Abstract
Background
Narrow fragmented QRS (fQRS) has recently been recognized as a significant predictor of prognosis in various cardiovascular diseases.
Hypothesis
We hypothesized that the presence of narrow fQRS on admission electrocardiogram (ECG) in patients with decompensated systolic heart failure (HF) of any cause would be associated with long‐term prognosis.
Methods
Patients hospitalized for decompensated HF due to ischemic or nonischemic dilated cardiomyopathy (left ventricular ejection fraction <35%) were retrospectively analyzed. The primary clinical end points were cardiovascular mortality, sudden cardiac death, and rehospitalization for HF.
Results
The mean duration of follow‐up was 3.73 ± 1.41 years. Patients were classified as fQRS(+) group (n = 114; mean age, 63.49 ± 12.04 years) and fQRS(−) group (n = 113 patients; mean age, 65.04 ± 11.95 years). fQRS on ECG was significantly correlated with New York Heart Association (NYHA) functional class (P = 0.001). In multivariate Cox proportional hazard analysis, narrow fQRS (odds ratio [OR]: 3.130, 95% confidence interval [CI]: 1.560‐2.848, P = 0.001), chronic renal failure (OR: 2.455, 95% CI: 1.120‐5.381, P = 0.025), NYHA class (OR: 8.305, 95% CI: 2.568‐26.855, P < 0.0001), and hypoalbuminemia (OR: 2.099, 95% CI: 1.122‐3.926, P = 0.020) were independent predictors of cardiovascular mortality. In Kaplan‐Meier survival analysis, narrow fQRS on admission ECG predicted worse survival rate at 84 months; survival probability significantly decreased in the fQRS(+) group compared with fQRS(−) group (P < 0.0001).
Conclusions
Presence of narrow fQRS is associated with worse NYHA functional class in patients hospitalized for decompensated HF. Narrow fQRS predicts cardiovascular mortality in a specific subgroup of systolic HF patients, namely those hospitalized for decompensated HF of both ischemic and nonischemic causes.
Introduction
Despite recent advances in the treatment of heart failure (HF), prognosis still remains poor. Half of the patients diagnosed with new onset HF die within 5 years; in cases with advanced HF the outlook is worse; the 1‐year survival rate is approximately 50%.1, 2, 3 Standard 12‐lead electrocardiogram (12 L‐ECG) is an important prognostic and diagnostic tool in patients with HF.
Patients with organic heart disease often have wide QRS complexes due to bundle branch blocks, a finding associated with adverse prognosis.4 Fragmentation in the QRS complex, irrespective of duration, is a result of myocardial scarring that causes heterogeneous ventricular activation and dyssynchronous contraction. Narrow fragmented QRS (fQRS), caused by nonspecific electrical deviation and/or deformation of QRS morphology, has recently been recognized as a significant predictor of cardiovascular events and prognosis in patients with coronary artery disease, acute coronary syndrome (ACS), and ischemic (ICM) and nonischemic dilated cardiomyopathy (DCM).5, 6, 7, 8, 9, 10, 11, 12 Prognostic utility of fQRS has also been demonstrated in Brugada syndrome and arrhythmogenic right ventricular dysplasia (ARVD).13, 14
To diagnose fQRS, typical complete bundle branch blocks (QRS duration [QRSd] ≥ 120 ms) and incomplete right bundle branch blocks (QRSd <120 ms) must be ruled out. Fragmented QRS has also been described in wide QRS situations (QRSd ≥120 ms) such as bundle branch blocks, premature ventricular ectopic beats, and paced QRS complexes.4
The prognostic significance of narrow fQRS has been previously evaluated in left ventricular (LV) systolic dysfunction. Presence of fQRS was found to be an independent predictor of the high sudden cardiac death (SCD) risk in patients with ICM.10 Wide and narrow fQRS on ECG strongly predicts event‐free survival rates in patients with DCM with ejection fraction (EF) <40%.11 The significance of fQRS in patients receiving device‐based therapy for HF was also examined. In a study of ICM and DCM, patients with implanted intracardiac defibrillators (ICD) were investigated, and fQRS was found to be a predictor of fatal arrhythmic events.15
We hypothesized that the presence of narrow fQRS on admission ECG in patients with decompensated systolic HF of any cause would be associated with long‐term prognosis in terms of cardiovascular mortality and SCD.
Methods
Patients hospitalized for ICM or DCM (LV ejection fraction [LVEF] <35%]) in a university hospital between the years 2005 and 2009 were retrospectively analyzed. All patients had decompensated HF, with New York Heart Association (NYHA) class II–IV symptoms despite optimal medical therapy. DCM was diagnosed according to current guideline criteria,16 and ICM was diagnosed if ≥70% stenosis was demonstrated in at least 1 major epicardial coronary artery or with positive history for previous myocardial infarctions.
A 12‐lead admission ECG was obtained from all patients. All ECGs (filter range 0.5 Hz to 150 Hz, AC filter 60 Hz, 25 mm/s, 10 mm/mV) were analyzed by 2 independent clinicians who were blinded to the study design and clinical data. Narrow fQRS was described as narrow QRS complex duration (<120 ms) in addition to notching in R′ or QRS complex. The presence of various RSR′ patterns, including an additional R wave (R′) or notching of the R wave or S wave, or the presence of more than 1 R′ (fragmentation) without typical bundle branch block in 2 contiguous leads corresponding to a major lead set for major coronary artery territory, was sought for the diagnosis of fQRS (Figure 1). A notch on an R or S wave was defined as a definite but transient reversal of direction of the main deflection.4 A 12 L‐ECG of each patient was analyzed without using any magnification by 2 clinicians blinded to clinical and angiographic characteristics. The interobserver concordance rate in detection of fQRS was 96%. In case of disagreement, the final diagnosis was achieved by mutual agreement. The intraobserver concordance rate was 97%. The QRS duration was determined by measuring the longest QRS in any lead by both manual readings and digital records obtained by the ECG machine.
Figure 1.
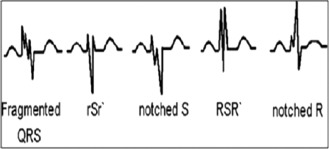
Examples of fragmented QRS pattern.
Patients with the following characteristics were ruled out: (1) presence of bundle branch block, Wolff‐Parkinson‐White syndrome, Brugada syndrome, or QRSd >120 ms on 12 L‐ECG; (2) patients with suspected subclinical myocardial involvement such as positive history of chronic inflammatory disease states or acute infective conditions; and (3) presence of significant valvular heart disease or permanent pacemaker.
Patient files were examined to evaluate demographic and clinical characteristics such as age, gender, functional status in terms of NYHA class, hypertension, hyperlipidemia, diabetes mellitus, smoking habits, peripheral arterial disease, cerebrovascular disease, and potential etiological factors for the development of HF, such as coronary artery disease, pregnancy, previous cardiotoxic drug exposure, and chronic renal failure. Past history, including previous hospitalizations for HF, myocardial infarctions, and revascularization procedures, along with medications used in index hospitalization, were also recorded. Records were examined for biochemical tests including urea, creatinine, electrolytes, fasting glucose, serum protein and albumin, troponin T, and C‐reactive protein.
Patients underwent standard transthoracic echocardiographic examinations using a System V echocardiography machine (GE, Vingmed Ultrasound AS; Horten, Norway). Echocardiography reports were examined for: LV mass, left atrial size, presence and severity of mitral, aortic and tricuspid regurgitations, pulmonary artery systolic pressure, mitral inflow E/A ratio, and EF determined with Simpson's method of discs using 2‐dimensional images.
The primary clinical end points consisted of cardiovascular mortality, SCD, and rehospitalization for decompensated HF. SCD was defined as death occurring in <1 hour after presentation of acute symptoms in a patient known to be in stable medical condition in the previous 24 hours.17 Secondary clinical end points were stroke and hemodynamically significant ventricular arrhythmias. Information regarding clinical end points was gathered from patient records or telephone interviews with patients or relatives. All patients were above 18 years of age and able to provide written informed consent, which was a prerequisite for enrollment. The study complies with the Declaration of Helsinki, and the trial protocol was approved by the local ethics committee.
Statistical Analysis
The values were expressed as mean ± standard deviation. Variables were analyzed for the presence of normal distribution using Kolmogorov‐Smirnov and Shapiro‐Wilk tests. Differences of continuous variables and categorical variables between the 2 groups were assessed using the independent samples Student t or Mann–Whitney U test. Associations between categorical variables were evaluated using the χ2 or Fisher exact tests. The associations between prognostic predictors of HF and cardiovascular mortality were evaluated using multivariate Cox proportional hazard analysis. Correlations between categorical variables and fQRS were assessed using the Pearson test. The impact of presence of fQRS on survival was determined using Kaplan‐Meier survival analysis. A P value <0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS version 16 (SPSS Inc., Chicago, IL).
Results
In this study, 227 patients (mean age, 64.26 ± 11.99 years) were enrolled. Patients were classified as fQRS(+) group (n = 114 patients; mean age, 63.49 ± 12.04 years) and fQRS(−) group (n = 113 patients; mean age, 65.04 ± 11.95 years) according to the presence of narrow fQRS on admission ECG. There was no difference in terms of age, gender, and other clinical characteristics between 2 groups (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics of fQRS(+) and fQRS(−) Patient Groups
| Variables | fQRS(+), n = 114 | fQRS(−), n = 113 | P |
|---|---|---|---|
| Age, y | 63.49 ± 12.04 | 65.04 ± 11.95 | 0.331 |
| Gender | |||
| Female, n (%) | 35 (30.7) | 36 (31.9) | |
| Male, n (%) | 79 (69.3) | 77 (68.1) | 0.851 |
| Smoking, n (%) | 60 (52.6) | 60 (53.1) | 0.944 |
| DM, n (%) | 49 (43.0) | 43 (38.1) | 0.449 |
| HT, n (%) | 55 (48.2) | 63 (55.8) | 0.258 |
| HPL n (%) | 7 (6.1) | 7 (6.2) | 0.986 |
| CAD, n (%) | 71 (62.3) | 71 (62.8) | 0.932 |
| CVD, n (%) | 7 (6.1) | 11 (9.7) | 0.316 |
| CRD, n (%) | 11 (9.6) | 15 (13.3) | 0.391 |
| PAD, n (%) | 3 (2.6) | 2 (1.8) | 1.000 |
| NYHA, n (%) | |||
| II | 31 (27.2) | 52 (46.0) | |
| III | 54 (47.4) | 50 (44.2) | |
| IV | 29 (25.4) | 11 (9.7) | |
| ACE‐I, n (%) | 72 (63.2) | 70 (61.9) | 0.850 |
| ARB, n (%) | 10 (8.8) | 16 (14.2) | 0.203 |
| MRA, n (%) | 39 (34.2) | 23 (20.4) | 0.019 |
| Diuretic, n (%) | 82 (71.9) | 60 (53.1) | 0.003 |
| ASA, n (%) | 86 (75.4) | 79 (69.9) | 0.350 |
| β‐Blocker, n (%) | 84 (73.7) | 87 (77.0) | 0.563 |
| Digoxin, n (%) | 29 (25.9) | 25 (22.1) | 0.558 |
| Insulin, n (%) | 25 (21.9) | 23 (20.4) | 0.771 |
| OAD, n (%) | 12 (10.5) | 17 (15) | 0.308 |
| Warfarin, n (%) | 30 (26.3) | 19 (16.8) | 0.082 |
| Statin, n (%) | 63 (55.3) | 51 (45.1) | 0.127 |
| Albumin, g/dL | 3.17 ± 0.58 | 3.36 ± 0.57 | 0.013 |
| BUN, mg/dL | 74.33 ± 44.77 | 66.55 ± 42.18 | 0.100 |
| Creatinine, mg/dL | 1.36 ± 0.63 | 1.34 ± 0.89 | 0.13 |
| Fasting glucose, mg/dL | 142.93 ± 68.24 | 133.31 ± 69.1 | 0.144 |
| Sodium, mEq/L | 135.46 ± 5.84 | 136.06 ± 4.75 | 0.362 |
| Potassium, mEq/L | 4.23 ± 0.67 | 4.29 ± 0.56 | 0.427 |
| Hemoglobin, g/dl | 12.35 ± 1.99 | 12.13 ± 2.19 | 0.43 |
| Hematocrit, % | 37.94 ± 6.60 | 36.47 ± 7.19 | 0.111 |
| CRP, mg/L | 34.21 ± 52.61 | 33.95 ± 51.37 | 0.93 |
| Troponin I, ng/mL | 9.99 ± 26.40 | 11.87 ± 27.18 | 0.949 |
Abbreviations: ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, acetylsalicyclic acid; BUN, blood urea nitrogen; CAD, coronary artery disease; CRD, chronic renal disease; CRP, C‐reactive protein; CVD, cerebrovascular disease; DM, diabetes mellitus; fQRS: fragmented QRS complex; HPL, hyperlipidemia; HT, hypertension; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association class; OAD, oral antidiabetic; PAD, peripheral arterial disease.
HF etiologies were as follows: ICM (n = 142, 62.56%), DCM (n = 33, 14.54%), HF due to other causes (n = 52, 22.90%). There were 11 patients with ICDs without a significant difference in distribution between the groups.
When patients were compared in terms of NYHA class, there were more patients with NYHA III and IV symptoms in the fQRS(+) group (P = 0.001, Table 1). Presence of narrow fQRS on ECG was significantly correlated with NYHA functional class (P = 0.001).
Patients were given standard medical therapy for HF according to current guidelines.15 There were no differences between the 2 groups with regard to the specific drugs used with the exception of diuretics and mineralocorticoid receptor antagonists (MRA) (Table 1). More patients in the fQRS(+) group were on diuretics and MRA (P = 0.003 and P = 0.019, respectively).
Laboratory values of fQRS(+) and fQRS(−) patients are given in Table 1. Patients in the fQRS(+) group had lower mean albumin values (3.17 ± 0.58 g/dL vs 3.36 ± 0.57 g/dL, P = 0.013) compared to the fQRS−) group. There were no differences between the groups regarding other biochemical parameters. No significant differences were observed between fQRS(+) and fQRS(−) patients in terms of echocardiographic parameters (Table 2).
Table 2.
Echocardiographic Parameters of fQRS(+) and fQRS(−) Patient Groups
| Variables | fQRS(+), n = 115 | fQRS(−), n = 114 | P |
|---|---|---|---|
| LVEF, % | 0.26 ± 0.06 | 0.27 ± 0.06 | 0.463 |
| AR, n (%) | 38 (33.3) | 51 (45.1) | 0.069 |
| MR, n (%) | 99 (86.8) | 101 (89.4) | 0.555 |
| TR, n (%) | 74 (64.9) | 64 (56.6) | 0.202 |
| DD, n (%) | 38 (33.6) | 36 (31.9) | 0.777 |
| PASP, mm Hg | 44.7 ± 12.65 | 41.4 ± 14.16 | 0.290 |
| LV MASS, g | 270.61 ± 112.9 | 262,25 ± 81.9 | 0.952 |
Abbreviations: AR, aortic regurgitation; DD, diastolic dysfunction; fQRS: fragmented QRS complex; LVEF, left ventricular ejection fraction; LV MASS, left ventricular mass; MR, mitral regurgitation; PASP, pulmonary artery systolic pressure; TR, tricuspid regurgitation.
A total of 49 patients (21.6%) died, 47 (20.7%) of whom were due to cardiovascular causes, and 2 (0.9%) were due to noncardiovascular causes. SCD was reported in 8 (3.52 %) patients, whereas stroke was reported in 18 patients (7.92 %) and rehospitalization for HF in 87 (38.33 %) patients.
The mean duration of follow‐up for the occurrence of clinical end points was 3.73 ± 1.41 years (range, 2–7 years). When end points were evaluated in relation to the presence of narrow fQRS on admission ECG, 39 patients (34.21%) died in the fQRS(+) group and 10 patients (8.85 %) in the fQRS(−) group (P < 0.0001). Fifty‐five patients in the fQRS(+) group (48.3%) and 32 patients in the fQRS(−) group (28.3%) were re‐hospitalized for worsening HF (P = 0.002). When hemodynamically significant ventricular arrhythmias were compared, no significant difference was found in 18 cases (15.8 %) in the fQRS(+) and 7 (6.19%) cases in the fQRS(−) groups (P = 0.2). The relation between fQRS and SCD or stroke did not reach statistical significance.
When the correlations between narrow fQRS and clinical end points were assessed, fQRS was found to be significantly correlated with cardiovascular mortality (P < 0.0001), rehospitalization for HF (P = 0.002), and hemodynamically significant ventricular arrhythmias (P = 0.021).
In multivariate Cox proportional hazard analysis, narrow fQRS (odds ratio [OR]: 3.130, 95% confidence interval (CI): 1.560‐2.848, P = 0.001), chronic renal failure (OR: 2.455, 95% CI: 1.120‐5.381, P = 0.025), NYHA class (OR: 8.305, 95% CI: 2.568‐26.855, P < 0.0001), and hypoalbuminemia (OR: 2.099, 95% CI: 1.122‐3.926, P = 0.020) were independent predictors of cardiovascular mortality (Table 3). In Kaplan‐Meier survival analysis, the presence of narrow fQRS on admission ECG predicted worse survival rates at 84 months; survival probability significantly decreased in the fQRS(+) group compared with fQRS(−) group (P < 0.0001) (Figure 2).
Table 3.
Multivariate Cox Proportional Hazard Analysis of Predictors of Cardiovascular Mortality in Systolic Heart Failure Patients
| P | OR | 95% CI | |
|---|---|---|---|
| CRF | 0.025 | 2.455 | 1.120‐5.381 |
| NYHA | <0.0001 | 8.305 | 2.568‐26.855 |
| fQRS | 0.001 | 3.130 | 1.560‐2.848 |
| Hypoalbuminemia | 0.020 | 2.099 | 1.122‐3.926 |
| Arrhythmia | 0.450 | 1.338 | 0.629‐2.848 |
| Age | 0. 843 | 1.003 | 0.97‐1.029 |
Abbreviations: CI, confidence interval; CRF, chronic renal failure; fQRS, fragmented QRS complex; NYHA, New York Heart Association class; OR, odds ratio.
Figure 2.
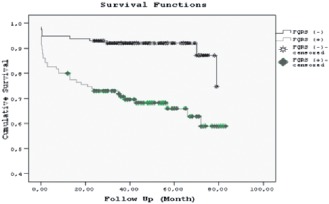
In Kaplan‐Meier survival analysis, narrow fQRS on admission electrocardiogram predicted a worse survival rate at 84 months.
Discussion
Acute decompensated HF is a significant cause of morbidity and is associated with poor long‐term prognosis in patients with systolic LV dysfunction. To optimize future medical treatments, easily accessible, cost‐effective, and readily available prognostic markers are required in these patients.
The presence of wide QRS complexes on ECG has been accepted as a traditional prognostic clinical marker in a wide variety of clinical settings.4 In recent clinical studies, narrow fQRS has also been implicated as a prognostic marker in various situations such as coronary artery disease, including ACS, Brugada syndrome, ARVD, and ICM and DCM.5, 6, 7, 8, 9, 10, 11, 12 Myocardial scar and/or ischemia have been implicated in the formation of fragmentation of the QRS complex, leading to inhomogeneous ventricular activations. Different fQRS morphologies are caused by shifting QRS vector during depolarization in and around scars or myocardial ischemic areas, depending on their extent and ventricular locations.11, 15, 17 Narrow fQRS was also associated with significant dyssynchrony in patients with DCM.17, 18
In this study, the presence of narrow fQRS was evaluated in patients admitted with decompensated systolic HF of any cause to investigate its relation with clinical symptoms, types of medical therapies provided, and echocardiographic parameters of cardiac function. The presence of narrow fQRS on ECG was significantly correlated with NYHA functional class, and the proportion of patients with narrow fQRS increased with worsening NYHA symptom status. The relation between NYHA class and fQRS has been previously investigated in patients with mitral stenosis, and fQRS was found to be associated with poor functional NYHA class.19 To the best of our knowledge, our study is the first study to present such a relationship in patients with dilated cardiomyopathy of any cause. In our study, the presence of narrow fQRS was associated with a poor overall clinical presentation besides NYHA class, including increased usage of diuretics and MRAs, and lower mean serum albumin levels, but we failed to demonstrate a similar association in terms of echocardiographic parameters including LVEF. As in our study, Cheema et al could not demonstrate a relationship between fQRS and EF in patients receiving ICDs for primary prevention due to ICM and DCM.20 Conversely, Sha et al demonstrated a significant association between fQRS and LVEF in DCM patients.11 In a study involving patients with ACS, the presence of fQRS was found not to be associated with LVEF.21
The prognostic significance of fQRS has been previously investigated in patients with LV systolic dysfunction having different clinical presentations. In a retrospective analysis carried out only in DCM patients (EF ≤40%), fQRS was found to have a predictive value for all‐cause mortality and ventricular arrhythmias.11 In our study, which was carried out in patients presenting with decompensated HF of any cause, the presence of fQRS on admission 12 L‐ECG was significantly correlated with cardiovascular mortality, rehospitalization for worsening HF, and hemodynamically significant ventricular arrhythmias. On the other hand, in ICD‐eligible stable patients with both ICM and DCM (EF ≤35%), fQRS was not associated with higher risk of either all‐cause or arrhythmic mortality, in contrast to our findings.20 The differences in clinical characteristics of the enrolled patients may be a likely explanation for this discrepancy. Moreover, in a similar study enrolling both ICM (EF ≤50 %) and DCM (EF ≤45 %) patients, fQRS in inferior leads was found to be an independent predictor for SCD.10 In another study carried out in ischemic HF patients, the number of leads with fQRS was an independent predictor of cardiovascular mortality or rehospitalization for HF, a finding mainly driven by the number of hospitalized patients.8 We were able to demonstrate a similar correlation between narrow fQRS and rehospitalization for HF in our study. In patients receiving cardiac resynchronization therapy (CRT) for HF, increased number of leads with fQRS predicted response to treatment.22
We found narrow fQRS to be an independent predictor of cardiovascular mortality along with NYHA class, albumin levels, and chronic renal failure. In Kaplan‐Meier survival analysis, the presence of fQRS on admission ECG predicted worse survival rates at 84 months after index hospitalization, similar to the study of Sha et al carried out in DCM patients.11
The limitations of the study include retrospective and single‐center design, probably leading to significant referral bias, and a small number of patients with CRT or ICD devices, precluding assessment of the relationship between fQRS and the impact of device‐based therapies. We did not perform analyses based on the number of leads with fQRS on ECG, which may have limited the predictive value of fQRS.
Conclusion
The presence of narrow fQRS is associated with worse NYHA functional class in patients hospitalized for decompensated HF. Our results are in accordance with most of the previously reported studies regarding the association between fQRS and mortality.8, 10, 11, 22 Narrow fQRS predicts cardiovascular mortality in a specific subgroup of HF patients, namely those hospitalized for decompensated HF. Because fQRS has recently been demonstrated to be a predictor of response to device‐based treatment for HF, its presence should be among the evaluated clinical variables during any hospitalizations for decompensated systolic HF.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Houser SR, Margulies KB. Is depressed myocyte contractility centrally involved in heart failure. Circ Res. 2003;92:350–358. [DOI] [PubMed] [Google Scholar]
- 2. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Eng J Med. 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 3. Stevenson LW. Recognition and management of patients with heart failure In: Goldman L, Braunwald E, eds. Primary Cardiology. Philadelphia, PA: WB Saunders; 1998:310–329. [Google Scholar]
- 4. Take Y, Morita H. Fragmented QRS: what is the meaning? Indian Pacing Electrophysiol J. 2012;12:213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frijo J, Mangalath K. Fragmented QRS electrocardiogram—the hidden talisman? Indian Pacing and Electrophysiol J. 2009;9:238–240. [PMC free article] [PubMed] [Google Scholar]
- 6. Grzegorz P, Ilan G, Joanna Z, et al. Prognostic Significance of Fragmented QRS complex for predicting the risk of recurrent cardiac events in patients with Q‐wave myocardial infarction. Am J Cardiol. 2007;100:583–586. [DOI] [PubMed] [Google Scholar]
- 7. Mithilesh KD, Mark AM, Hussam S, et al. Usefulness of fragmented QRS on 12‐lead electrocardiogram in acute coronary syndrome for predicting mortality. Am J Cardiol. 2009;104:1631–1637. [DOI] [PubMed] [Google Scholar]
- 8. Torigoe K, Tamura A, Kawano Y, et al. The number of leads with fragmented QRS is independently associated with cardiac death or hospitalization for heart failure in patients with prior myocardial infarction. J Cardiol. 2012;59:36–41. [DOI] [PubMed] [Google Scholar]
- 9.9.Mithilesh KD, Bilal K, Sony J, et al. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–2501. [DOI] [PubMed] [Google Scholar]
- 10. Pei J, Li N, Gao Y, et al. The J wave and fragmented QRS complexes in inferior leads associated with sudden cardiac death in patients with chronic heart failure. Europace. 2012;14:1180–1187. [DOI] [PubMed] [Google Scholar]
- 11. Sha J, Zhang S, Tang M, et al. Fragmented QRS is associated with all‐cause mortality and ventricular arrhythmias in patient with idiopathic dilated cardiomyopathy. Ann Noninvasive Electrocardiol. 2011;16:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chatterjee S, Changawala N. Fragmented QRS complex: a novel marker of cardiovascular disease. Clin Cardiol. 2010;33:68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hiroshi M, Kengo FK, Daiji M, et al. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118:1697–1704. [DOI] [PubMed] [Google Scholar]
- 14. Peters S, Trummel M, Koehler B. QRS fragmentation in standard ECG as a diagnostic marker of arrhythmogenic right ventricular dysplasia‐cardiomyopathy. Heart Rhythm. 2008;5:1417–1421. [DOI] [PubMed] [Google Scholar]
- 15. Das MK, Maskoun W, Shen C, et al. Fragmented QRS on twelve‐lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm. 2010;7:74–80. [DOI] [PubMed] [Google Scholar]
- 16. McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Committee for Practice Guidelines: ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J. 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 17. John RM, Tedrow UB, Koplan BA, et al. Ventricular arrhythmias and sudden cardiac death. Lancet. 2012;380:1520–1529. [DOI] [PubMed] [Google Scholar]
- 18. Tigen K, Karaahmet T, Gurel E, et al. The utility of fragmented QRS complexes to predict significant intraventricular dyssynchrony in nonischemic dilated cardiomyopathy patients with a narrow QRS interval. Can J Cardiol. 2009;25:517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yuce M, Davutoglu V, Ozbala B, et al. Fragmented QRS is predictive of myocardial dysfunction, pulmonary hypertension and severity in mitral stenosis. Tohoku J Exp Med. 2010;220:279–283. [DOI] [PubMed] [Google Scholar]
- 20. Cheema A, Khalid A, Wimmer A, et al. Fragmented QRS and mortality risk in patients with left ventricular dysfunction. Circ Arrhythm Electrophysiol. 2010;3:339–344. [DOI] [PubMed] [Google Scholar]
- 21. Guo R, Li Y, Xu Y, et al. Significance of fragmented QRS complexes for identifying culprit lesions in patients with non‐ST‐elevation myocardial infarction: a single‐center, retrospective analysis of 183 cases. BMC Cardiovasc Disord. 2012;12:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Celikyurt U, Agacdiken A, Sahin T, et al. Number of leads with fragmented QRS predicts response to cardiac resynchronization therapy. Clin Cardiol. 2013;36:36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]


