| STEMI | 18 (4) | 13 (5) | ||
| CHF | 16 (3) | 13 (5) | ||
| Arrhythmia | 13 (3) | 4 (2) | ||
| Weight, lb (±SD) | 205.7 (43.8) | 199.2 (45) | <0.001 | <0.001 |
| HTN, mm Hg (±SD) | ||||
| Mean SBP | 134 (22) | 132 (22) | 0.14 | 0.57 |
| Mean DBP | 72 (13) | 73 (12) | 0.33 | 0.6 |
| Diabetes, n (%) | 222 (44) | 76 (30) | <0.001 | <0.001 |
| HGB A1c, % (±SD) | 7.6 (1.8) | 7.72 (2.4) | 0.5 | 0.42 |
| Glucose, mg/dL (±SD) | 134 (61) | 128 (62) | 0.2 | 0.04 |
| Creatinine, mg/dL (±SD) | 1.4 (1.1) | 1.2 (1.0) | 0.02 | 0.21 |
| Lipids, mg/dL (±SD) | ||||
| Total cholesterol | 162 (41) | 173 (50) | 0.001 | 0.15 |
| HDL | 40 (16) | 39 (19) | 0.71 | 0.58 |
| LDL | 94 (35) | 104 (42) | 0.001 | 0.09 |
| Triglycerides | 148 (99) | 162 (139) | 0.12 | 0.61 |
| Medications, n (%) | ||||
| β‐Blocker | 376 (75) | 171 (66) | 0.01 | 0.09 |
| Calcium channel blocker | 180 (36) | 77 (30) | 0.1 | 0.14 |
| Diuretic | 214 (43) | 95 (37) | 0.12 | 0.2 |
| Nitrate | 132 (26) | 42 (16) | 0.002 | 0.05 |
| ACEI/ARB | 340 (68) | 143 (56) | <0.001 | 0.003 |
| Other anti‐HTN | 78 (16) | 30 (12) | 0.12 | 0.25 |
| Aspirin | 379 (76) | 180 (70) | 0.1 | 0.17 |
| Statin | 376 (75) | 161 (63) | 0.001 | 0.007 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CHF, congestive heart failure; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; HGB A1c, hemoglobin A1c; HTN, hypertension; LDL, low‐density lipoprotein; NSTEMI, non– ST‐elevation myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SD, standard deviation; STEMI, ST‐elevation myocardial infarction.
Unadjusted Results
Although smokers were more likely to have undergone PCI for MI, there was a statistically significant difference between the 2 groups for indications for their interventions (Table 1). No significant differences were observed in baseline weight and HTN management between the 2 cohorts. Although diabetes was more prevalent in the nonsmoking group (44% vs 33%, P ≤ 0.001), there were no statistical differences in glycosylated hemoglobin (HgbA1c), glucose, and creatinine between the 2 groups. Medication profile comparison between the groups showed an increased overall use of medications in the nonsmoking cohort. As outlined in Table 1, nonsmokers were on a higher number of medications.
Results After Controlling for Age
Because the mean age of the smoking cohort was 7 years younger than the nonsmoking cohort, age as a confounding factor in the above variables was considered. After controlling for age, multiple variables lost their statistical significance (Table 1). The following groups emerged as having statistically significant differences between the cohorts: weight (P ≤ 0.001), diabetes (P ≤ 0.001), glucose (P = 0.04), and the use of following medications: nitrates (P = 0.05), ACEI/ARB receptor blocker (P = 0.003), and statins (P = 0.007).
Six‐Month Clinical Follow‐up
Primary End Points
There was a statistically significant increase in MI in the nonsmoking cohort (2.4% vs 0.4%, P = 0.04). The incidence of death (5.6% vs 2.7%, P = 0.07), unplanned cardiac hospitalizations (18.2 vs 15.5%, P = 0.36), and MACE (25% vs 19%, P = 0.08) showed a trend toward higher event rates in nonsmokers. Unplanned cardiac intervention rates were higher in smokers (3.6% vs 5.8%, P = 0.16) (Figure 2). However, after controlling for age, no statistically significant difference was seen between the 2 groups (Table 2).
Figure 2.
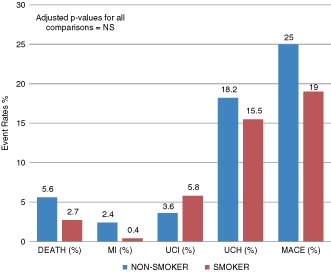
Impact of smoking status on 6‐month clinical outcomes. Abbreviations: MACE, major adverse cardiac events; MI, myocardial infarction; NS, nonsignificant; UCH, unplanned cardiac hospitalization; UCI, unplanned cardiac intervention.
Table 2.
Impact of Smoking Status on 6‐Month Clinical Outcomes (Primary End Points)
| Events | Nonsmokers, n = 501 | Smokers, n = 258 | P Value | Age‐Adjusted P Value |
|---|---|---|---|---|
| Death, n (%) | 28 (5.6) | 7 (2.7) | 0.07 | 0.62 |
| Myocardial infarction, n (%) | 12 (2.4) | 1 (0.4) | 0.04 | 0.09 |
| Unplanned cardiac intervention, n (%) | 18 (3.6) | 15 (5.8) | 0.16 | 0.18 |
| Unplanned cardiac hospitalization, n (%) | 91 (18.2) | 40 (15.5) | 0.36 | 0.83 |
| Composite events, n (%) | 125 (25) | 50 (19) | 0.08 | 0.90 |
Secondary End Points
At 6 months, marked improvements in clinical profiles that pertain to secondary cardiovascular risk reduction were seen in both arms (Table 3). Although there were differences in baseline medication usages, particularly statins and ACEI/ARB, between the two groups at the beginning of the study, no such age‐adjusted statistical differences were noted at 6 months. Glycemic control remained poor in both groups with mean HgbA1c for both cohorts remaining steady at 7.7%.
Table 3.
Characteristics of Nonsmokers and Smokers at 6 Months
| Nonsmokers, n = 473 | Smokers, n = 251 | P Value | Age‐Adjusted P Value | |
|---|---|---|---|---|
| Weight, lb (±SD) | 206 (43) | 200 (43) | 0.08 | <0.001 |
| HTN, mm Hg (±SD) | ||||
| Mean SBP | 130 (20) | 125 (18) | 0.006 | 0.01 |
| Mean DBP | 70 (12) | 71 (11) | 0.36 | 0.19 |
| HGB A1c, % (±SD) | 7.7 (1.9) | 7.7 (2.5) | 0.9 | 0.54 |
| Glucose, mg/dL (±SD) | 132 (60) | 132 (70) | 0.9 | 0.35 |
| Creatinine, mg/dL (±SD) | 1.4 (0.8) | 1.3 (0.9) | 0.06 | 0.41 |
| Lipids, mg/dL, n (%) | ||||
| Total cholesterol | 149 (41) | 156 (42) | 0.03 | 0.5 |
| HDL | 38 (12) | 38 (13) | 0.7 | 0.22 |
| LDL | 85 (33) | 90 (31) | 0.05 | 0.3 |
| Triglycerides | 141 (102) | 162 (167) | 0.07 | 0.84 |
| Medications, n (%) | ||||
| β‐Blocker | 440 (88) | 228 (90) | 0.4 | 0.98 |
| Calcium channel blocker | 153 (31) | 74 (29) | 0.7 | 0.81 |
| Diuretic | 221 (44) | 102 (40) | 0.3 | 0.69 |
| Nitrate | 157 (31) | 61 (24) | 0.04 | 0.14 |
| ACEI/ARB | 381 (76) | 189 (75) | 0.7 | 0.37 |
| Other anti‐HTN | 101 (20) | 33 (13) | 0.02 | 0.07 |
| Aspirin | 470 (94) | 240 (94) | 0.96 | 0.55 |
| Statin | 440 (89) | 223 (89) | 0.9 | 0.62 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; HGB A1c, hemoglobin A1c; HTN, hypertension; LDL, low‐density lipoprotein; SBP, systolic blood pressure; SD, standard deviation.
Discussion
One of the goals of this study was to address a clinical phenomenon often referred to as the “smoker's paradox”. Multiple studies in the past have shown that smokers who underwent PCI seem to have paradoxically better outcomes than nonsmokers. Barbash et al. found that smokers undergoing thrombolytic therapy had better 30‐day mortality rates and less in‐hospital complications than nonsmokers.19 These results were also replicated by other studies.20, 21, 22 In the PCI era, similar unconventional results have been seen. However, most of these studies were done in the 1980s and 1990s, when balloon angioplasty (BA) was the standard of care, and focused on angiographic outcomes post‐PCI. Cohen et al. studied the impact of angiographic restenosis and target lesion revascularization in smokers, and determined that smokers had better outcomes.7 Weisz et al, whose cohort consisted of patients undergoing coronary stenting and BA, found similar results in smokers.23
In this contemporary study of PCI outcomes within a VA population, the results reaffirmed the finding of previous studies. After initial analysis, smokers appeared to do better than nonsmokers and showed a strong favorable statistical trend in almost all primary end points (Table 2). However, after controlling for the significant age disparity, any suggestion of a protective effect of smoking disappeared. Prior studies in different populations demonstrated similar findings.19, 20, 21, 22, 23, 24 Furthermore, in this study, nonsmokers had a higher baseline risk profile than smokers. Nonsmokers had an elevated incidence of diabetes and were on more medications, particularly statins for hyperlipidemia and ACEI/ARB for hypertension, than smokers. These observations should lead to worst outcomes in nonsmokers than smokers. Although a higher baseline statin use may have theoretically provided a better degree of cardioprotective effect in nonsmokers, both cohorts were aggressively treated, and there were no statistical differences in statin usage between the two groups at 6 months.
The impact of age cannot be emphasized enough. Smoking likely contributed to these patients requiring coronary revascularization at a much younger age.25, 26 Although some may look at these data and conclude that there is no difference between smokers and nonsmokers, one should note that by smoking, these patients had outcomes similar to relatively sicker nonsmokers who were 7 years older in age.
What is unique about this trial is that it was conducted at a VA facility, where access to care is not constrained by economic and social factors. Veterans have equal access to care regardless of their ability to pay. Furthermore, all VA facilities are linked through online electronic medical records, which can readily be accessed, thus minimizing the number of patients who were lost to follow‐up. The ability to provide universal healthcare access to veterans is what made the secondary end points of this study a special aspect of this trial. Aggressive approaches for treatment and control of coronary artery disease (CAD) risk factors for secondary prevention in smokers was noted. More has to be done to improve glycemic control in high‐risk cohorts.
Clinical Applicability
The apparent smoker's paradox seen in short‐term outcomes in smokers undergoing PCI should be interpreted with caution. Lack of age‐adjusted statistical significance should focus on the deleterious effects of smoking on CAD, manifested by an earlier need for coronary artery interventions. Long‐term outcomes continue to favor nonsmokers, and every attempt should be made to encourage smoking cessation and abstinence in all patients. Increased physician vigilance is required to control modifiable risk factors for secondary prevention. More has to be done in veterans to control diabetes.
Limitations
Our study had several limitations. First, this was a retrospective study and therefore subjected to limitations inherent in any retrospective study. The sample size was small, and the follow‐up period was only 6 months long. All patients who quit after PCI were randomized to the smoking cohort. No quantitative analyses were done to categorize the degree of smoking in patients. Data on angiographic outcomes of coronary arteries on patients undergoing repeat revascularization were not evaluated. Over 98% of the patients in the study were males. Some patients may have left the VA system to obtain care at other institutions; however, remote access was used to check all other VA facilities and read subsequent notes to see if any care was provided outside the VA. Although there is a small chance that patients who died at other institutions may have not been accounted for in this study, due to the unique integrated aspects of the VA's notification system, patients who die at home or other hospitals are usually entered into the VA medical records.
Conclusion
Smokers, despite having a healthier baseline profile than nonsmokers, underwent coronary interventions at a much younger age. The famous smoker's paradox does not hold true once the data are controlled for age. Long‐term studies have continued to demonstrate adverse outcomes in smokers, and every attempt should be made to encourage cessation and abstinence from smoking for both primary and secondary prevention of CAD.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1.US Surgeon General Clinical Practice Guidelines. Treating tobacco use and dependence: 2008 update. http://www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf. Accessed June 5, 2010.
- 2.Healthy People 2010. Understanding and improving health. http://www.healthypeople.gov/document/pdf/uih/uih.pdf. Accessed June 5, 2010.
- 3.American Heart Association. Cigarrete smoking statistics. http://www.americanheart.org/presenter.jhtml?identifier=4559. Accessed June 5, 2010.
- 4.Department of Veterans Affairs. VA stats at a glance (April 2010). VA benefits and health care utilization. http://www1.va.gov/VETDATA/Pocket‐Card/4X6_spring10_sharepoint.pdf. Accessed June 5, 2010.
- 5.Spigel S. Old research report. Smoking among veterans. September 7, 2007. http://www.cga.ct.gov/2007/rpt/2007‐R‐0534.htm. Accessed June 5, 2010.
- 6. McKinney WP, McIntire DD, Carmody TJ, et al. Comparing the smoking behavior of veterans and nonveterans. Public Health Rep. 1997;112:212–217. [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen DJ, Doucet M, Cutlip DE, et al. Impact of smoking on clinical and angiographic restenosis after PCI. Another smoker's paradox. Circulation. 2001;104:773–778. [DOI] [PubMed] [Google Scholar]
- 8. Quillen JE, Rossen JD, Oskarsson HJ, et al. Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am Coll Cardiol. 1993;22:642–647. [DOI] [PubMed] [Google Scholar]
- 9. Nadler JL, Brinkman HJ, van Mourik JA, et al. Cigarette smoking inhibits prostacyclin formation. Lancet. 1983;1:1248–1250. [DOI] [PubMed] [Google Scholar]
- 10. Benowitz NL. Pharmacologic aspects of cigarette smoking and nicotine addiction. N Engl J Med. 1988;319:1318–1330. [DOI] [PubMed] [Google Scholar]
- 11. Hasdai D, Garratt KN, Grill DE, et al. Effect of smoking status on the long‐term outcome after successful percutaneous coronary revascularization. N Engl J Med. 1997;336:755–761. [DOI] [PubMed] [Google Scholar]
- 12. Macdonald RG, Henderson MA, Hirshfeld JW Jr, et al. Patient‐related variables and restenosis after percutaneous transluminal coronary angioplasty: a report from the M‐HEART Group. Am J Cardiol. 1990;66:926–931. [DOI] [PubMed] [Google Scholar]
- 13. Violaris AG, Thury A, Regar E, et al. Influence of a history of smoking on short term (six month) clinical and angiographic outcome after successful coronary angioplasty. Heart. 2000;84:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galan KM, Deligonul U, Kern MJ, et al. Increased frequency of restenosis in patients continuing to smoke cigarettes after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1988;61:260–263. [DOI] [PubMed] [Google Scholar]
- 15. Ishikawa T, Yagi H, Ogawa T, et al. Deteriorative effect of smoking on target lesion revascularization after implantation of coronary stents with diameter of 3.0 mm or less. Circ J. 2005;69:227–231. [DOI] [PubMed] [Google Scholar]
- 16. Vartiainen E, Seppala T, Lillsunde P, et al. Validation of self reported smoking by serum cotinine measurement in a community‐based study. J Epidemiol Community Health. 2002;56:167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atterbring J, Herlitz J, Berndt AK, et al. Are patients truthful about their smoking habits? A validation of self‐report about smoking cessation with biochemical markers of smoking activity amongst patients with ischaemic heart disease. J Intern Med. 2001;249:145–151. [DOI] [PubMed] [Google Scholar]
- 18. Studts J, Ghate SR, Jill JL, et al. Validity of self‐reported smoking status among participants in a lung cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2006;15:1825–1828. [DOI] [PubMed] [Google Scholar]
- 19. Barbash GI, Reiner J, White HD. Evaluation of paradoxic beneficial effects of smoking in patients receiving thrombolytic therapy for acute myocardial infarction: mechanism of the “smoker's paradox” from the GUSTO‐I trial, with angiographic insights. Global Utilization of Streptokinase and Tissue‐Plasminogen Activator for Occluded Coronary Arteries. J Am Coll Cardiol. 1995;26:1222–1229. [DOI] [PubMed] [Google Scholar]
- 20. Grines CL, Topol EJ, O'Neill WW, et al. Effect of cigarette smoking on outcome after thrombolytic therapy for myocardial infarction. Circulation. 1995;91:298–303. [DOI] [PubMed] [Google Scholar]
- 21. Zahger D, Cercek B, Cannon CP, et al. How do smokers differ from nonsmokers in their response to thrombolysis? (the TIMI‐4 trial). Am J Cardiol. 1995;75:232–236. [DOI] [PubMed] [Google Scholar]
- 22. Gottlieb S, Boyko V, Zahger D, et al. Smoking and prognosis after acute myocardial infarction in the thrombolytic era (Israeli Thrombolytic National Survey). J Am Coll Cardiol. 1996;28:1506–1513. [DOI] [PubMed] [Google Scholar]
- 23. Weisz G, Cox DA, Garcia E, et al. Impact of smoking status on outcomes of primary coronary intervention for acute myocardial infarction—the smoker's paradox revisited. Am Heart J. 2005;150:358–364. [DOI] [PubMed] [Google Scholar]
- 24. Hasdai D, Larman A, Rihal CS, et al. Smoking status and outcome after primary coronary angioplasty for acute myocardial infarction. Am Heart J. 1999;137(4 pt 1):612–620. [DOI] [PubMed] [Google Scholar]
- 25. Moran A, Gu D, Zhao D, et al. Future cardiovascular disease in China: Markov model and risk factor scenario projections from the coronary heart disease policy model‐China. Circ Cardiovasc Qual Outcomes. 2010;3:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kunitomo M, Yamaguchi Y, Kagota S, et al. Biochemical evidence of atherosclerosis progression mediated by increased oxidative stress in apolipoprotein E‐deficient spontaneously hyperlipidemic mice exposed to chronic cigarette smoke. J Pharmacol Sci. 2009;110:354–361. [DOI] [PubMed] [Google Scholar]


