Abstract
Background
Cardiovascular and cerebrovascular (CVD) events/diseases are a common cause of non–acquired immunodeficiency syndrome (AIDS)‐related mortality in the aging human immunodeficiency virus (HIV)‐infected population. The incidence rate and clinical correlates of CVD in people living with HIV/AIDS compared to the general population warrants further investigation.
Hypothesis
HIV/AIDS is associated with increased risk CVD compared to general population.
Methods
CVD events in a matched cohort of HIV‐infected and non–HIV‐infected adults, ≥18 years old, served through the South Carolina Medicaid program during 1994 to 2011 were examined using time‐dependent proportional hazards regression and marginal structural modeling.
Results
A retrospective cohort of 13 632 adults was followed longitudinally for an average of 51 months. The adjusted hazard ratio (aHR) of incident CVD events was higher among HIV‐infected individuals exposed to combination antiretroviral therapy (cART) (aHR = 1.15) compared to the non–HIV‐infected group, but did not differ from the subgroup of cART‐naïve HIV‐infected adults. A higher aHR of incident CVD was associated with comorbid hypertension (aHR = 2.18), diabetes (aHR = 1.38), obesity (aHR = 1.30), tobacco use (aHR = 1.47), and hepatitis C coinfection (aHR = 1.32), and older age (aHR = 1.26), but with a lower risk among females (aHR = 0.86). A higher risk of incident CVD events was also apparent in HIV‐infected individuals with exposure to both protease inhibitors (adjusted risk ratio [aRR] = 1.99) and non‐nucleoside reverse transcriptase inhibitors (aRR = 2.19) compared to those with no exposure. Sustained viral load suppression was associated with a lower risk of incident CVD events (aRR = 0.74).
Conclusions
After adjusting for traditional risk factors and sociodemographic differences, there is higher risk of incident cardiovascular events among HIV‐infected individuals exposed to combined antiretroviral medications compared to the general population.
Introduction
Cardiovascular and cerebrovascular events/diseases (CVD) are the second most common causes of non–acquired immunodeficiency syndrome (AIDS)‐related mortality and morbidity in an aging human immunodeficiency virus (HIV) population.1, 2 A complex interplay of proinflammatory immune responses secondary to HIV viremia and the metabolic adverse effects of antiretroviral medications are thought to promote atherosclerosis, which then increases the risk of CVD.3 Recent studies and reviews of individual investigations have consistently demonstrated that HIV‐infected persons are at increased risk of CVD, although few studies calculated the CVD risk rates after adjusting for traditional cardiovascular risk factors.4, 5, 6, 7
Moreover, despite some recent investigations suggesting an increased relative risk (RR) of CVD with exposure to combined antiretroviral therapies (cART), other large longitudinal cohort studies and meta‐analyses of individual clinical trials have failed to confirm this association.5, 7, 8, 9, 10 Among the different classes of cART, protease inhibitors (PIs) have been most closely associated with CVD adverse events, whereas comparative results for non‐nucleoside reverse transcriptase inhibitors (NNRTIs) and nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) have been conflicting.7, 8, 9, 10 Although older antiretroviral therapy regimens (ARTs) were associated with drug‐specific risks for CVD, newer ARTs may not carry the same CVD risks.10, 11 In clinical practice, HIV‐infected patients may typically require several changes in cART regimens to achieve targeted viro‐immunological control, and may also develop cardio‐metabolic comorbidities (eg, diabetes, dyslipidemia, and hypertension), which predispose them to CVD events. Few studies have systematically accounted for such time‐dependent changes in clinical status and therapeutic factors.5, 7, 8, 9, 10, 12
The purpose of this investigation was to further elucidate this important clinical issue by determining the incidence rates and adjusted RR of incident CVD events in a large, population‐based cohort of HIV‐infected individuals compared to a non–HIV‐infected control cohort over time, controlling for traditional risk factors, and within the cohort of HIV‐infected individuals by comparing the adjusted RR of CVD events associated with exposure to PIs and NNRTIs, controlling for changes in viro‐immunological status.
Methods
A final cohort of 6816 HIV‐infected individuals ≥18 years old served through the South Carolina (SC) Medicaid program from January 1, 1994 through December 31, 2011, and who did not meet the exclusion criteria for this study (ie, death within 6 months of selection into the cohort [washout period], <30 days between the first and last visit in the cohort, or evidence of cocaine use) was selected. A 1:1 propensity score matching was used to randomly select non–HIV‐infected persons matched on age and year at entry into the Medicaid system, race/ethnicity, gender, and total months of enrollment in Medicaid, yielding a total study cohort of 13 632 persons.13 Medical claims were used to identify all service encounters (inpatient, outpatient, or emergency), date of service, and the International Classification of Diseases, 9th Revision (ICD‐9), Clinical Modification diagnosis codes related to that visit, and any pharmacy fills/refills during the enrollment time period for each person in the total cohort. Medicaid data were then linked with the enhanced HIV/AIDS Reporting System surveillance database maintained by the SC Department of Health and Environmental Control to perform the analyses presented herein.14 This study was approved by the SC Health and Human Services Research Committee and the SC Department of Health and Environmental Control Internal Review Board.
HIV‐infected individuals were categorized as cART‐treated if they received antiretroviral drugs cumulatively for at least 30 days, whereas those who did not receive any antiretroviral treatment or received it for <30 days were categorized as cART‐naïve. The incidence of CVD events was defined as an inpatient, emergency, or outpatient medical encounter, not within 6 months of the first date of selection into the cohort (washout period), associated with ICD‐9 and/or procedural codes for acute myocardial infarction, angina pectoris, percutaneous coronary intervention, and nonhemorrhagic stroke. At least 2 visits, 30 days apart, or the prescription of related medications for at least 30 days were required to ascertain relevant comorbidities including diabetes, essential hypertension, obesity/overweight, dyslipidemia, hepatitis B coinfection, and hepatitis C coinfection. Tobacco use was ascertained if there was at least 1 visit associated with ICD‐9 codes related to tobacco abuse or any prescription for smoking cessation agents.
Descriptive analyses were performed to assess the univariate association of covariates with incident CVD events. A time‐dependent, proportional hazards analysis was then used to estimate the harzard ratio (HR) of new‐onset CVD among the 3 exposure groups (ie, cART naïve, HIV‐infected; cART‐treated, HIV‐infected; and non–HIV‐infected groups). In this analysis, preexisting conditions (ie, essential hypertension, diabetes, dyslipidemia, and obesity that developed prior to a CVD event), hepatitis B coinfection, and hepatitis C coinfection were used as time‐dependent covariates, and onset status (ie, incident development of each comorbid condition) was updated during each person‐month of observation prior to any documented CVD events. Gender, race/ethnicity, age at enrollment, year of enrollment in Medicaid, and tobacco use variables were included in the analyses as fixed covariates.
To explore the association of cART or viro‐immunological control per month with the development of CVD, a subgroup analysis was conducted within the HIV‐infected cohort. Because NRTI medications are common components of both PI‐ and NNRTI‐based treatment regimens, exposure to NRTI medications was not included as a separate covariate to avoid collinearity, as confirmed with sensitivity analysis. However, time‐dependent exposure to PIs and NNRTIs, and viro‐immunological status per person month were included in the analyses as predictor variables, which allowed us to account for those treated with both types of regimens during the study period. Furthermore, to estimate more precisely the effect of exposure to PI and NNRTI drugs with development of CVD, we used marginal structural models (MSM) with the inverse probability of treatment weighted (IPTW) estimators as performed by Hernán et al.15
To obtain a final parsimonious model, each multivariable model was reduced by backward elimination using a cutoff P value of 0.08 and ensuring that removal of a variable did not result in more than a 10% change in the dependent variable. For each multivariable Cox proportional hazards model, interaction terms with the time variable were included. All statistical analyses were performed in SAS version 9.2 software (SAS Institute, Cary, NC).
Results
Due to the matching procedure, there were no significant differences in demographic characteristics and baseline comorbid conditions between the HIV‐infected group and the matched non–HIV‐infected group (Table 1). Overall, the median age of the study cohort was 39 years (interquartile range [IQR], 31–46 years), and the majority were males (57%) and African American (71%). The median number of months of follow‐up was 51 (IQR, 23–105 months). In our study cohort, 20.6% had comorbid hypertension, 19% had dyslipidemia, 14.5% had diabetes, 10% had documented obesity, 8.2% had hepatitis C virus coinfection, 3.5% had hepatitis B virus coinfection, and 30% had documented tobacco use/disorder (Table 1).
Table 1.
Comparison of Demographic Characteristics and Baseline Medical Conditions Between HIV‐Infected and the Matched Non–HIV‐Infected Study Cohorts
| Variable | HIV Infected, Case, n = 6816 | Non–HIV Infected, Control, n = 6816 |
|---|---|---|
| Gender, N (%) | ||
| Female | 2953 (21.7) | 2940 (21.6) |
| Male | 3863 (28.3) | 3876 (28.4) |
| Race, N (%) | ||
| Black | 4856 (35.6) | 4786 (35.1) |
| Others | 560 (4.1) | 587 (4.3) |
| White | 1400 (10.3) | 1443 (10.6) |
| Age categories, N (%) | ||
| 18–29 years | 186 (1.4) | 187 (1.4) |
| 30–44 years | 4705 (34.5) | 4638 (34.0) |
| 45–64 years | 1857 (13.6) | 1905 (14.0) |
| ≥65 years | 68 (0.5) | 86 (0.6) |
| Median age at study entry, y (IQR) | 38 (31–46 ) | 38 (30–46) |
| Median months enrolled (IQR) | 53 (22–106) | 50 (23–103) |
| Baseline hypertension, yes, N (%) | 921 (6.8) | 973 (7.1) |
| Baseline diabetes, yes, N (%) | 387 (2.8) | 377 (2.8) |
| Baseline dyslipidemia, yes, N (%) | 192 (1.4) | 208 (1.5) |
| Obesity, yes, N (%) | 666 (9.8) | 674 (9.9) |
| Comorbid hepatitis B, yes, N (%) | 431 (3.2) | 46 (0.3)a |
| Comorbid hepatitis C, yes, N (%) | 927 (6.8) | 187 (1.4)a |
| Documented tobacco use, yes, N (%) | 1994 (14.6) | 2100 (15.4)b |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
Significant at P ≤ 0.001.
Significant at P ≤ 0.05.
In the HIV‐infected group, 80% were treated with at least 1 ART medication during the study period. Of those treated, 66% and 46% were treated with PIs and NNRTIs, respectively. Furthermore, 35% were treated with both PIs and NNRTIs. Rates of usage for individual medications within the 3 classes of antiretroviral medications (ie, NRTIs, PIs, and NNRTIs) during 1994 to 2003 and 2004 to 2011 were analyzed, along with overall rates during the study period. These data suggest that among NRTIs, emtricitabine and tenofovir were used more in the 2004 to 2011 period compared to didanosine, stavudine, and zidovudine. For PIs, atazanavir and ritonavir were used more in the 2004 to 2011 period compared to other agents such as amprenavir, indinavir, nelfinavir, and saquinavir. Among NNRTIs, efavirenz was used more in the 2004 to 2011 period compared to nevirapine. (A detailed table is available from the first author.)
Unadjusted incidence rates per 1000 person‐years (PY) suggest a higher incidence rate of CVD events in the HIV‐infected group as compared to the matched non–HIV‐infected group (22 vs 20 per 1000 PY). (The stratified incidence rates are available from the first author.) The proportion of incident CVDs by type of event did differ across the cohort subgroups. Within the total cohort, incident acute myocardial infarction was identified in 4.2% (566 individuals), angina in 9.8% (1331 individuals), and stroke/transient ischemic attacks in 5.3% (718 individuals). The distribution of these incident events/disorders across the HIV‐infected vs noninfected control groups was very similar: acute myocardial infarction (4.5% vs 4.2%), angina (10.5% vs 9.7%), and stroke/transient ischemic attacks (6.3% vs 4.7%). However, the proportion of individuals in the HIV‐infected cART‐naïve group demonstrating each of these incident events/disorders was significantly lower (2.8%, 6.9%, and 4.0%, respectively).
The median log10 viral load (VL) over the study period was 3.37 (IQR, 2.36–4.42) and median CD4+ T‐cell count over the study period was 208 cells/mm3 (IQR, 78–426 cells/mm3). Median log10 VL for cART‐naïve and cART‐treated HIV‐infected persons over the observation period was 3.71 (IQR, 2.57–4.76) and 3.30 (IQR, 2.32–4.34), respectively. Similarly, median CD4+ T‐cell count for cART‐naïve vs cART‐treated HIV‐infected persons over the study observation period was 185 (IQR, 38–454) and 213 (IQR, 88–420) cells/mm3.
Results from the time‐dependent proportional hazards analysis suggested that after accounting for individual characteristics, preexisting conditions, and tobacco use, the RR of incident CVD events was 15% higher in the cART‐treated HIV‐infected group as compared to the non–HIV‐infected group (adjusted hazard ratio [aHR] = 1.15) (Table 2). The Figure 1 presents the adjusted time to event (proportional hazards) curves for incident CVD comparing the HIV cART exposed and naïve cohort with the non–HIV‐infected cohort. However, the RR was not significantly different in cART‐naïve HIV‐infected individuals as compared to the non–HIV‐infected group. Furthermore, these results suggest a significantly higher risk associated with other risk factors, including preexisting hypertension (aHR = 2.18), preexisting diabetes (aHR = 1.38), preexisting obesity (aHR = 1.30), any tobacco use (aHR = 1.47), and hepatitis C coinfection (aHR = 1.32). The RR of incident CVD events was also significantly higher among older individuals (aHR = 1.26) but lower among females (aHR = 0.86) compared to males (Table 2).
Table 2.
Adjusted Hazard Ratios for Incident Cardiovascular and Cerebrovascular Events Associated With HIV‐Infection Status and Medications, Comorbid Conditions, and Individual Risk Factors
| Parameter | Adjusted Hazard Ratio | 95% Confidence Interval |
|---|---|---|
| Sex (female)c | 0.86a | 0.78‐0.95 |
| Ethnicity (African American)d | 0.92 | 0.82‐1.04 |
| Ethnicity (other nonwhite)d | 1.18 | 0.98‐1.42 |
| Age at study entry | 1.26b | 1.23‐1.28 |
| HIV infected taking cART | 1.15a | 1.04‐1.27 |
| HIV infected cART naive | 1.18 | 0.98‐1.41 |
| Preexisting diabetes | 1.38b | 1.19‐1.61 |
| Preexisting obesity | 1.30b | 1.11‐1.52 |
| Comorbid hepatitis C | 1.32b | 1.08‐1.61 |
| Tobacco used | 1.47b | 1.33‐1.62 |
Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus.
Significant at P ≤ 0.01.
Significant at P ≤ 0.001.
Compared to males.
Compared to White.
Figure 1.
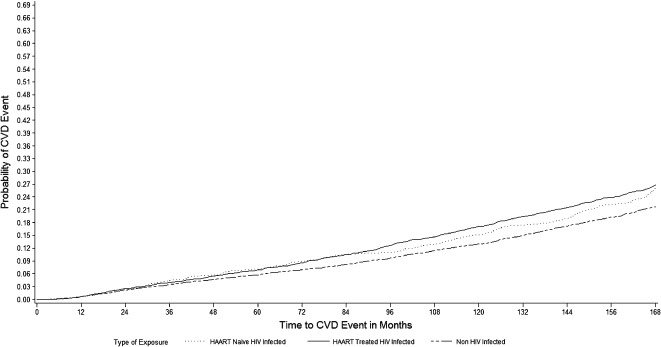
Adjusted time to event (proportional hazards) curves for incident cardiovascular and cerebrovascular events/diseases (CVD) comparing the human immunodeficiency virus (HIV) combination antiretroviral therapy (cART) exposed and naïve cohort with the non–HIV‐infected cohort. Abbreviations: HAART, highly active antiretroviral therapy.
A significant association between incident CVD events and length of exposure to PIs (adjusted RR [aRR] = 1.99) and to NNRTIs (aRR = 2.21) was found in the MSM analyses (Table 3). Furthermore, after accounting for other confounding factors, including exposure to cART medications, increasing age (aRR = 1.04), preexisting hypertension (aRR = 2.30), and preexisting diabetes (aRR = 1.81) were significantly associated with a higher risk for incident CVDs, whereas mean VL (sustained, suppressed) (aRR = 0.74) and the presence of comorbid hepatitis C (aRR = 0.55) were significantly associated with a lower risk of CVD events in the HIV‐infected cohort.
Table 3.
Adjusted Risk Ratios for Incident Cardiovascular and Cerebrovascular Events Associated With cART Medication Classes, HIV‐Infection Status, Comorbid Conditions, and Individual Risk Factors
| Parameter | Adjusted Risk Ratio | 95% Confidence Interval |
|---|---|---|
| Ethnicity (African American)c | 0.54a | 0.41‐0.71 |
| Age at study entry | 1.04a | 1.03‐1.06 |
| Months treated PIs | 1.99a | 1.53‐2.60 |
| Months treated NNRTIs | 2.48a | 1.91‐3.23 |
| Preexisting hypertension | 2.30a | 1.68‐3.14 |
| Preexisting dyslipidemia | 0.06a | 0.03‐0.12 |
| Preexisting diabetes | 1.81b | 1.13‐2.91 |
| Comorbid hepatitis C | 0.55b | 0.31‐0.97 |
| Log viral load | 0.74a | 0.67‐0.83 |
Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; NNRTIs, nucleoside/nucleotide reverse transcriptase inhibitors; PIs, protease inhibitors.
Significant at P ≤0.001.
Significant at P ≤ 0.01.
Compared to White.
Discussion
Despite the growing concern about cardiometabolic conditions in HIV infection, the absolute or unadjusted/raw incidence rates of CVD events in our cohort were not substantially higher among people with HIV as compared to a sociodemographically similar general population group until the influence of comorbid metabolic conditions and other traditional risk factors were accounted for. Our multivariable analyses suggested that cART‐exposed, HIV‐infected individuals had a significantly higher risk of CVD events (15%) as compared to the non–HIV‐infected control group, but not when compared to the cART‐naïve HIV‐infected group. Published risk ratio estimates between HIV‐infected and noninfected adults yielded a 52% higher risk for HIV‐infected, ART‐naïve patients, and a 100% higher risk for HIV‐infected patients taking ART.7 Other investigators have reported significantly higher absolute/raw CVD incidence rates in HIV‐infected populations compared to the non–HIV‐infected population, but did not employ a control group, did not use matching procedures, or did not adjust their estimates with traditional risk factors or worsening clinical status, which may be partially responsible for our observed differences.4, 5, 7, 9, 16, 17
The development of metabolic conditions that increase the risk of CVD was also confirmed in our cohort. Preexisting obesity contributed a 30% increase, and preexisting diabetes contributed a 38% increase in CVD risk in the comparison of HIV‐infected vs noninfected controls. Within the HIV‐infected cohort, preexisting hypertension was associated with a 130% CVD risk increase, whereas preexisting diabetes was associated with an 81% CVD risk increase. These results comport with other studies, which have shown an increased RR of CVD events and the development of cardiometabolic conditions, such as diabetes and dyslipidemia, among cART‐exposed, HIV‐infected individuals as compared to cART‐naïve individuals.4, 8, 9, 15, 16, 17
Our results also suggest that within the HIV‐infected cohort, after accounting for traditional risk factors, along with viro‐immunological control and coprescription of different classes of antiretrovirals, length of exposure to both PIs and NNRTIs was associated with a higher risk of incident CVD. However, because an NRTI backbone is commonly prescribed along with PI or NNRTI regimens, it is possible that the measure of association estimated by the MSM analyses is partly shared or inflated.9, 18, 19 The RR of CVD (defined broadly and comparable to our definition) among classes of ARTs estimated in a recent meta‐analysis were significantly greater for PIs (RR = 1.11) and NRTIs (RR = 0.05), but not for NNRTIs (RR = 1.04).7 However, their estimated RRs were not adjusted for traditional risk factors or worsening clinical status. Nevertheless, our finding not only extends the current literature regarding different classes of ART, but also underscores that MSM, which simulates randomized trial methods in observational data, is useful in mitigating some underlying bias in estimating RRs due to time‐dependent confounders in longitudinal data analysis.11
Based on previous clinical studies and practice reports, NNRTIs are thought to have the safest cardiometabolic profiles, but a few studies have shown higher metabolic dysfunction associated with efavirenz as compared to other NNRTIs, such as nevirapine.20 In our cohort, the NNRTI efavirenz was used more in the 2004 to 2011 period than nevirapine. Therefore, the strong association of NNRTIs with the development of incident CVD events demonstrated in these analyses may indicate that among practitioners there is inadequate use of NNRTI regimens with less toxic cardiometabolic profiles. Therefore, this finding warrants not only a reassessment of clinical practice quality and outcomes, but also further investigation into the relative side effects of newer drugs within the NNRTI class in clinical practice settings.
Furthermore, our results suggest an independent association between hepatitis C coinfection both in the HIV‐infected and non–HIV‐infected cohorts, as well as an association between sustained suppression of HIV viremia and the development of CVD events in the HIV‐infected cohort. A lower mean VL (sustained, suppressed) and the presence of comorbid hepatitis C were significantly associated with a lower risk of CVD events in the HIV‐infected cohort. Few other studies suggest similar results (eg, Bedimo et al recently reported that hepatitis C coinfection was associated with a significantly higher risk of cerebrovascular disorders but not with myocardial infarction).9, 21 Moreover, although sustained viral suppression is generally accepted to be related to fewer CVD events, fewer investigations have systematically incorporated VL or CD4+ T‐cell counts as covariates in determining risk for CVD.6, 7, 22
Finally, in developed countries, cART availability has changed the pattern of cardiac diseases associated with HIV infection from cardiomyopathy, pericardial effusion, and pulmonary hypertension to premature coronary artery disease and other manifestations of atherosclerosis, which are partly attributable to medication‐induced metabolic problems such as insulin resistance and dyslipidemia. Coupled with the extent of comorbid metabolic disorders, the higher frequency of CVD events identified in this study (ie, myocardial infarction, angina, and stroke/transient ischemic attacks) in the HIV‐infected population constitute important findings for clinical cardiologists collaborating with infectious disease specialists in the management of aging HIV patients. It has been suggested that any HIV‐positive patient who is at high risk of developing or who demonstrates any potential clinical manifestation of cardiovascular disease should have a baseline echocardiogram performed with serial echocardiography performed biannually thereafter.23 Based on our results, these assessments should be a high priority among HIV‐infected individuals with increased risk of cardiac involvement (ie, previous exposure to relatively more cardiotoxic ARTs); development of cardiometabolic disorders such as diabetes, dyslipidemia, obesity, and hypertension; worsening clinical status (increasing VL and lower CD4+ T‐cell count); and advancing age.
Several study limitations need to be considered, however, in interpreting these results. Information bias is of concern because the data were collected for administrative and billing purposes, and neither the recruitment of patients nor the data collection were under the control of the investigators. Identification of all medical conditions was based on spontaneous reporting to or observation by a physician, and their designation of each diagnosis in the Medicaid billing system. No structured research and clinical interviews were employed to confirm any of the assigned clinical diagnoses. Nevertheless, conservative criteria were employed for defining both the dependent and predictor variables to mitigate the risk of dependent variable misclassification (ie, both ICD‐9 codes and prescribed medications were used to identify cases), a relatively long washout period of 6 months was used to reduce the risk of misclassifying a prevalent disorder as incident, and a matched control group of non–HIV‐infected patients was employed. Reliable information about diet, physical activity, and family history of metabolic conditions, which are also significant predictors of incident CVD events, was lacking in this dataset. Patients who dropped out of treatment or who were periodically ineligible for Medicaid are not represented in this dataset. These results report associations and, as a result, directions of causality cannot be inferred. Furthermore, although many significant covariates have been controlled for, other unmeasured differences in these patients may explain the findings. Finally, studies that utilize Medicaid data may not be generalizable to non‐Medicaid populations. However, the findings of this study may still be beneficial in facilitating public health interventions aimed at lowering the risk of CVD events and disorders in other HIV‐infected populations.24
Conclusion
Taken together, these findings may indicate that HIV infection per se appears to increase the risk of incident CVD events, but less so in the earlier stages of the disease, especially in younger adults. However, the risk of incident CVD events increases substantially as the HIV disease progresses, comorbid metabolic conditions develop, and as patients age and have longer periods of exposure to cART. Healthcare providers (cardiologists and infectious disease specialists) should remain aware and vigilant of the higher risk for development of CVD events among HIV‐infected patients whose status is worsening over time, who have been exposed to more cardiotoxic ART regimens, or who have been treated with cART over longer periods of time. HIV‐infected patients should be screened periodically, modifiable individual and clinical risk factors should be continually addressed, and alternate cART therapies with safer cardiometabolic profiles should be prescribed, where possible, to mitigate the long‐term risk of potentially fatal CVD events.
This work was partially supported by funding from the Mid‐Atlantic American Heart Association Pre‐Doctoral Fellowship to Dr. Tripathi at the University of South Carolina, Arnold School of Public Health.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Bozzette SA, Ake CF, Tam HK, et al. Long‐term survival and serious cardiovascular events in HIV‐infected patients treated with highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:338–341. [DOI] [PubMed] [Google Scholar]
- 2. Antiretroviral Therapy Cohort Collaboration . Causes of death in HIV‐1‐infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010;50:1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palios J, Kadoglou NP, Lampropoulos S. The pathophysiology of HIV‐/HAART‐related metabolic syndrome leading to cardiovascular disorders: the emerging role of adipokines. Exp Diabetes Res. 2012;2012:103063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Triant VA, Lee H, Hadigan C, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klein D, Hurley LB, Quesenberry CP Jr, et al.. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV‐1 infection? J Acquir Immune Defic Syndr. 2002;30:471–477. [DOI] [PubMed] [Google Scholar]
- 6. Durand M, Sheehy O, Baril JG, et al. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case–control study using Québec's public health insurance database. J Acquir Immune Defic Syndr. 2011;57:245–253. [DOI] [PubMed] [Google Scholar]
- 7. Islam FM, Wu J, Jansson J, et al. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta‐analysis. HIV Med. 2012;13:453–468. [DOI] [PubMed] [Google Scholar]
- 8. Friis‐Moller N, Reiss P, Sabin CA, et al; DAD Study Group . Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. [DOI] [PubMed] [Google Scholar]
- 9. Ho JE, Hsue PY. Cardiovascular manifestations of HIV infection . Heart. 2009;95:1193–1202. [DOI] [PubMed] [Google Scholar]
- 10. Bavinger C, Bendavid E, Niehaus K, et al. Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review. PLoS One. 2013;8(3):e59551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Longenecker CT, Triant VA. Initiation of antiretroviral therapy at high CD4 cell counts: does it reduce the risk of cardiovascular disease? Curr Opin HIV AIDS. 2014;9:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feleke Y, Fekade D, Mezegebu Y. Prevalence of highly active antiretroviral therapy associated metabolic abnormalities and lipodystrophy in HIV infected patients. Ethiop Med J. 2012;50:221–230. [PubMed] [Google Scholar]
- 13.Coca‐Perraillon M. Local and global optimal propensity score matching. Statistics and Data Analysis, SAS Global Forum 2007. http://www2.sas.com/proceedings/forum2007/185‐2007.pdf. Accessed July 03, 2013.
- 14.HIV and STD division surveillance report. 2012. South Carolina Department of Health and Environmental Control. http://www.scdhec.gov/Health/DiseasesandConditions/InfectiousDiseases/HIVandSTDs/DataandReports. Accessed June 30, 2013.
- 15. Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV‐positive men . Epidemiology. 2000;11:561–570. [DOI] [PubMed] [Google Scholar]
- 16. Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV‐infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–512. [DOI] [PubMed] [Google Scholar]
- 17. Corral I, Quereda C, Moreno A, et al. Cerebrovascular ischemic events in HIV‐1‐infected patients receiving highly active antiretroviral therapy: incidence and risk factors. Cerebrovasc Dis. 2009;27:559–563. [DOI] [PubMed] [Google Scholar]
- 18. Grinspoon S, Carr A. Cardiovascular risk and body‐fat abnormalities in HIV‐infected adults . N Engl J Med. 2005;352:48–62. [DOI] [PubMed] [Google Scholar]
- 19. US Department of Health and Human Services . Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV‐1‐infected adults and adolescents. December 1, 2009. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed November 03, 2012.
- 20. Buchacz K, Weidle PJ, Moore D, et al. Changes in lipid profile over 24 months among adults on first‐line highly active antiretroviral therapy in the home‐based AIDS care program in rural Uganda. J Acquir Immune Defic Syndr. 2008;47:304–311. [DOI] [PubMed] [Google Scholar]
- 21. Bedimo R, Westfall AO, Mugavero M, et al. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV‐infected patients. HIV Med. 2010;11:462–468. [DOI] [PubMed] [Google Scholar]
- 22. Hemkens LG, Bucher HC. HIV infection and cardiovascular disease. Eur Heart J. 2014;35:1373–1381. [DOI] [PubMed] [Google Scholar]
- 23. Volberding PA, Murphy RL, Barbaro G, et al. The pavia consensus statement. AIDS . 2003;17(suppl 1):S170–S179. [DOI] [PubMed] [Google Scholar]
- 24. US Center for Disease Control . Racial/ethnic disparities in diagnoses of HIV/AIDS—33 states, 2001–2004 . MMWR Morb Mortal Wkly Rep. 2006;55:121–125. [PubMed] [Google Scholar]


