Abstract
Objective
To investigate whether the early administration of intravenous second‐line immunotherapy correlates with improved long‐term cognition and the potential mechanisms via imaging in adult patients with moderate‐to‐severe anti‐N‐methyl‐D‐aspartate (NMDA) receptor encephalitis.
Methods
Sixteen adult patients with moderate‐to‐severe anti‐NMDA receptor encephalitis past the acute stage and 15 healthy controls (HCs) performed a set of comprehensive neuropsychological tests, and underwent a resting‐state fMRI study to analyze resting state functional connectivity (FC). In addition, correlation analyses were performed between hippocampal FC and cognitive performance. All patients were received intravenous first‐line immunotherapy, and nine of them were also given intravenous second‐line immunotherapy within 3 months of disease onset.
Results
The patients who only received first‐line immunotherapy showed significant verbal episodic memory impairments compared with HCs and those who received second‐line immunotherapy, while no significant differences were noted between the patients with second‐line immunotherapy and the HCs. In line with the results of neuropsychological tests, significant changes in bilateral hippocampal FC were observed in the patients who only received first‐line immunotherapy compared with both HCs and those who received second‐line immunotherapy. However, no significant differences in hippocampal FC were observed in the patients with second‐line immunotherapy compared with the HCs. Importantly, hippocampal‐medial prefrontal cortex (mPFC) connectivity positively correlated with memory performance.
Interpretation
In the long term, early administration of intravenous second‐line immunotherapy may be associated with more favorable verbal episodic memory outcomes in patients with moderate‐to‐severe anti‐NMDA receptor encephalitis. These results may provide some evidence and guidance for the use of immunotherapy in this population.
Introduction
First characterized in 2007, anti‐N‐methyl‐D‐aspartate (anti‐NMDA) receptor encephalitis is a potentially lethal (although it is reversible with treatment), immune‐mediated brain inflammation, characterized by the acute onset of various neurological and psychiatric manifestations.1, 2, 3, 4 This disease has been recognized in patients of all ages, but is more frequent in young adults and children, with or without teratoma.1, 5 The clinical picture is similar at the end of the first month in most cases, and several symptoms are present, such as the following: abnormal behavior and cognition; memory deficit; speech disorder; seizures; abnormal movements; loss of consciousness or autonomic dysfunction; and central hypoventilation.6 Generally, timely treatments, such as first‐line immunotherapy (steroids, intravenous immunoglobulin, plasmapheresis), which may be combined with second‐line immunotherapy (rituximab, cyclophosphamide), and tumour removal lead to substantial recovery, as reflected by low modified Rankin Scale (mRS) scores for approximately 80% of patients. 6 However, more than 75% of patients with anti‐NMDA receptor encephalitis are left with permanent cognitive deficits of varying severity, predominantly in the domains of memory, attention, and executive control, which become major determinants of long‐term morbidity.7 Currently, only early immunotherapy is consistently considered to produce favorable cognitive outcomes.7, 8 However, studies concerning the association of second‐line immunotherapy with cognitive outcomes are limited and have shown conflicting results for anti‐NMDA receptor encephalitis.
A large retrospective cohort study identified the use of second‐line immunotherapy as an additional factor for good outcomes, as reflected by low mRS scores.6 However, various cognitive function domains cannot be assessed systematically by the mRS. It is crucial to introduce comprehensive cognition evaluation tools, including a series of neuropsychological tests and functional MRI technology, to study long‐term cognitive outcomes in anti‐NMDAR encephalitis and furthermore supplement the mRS in the assessment of overall outcomes. In a recent systematic review, second‐line immunotherapy was least relevant for cognitive outcomes.7 Nevertheless, the treatment type, administration route, timing and treatment duration of second‐line therapy, disease severity, and neuropsychological tests varied significantly across the included studies.7 Therefore, conclusions from these data should be interpreted with caution. Conversely, sporadic case reports have shown that second‐line immunotherapy may improve cognitive outcomes.9 However, the cognitive outcomes in this study were based on medical history inquiries and clinical observations rather than neuropsychological assessments.
The early use of second‐line immunotherapy is increasingly recommended for reducing recurrence rates; however, the choice of timing for the use of second‐line immunotherapy varies greatly among physicians. Some physicians tend to postpone second‐line immunotherapy until disease relapse instead of initiating treatment during the acute stage. Therefore, it is important to determine whether the early administration of second‐line immunotherapy is associated with long‐term cognitive outcomes improvements, which could provide insights for selecting therapeutic options in patients with moderate‐to‐severe anti‐NMDA receptor encephalitis. Therefore, 16 adult patients with moderate‐to‐severe anti‐NMDA receptor encephalitis past the acute stage were recruited in this retrospective study. All patients received intravenous first‐line immunotherapy either alone or combined with intravenous second‐line immunotherapy within 3 months after disease onset. The goal of this study was to explore whether the early administration of intravenous second‐line immunotherapy contributes to long‐term cognitive improvements and the potential underlying mechanisms by using resting functional MRI.
Methods
Subjects
Sixteen patients with anti‐NMDA receptor encephalitis who were hospitalized or referred to the outpatient clinic for further counseling and treatment at the Department of Neurology, the First Affiliated Hospital, Zhejiang University School of Medicine were recruited between July 2016 and February 2018. The diagnosis was based on typical clinical features together with the presence of IgG antibodies for NMDA receptors.2, 10 All patients recruited in this study were considered to have moderate‐to‐severe anti‐NMDAR encephalitis, because the maximal mRS score was 4 or 5 points for all patients during the acute stage based on medical records. Assays for CSF NMDAR‐IgG, LGI1‐ IgG, CASPR2‐ IgG, AMPAR1/R2‐ IgG, and GABAB R‐IgG were performed at EUROIMMUN Diagnostic Laboratory, China by cell‐based indirect immune‐fluorescence test (IIFT) employing BIOCHIPs (EUROIMMUN AG, Luebeck, Germany). There were no abnormalities in structural MRI for any patient.
All patients were evaluated using neuropsychological assessments and neuropsychiatric inventory (NPI), except for one patient who received only first‐line immunotherapy due to refusal, and all patients underwent an MRI scan. The initial assessment was conducted after the acute stage of the disease (at least 6 months after initial discharge from the hospital, median/interquartile range (IQR), 9.5/7–34.75 months; range, 6–47 months).
Fifteen individuals with no history of psychiatric or neurologic disease served as healthy controls (HCs) in the experiments. The control participants were matched to the patients with respect to sex, educational level, and age. Demographic and clinical data are shown in Tables 1 and 2.
Table 1.
Patient characteristics.
| Patient | Sex | Age | IgGNMDA receptor antibodies (CSF titer) | Symptoms | Duration of unconsciousness, day | Treatment protocol | Hospital stay month | Paraneoplastic syndrome | mRS score | AEDs at study time point | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Total | Tumor | Resection | Before treatment | Study time point | ||||||||
| 1 | F | 22 | 1:32 | Behavior, seizure | LOC, dyskinesia, seizure, behavior, cognition | 15–20 | High‐dose steroids, IVIG, cyclophosphamide | 1.1 | None | / | 5 | 0 | Topiramate 50 mg/day |
| 2 | M | 28 | 1:32 | Behavior, dyskinesia | LOC, dyskinesia, seizure, behavior, cognition, autonomic instability | 20–25 | High‐dose steroids, IVIG, cyclophosphamide | 1.2 | None | / | 5 | 1 | None |
| 3 | M | 31 | 1:32 | Seizure, LOC | LOC, dyskinesia, seizure, behavior, cognition, autonomic instability | 25–30 | IVIG, cyclophosphamide | 1.4 | None | / | 5 | 0 | None |
| 4 | F | 15 | 1:32 | Dyskinesia, seizure | Dyskinesia, seizure, behavior, cognition | NA | High‐dose steroids, IVIG, rituximab | 1.2 | None | / | 4 | 1 | Oxcarbazepine 900 mg/day |
| 5 | F | 24 | 1:32 | Behavior | LOC, dyskinesia, seizure, behavior, cognition | 10–15 | High‐dose steroids, IVIG, cyclophosphamide | 1.2 | None | / | 5 | 0 | None |
| 6 | F | 34 | 1:32 | Behavior, cognition | LOC, dyskinesia, seizures, behavior, cognition, autonomic instability | 700+ | High‐dose steroids, plasmapheresis, IVIG, rituximab, cyclophosphamide | 26 | Mature cystic teratoma of left ovarian | yes | 5 | 0 | None |
| 7 | M | 22 | 1:32 | Behavior | LOC, dyskinesia, seizures, behavior, cognition | 10–15 | Cyclophosphamide | 0.8 | None | / | 4 | 0 | None |
| 8 | F | 25 | 1:32 | Behavior, seizure | LOC, dyskinesia, seizures, behavior, cognition | 20–25 | High‐dose steroids, IVIG, rituximab, cyclophosphamide | 1.2 | Mature cystic teratoma of bilateral ovarian | yes | 5 | 0 | None |
| 9 | F | 20 | 1:32 | Behavior, dyskinesia | LOC, dyskinesia, seizures, behavior, cognition | 20–25 | High‐dose steroids, IVIG, rituximab, cyclophosphamide | 1.3 | None | / | 5 | 2 | None |
| 10 | F | 30 | 1:32 | Behavior | LOC, behavior, cognition | 8–10 | High‐dose steroids, IVIG | 0.7 | None | / | 4 | 0 | None |
| 11 | M | 49 | 1:10 | Seizure, behavior | LOC, dyskinesia, seizure, behavior, cognition | 15–20 | High‐dose steroids, IVIG | 1.2 | None | / | 5 | 1 | None |
| 12 | F | 34 | 1:10 | Dyskinesia, cognition | Dyskinesia, behavior, cognition | NA | High‐dose steroids, IVIG | 0.8 | None | / | 4 | 0 | None |
| 13 | F | 42 | 1:10 | Seizure, behavior, | LOC, seizure, behavior, cognition, autonomic instability | 20–25 | High‐dose steroids, IVIG | 1.2 | None | / | 5 | 1 | None |
| 14 | F | 25 | 1:32 | Seizure, behavior, | Seizure, behavior, cognition | NA | High‐dose steroids, IVIG | 1.3 | Mature cystic teratoma of right ovarian | yes | 5 | 0 | None |
| 15 | M | 22 | 1:32 | Behavior, seizure | LOC, seizure, behavior, cognition | 20–25 | High‐dose steroids, IVIG | 1.3 | None | / | 4 | 0 | None |
| 16 | F | 52 | 1:32 | Behavior, seizure | Seizure, behavior, cognition | NA | High‐dose steroids, IVIG | 1.2 | None | / | 4 | 1 | None |
CSF, cerebrospinal fluid; LOC, loss of consciousness; IVIG, intravenous human immunoglobulin; mRS, modified Rankin Score; AEDs, antiepileptic drugs; NA, not applicable.
Table 2.
Demographic data and various putative predictive factors of the patients and healthy controls.
| Clinical variables | HC | First‐line only | Second‐line | F (P)a | χ 2 (P)b | t (P)c | U (P)d |
|---|---|---|---|---|---|---|---|
| Age (X ± SD, year) | 30.27 ± 7.70 | 34.43 ± 10.85 | 25.33 ± 4.82 | 2.7 (0.084) | / | / | / |
| Education (X ± SD, year) | 14.13 ± 2.07 | 11.14 ± 4.26 | 12.78 ± 3.63 | 2.23 (0.126) | / | / | / |
| Sex (Male/Female) | 5/10 | 2/5 | 3/6 | / | 0.056 (0.97) | / | / |
| EEG abnormality (Absent/Present) | / | 2/5 | 3/6 | / | 0.042 (0.838) | / | / |
| Seizures (Absent/Present) | / | 2/5 | 0/9 | / | 2.94 (0.086) | / | / |
| ICU treatment (Absent/Present) | / | 7/0 | 8/1 | / | 0.83 (0.362) | / | / |
| Etiology (Idiopathic/Paraneoplastic) | / | 6/1 | 7/2 | / | 0.163 (0.687) | / | / |
| Time of definite diagnosis (X ± SD, day) | / | 18.57 ± 7.61 | 16.89 ± 9.13 | / | / | ‐0.39 (0.70) | / |
| Time of follow‐up after initial discharge (median/IQR; range, month) | / | 31/7–38; 6–47 | 9/6.5–11.5; 6–44 | / | / | / | 18.50 (0.167) |
EDB, extreme delta brush; IQR, interquartile range; HC, healthy control group; first‐line only, the patients who received only first‐line immunotherapy; second‐line, the patients who received first‐line and second‐line immunotherapy;
one‐way analysis of variance (ANOVA);
Chi‐square test;
Two‐sample t‐test;
Mann–Whitney U test.
All subjects provided written informed consent, and the project was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine.
Treatment protocol
In the current study, the first‐line immunotherapy protocol at our institute was defined as high‐dose steroids (500–1000 mg of methylprednisolone daily for 3 days, followed by tapering) and intravenous immunoglobulin (IVIG) (0.4 g/kg for 5 days) with or without plasmapheresis (before IVIG, once), and second‐line immunotherapy was defined as intravenous rituximab (375 mg/m2 weekly for 4 weeks) and cyclophosphamide (750 mg/m2 monthly for 4–6 months, depending on the response), in combination or alone.
All patients received first‐line immunotherapy at 1–3 days after a definite diagnosis; nine of them also received second‐line immunotherapy at 7–56 days after first‐line immunotherapy. Treatment administered within 3 months of disease onset was considered early immunotherapy. The therapeutic strategy was selected at the treating clinicians’ discretion. Some physicians are more proactive and choose to apply second‐line immunotherapy at the acute stage when a patient’s condition is sufficiently severe, while others tend to postpone immunotherapy until disease relapse.
Neuropsychological assessment
A set of comprehensive neuropsychological tests was administered to assess working memory (digit span test) and verbal episodic memory (Chinese auditory verbal learning test, CAVLT), as well as the Stroop test, semantic fluency test, block design test, symbol‐digit modalities test (SDMT), self‐rating anxiety scale (SAS), and self‐rating depression scale (SDS).
Resting‐state functional connectivity analysis
Acquisition and initial image preprocessing of resting‐state fMRI data are provided in detail in the Data S1. The left and right hippocampal seed regions were extracted from the Anatomical Automatic Labeling (AAL) template. The Pearson correlation coefficient between the mean time courses of each seed region and those of each voxel throughout the whole brain was calculated for each individual.11 The resulting correlation coefficients were transformed into z‐values using Fisher’s transformation.
Statistical analysis
Statistical analyses were performed using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL). A Chi‐square test was used for categorical variables, and demographic variables were compared between groups using a one‐way analysis of variance (ANOVA), two‐sample T‐test, or Mann–Whitney U test.
One‐way ANOVA was used to compare the FC between the left and right hippocampus among the three groups within a whole‐brain mask (FA > 0.2) using the DPABI toolbox. The main effect F‐maps were set at a significance threshold of P < 0.001 (Gaussian Random Field theory correction (GRF) was used for multiple comparisons). Post hoc comparisons were performed in local clusters with significant group differences to compare the three groups. To estimate the relationship between FC and neuropsychological test results in all participants, we calculated the Pearson correlations between each neuropsychological tests score and the FC value of the left or right hippocampus in local clusters with significant group differences. The correlations were considered significant at a threshold of P < 0.05.
Results
Demographic and clinical data
The patients who received only first‐line therapy, the patients who received second‐line immunotherapy and the HCs did not differ significantly with regard to age (F = 2.84, P = 0.08), educational level (F = 1.70, P = 0.20) or sex (χ 2 = 0.64, P = 0.73) (Table 2). In addition, all patients (11 women; mean age, 28.81 ± 9.86 years; education, 12.38 ± 3.34 years) and HCs did not differ significantly regarding age (t = 0.98, P = 0.34), educational level (t = 1.51, P = 0.14), or sex (χ 2 = 0.015, P = 0.90). With regard to time of definite diagnosis, the patients with first‐line immunotherapy only and those with second‐line immunotherapy did not show a significant difference (t = −0.39, P = 0.70) (Table 2). According to the Mann–Whitney U test, the patients with first‐line immunotherapy only and those with second‐line immunotherapy did not show a significant difference (U = 18.50, P = 0.167) with regard to time of follow‐up after initial discharge (Table 2). In addition, the patients with second‐line immunotherapy and those with first‐line immunotherapy only did not differ significantly with regard to mRS scores (t = 1.43, P = 0.17) or potential predictive factors (EDB, χ 2 = 0.04, P = 0.84; seizures, χ 2 = 2.94, P = 0.09; ICU treatment, χ 2 = 0.83, P = 0.36; etiology, χ 2 = 0.16, P = 0.69). The demographic and clinical data are shown in Tables 1 and 2.
Neuropsychological assessment
No patients had any psychotic symptoms according NPI. The neuropsychological test results assessed by one‐way ANOVA are shown in Table 3. The patients showed significant impairments in the SDMT (F = 11.25, P = 0.000), digit span test (forward, F = 4.17, P = 0.026; backward, F = 6.93, P = 0.004), semantic fluency test (F = 4.34, P = 0.023), block design test (F = 4.61, P = 0.019), and CAVLT (immediate memory following interference, F = 8.49, P = 0.001; delayed recall, F = 5.23, P = 0.012; recognition, F = 4.65, P = 0.034), whereas scores of the Stroop test (F = 1.81, P = 0.183), self‐rating anxiety scale (F = 0.76, P = 0.493) and self‐rating depression scale (F = 0.85, P = 0.456) were normal.
Table 3.
Summary of the neuropsychological test results among the patients and healthy controls.
| Tests | HC | Second‐line | First‐line only | F | P | Post hoc analysis (P) | ||
|---|---|---|---|---|---|---|---|---|
| HC vs. second‐line | HC vs. first‐line only | Second‐line vs. first‐line only | ||||||
| Stroop test (dot) | 12.94 ± 2.64 | 14.05 ± 2.62 | 15.24 ± 2.36 | 1.81 | 0.183 | / | / | / |
| Stroop test (color word) | 24.04 ± 4.80 | 30.91 ± 10.41 | 37.89 ± 16.36 | 3.35 | 0.080 | / | / | / |
| SDMT | 62.87 ± 7.80 | 48.78 ± 8.97 | 43.83 ± 13.66 | 11.25 | 0.000 | 0.002 | 0.000 | 0.331 |
| Digit span test (forward) | 9.40 ± 1.12 | 8.00 ± 1.32 | 8.00 ± 1.78 | 4.17 | 0.026 | 0.019 | 0.038 | 1.000 |
| Digit span test (backward) | 7.53 ± 1.46 | 5.33 ± 1.00 | 6.00 ± 2.00 | 6.93 | 0.004 | 0.001 | 0.039 | 0.395 |
| Semantic Fluency Test | 21.87 ± 5.15 | 19.44 ± 4.77 | 15.33 ± 2.07 | 4.34 | 0.023 | 0.224 | 0.007 | 0.103 |
| Block Design Test | 40.60 ± 6.63 | 32.22 ± 6.57 | 31.67 ± 11.55 | 4.61 | 0.019 | 0.016 | 0.025 | 0.893 |
| CAVLT (immediate memory following interference) | 13.73 ± 1.10 | 13.56 ± 1.23 | 11.17 ± 1.94 | 8.49 | 0.001 | 0.754 | 0.000 | 0.002 |
| CAVLT (delayed recall) | 13.27 ± 1.71 | 12.78 ± 1.72 | 10.67 ± 1.51 | 5.23 | 0.012 | 0.495 | 0.003 | 0.024 |
| CAVLT (recognition) | 14.80 ± 0.41 | 14.89 ± 0.33 | 13.50 ± 1.05 | 4.65 | 0.034 | 0.834 | 0.062 | 0.049 |
| Self‐rating anxiety scale | 23.47 ± 3.04 | 25.11 ± 6.43 | 26.33 ± 5.96 | 0.76 | 0.493 | / | / | / |
| Self‐rating depression scale | 23.53 ± 3.83 | 26.89 ± 7.66 | 25.33 ± 5.68 | 0.85 | 0.456 | / | / | / |
SDMT, symbol‐digit modalities test; CAVLT, Chinese auditory verbal learning test; HC, healthy control group; first‐line only, the patients who received only first‐line immunotherapy; second‐line, the patients who received first‐line and second‐line immunotherapy.
Post hoc comparisons (least‐significant difference correction, LSD) showed that CAVLT did not differ significantly between the HCs and the patients with second‐line immunotherapy (immediate memory following interference, P = 0.754; delayed recall, P = 0.459; recognition, P = 0.843). However, the patients who received only first‐line immunotherapy showed significant verbal episodic memory impairments compared with both those who received second‐line immunotherapy (immediate memory following interference, P = 0.002; delayed recall, P = 0.024; recognition, P = 0.049) and HCs (immediate memory following interference, P = 0.000; delayed recall, P = 0.003). To consider whether time of follow‐up had an impact on verbal episodic memory improvements, we compared the memory performance between the patients with only 6–12 months of follow‐up and those with more than 1 year of follow‐up. The results showed that there were no significant differences regarding memory performance.
In addition, compared with the HCs, the patients with first‐line only and those with second‐line immunotherapy showed significant impairments in the following tests: the SDMT (HCs vs. first‐line only, P = 0.000; HCs vs. second‐line therapy, P = 0.002), digit span test (HCs vs. first‐line only, P = 0.038; HCs vs. second‐line therapy, P = 0.019), and block design test (HCs vs. first‐line only, P = 0.039; HCs vs. second‐line therapy, P = 0.001). Only the patients who received only first‐line immunotherapy showed significant impairments in the semantic fluency test (HCs vs. first‐line only, P = 0.007). However, no differences were observed between the patients with first‐line only and those with second‐line immunotherapy in the above tests.
Resting‐state FC
Voxel‐wise analysis of left hippocampal functional connectivity (FC) showed decreased FC in the left hippocampus with the medial prefrontal cortex (mPFC), and increased FC in the left hippocampus with the bilateral sensorimotor cortices (SMC) and supplementary motor area (SMA) (P < 0.001, GRF‐corrected, cluster size > 30; Fig. 1). A post hoc analysis demonstrated that the patients who received only first‐line immunotherapy showed a significant reduction in left hippocampal FC with the mPFC and significant increases in left hippocampal FC with the bilateral SMC and SMA compared with both the HCs and those who received second‐line immunotherapy (Fig. 1). No significant differences in left hippocampal FC were noted in the patients with second‐line immunotherapy compared with the HCs (Fig. 1).
Figure 1.
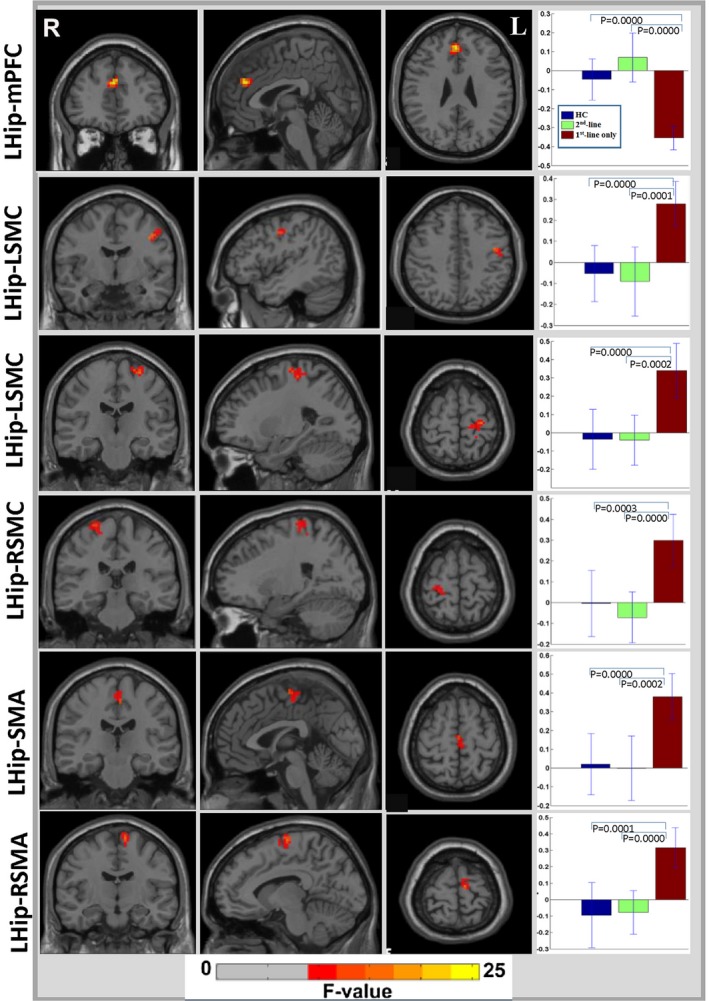
Significant resting‐state functional connectivity changes between the left hippocampus and cerebral cortex in patients with anti‐NMDA receptor encephalitis. Hip, hippocampus; L, left; R, right; mPFC, medial prefrontal cortex; SMC, sensorimotor cortex; SMA, supplementary motor area; HC, healthy control group; first‐line only, the patients who received only first‐line immunotherapy; second‐line, the patients who received first‐line and second‐line immunotherapy.
Similarly, voxel‐wise analysis of right hippocampal FC showed significant reductions in the right hippocampal FC with the mPFC, inferior‐parietal lobule (IPL), and bilateral dorsolateral prefrontal cortex (DLPFC), while significant increases were observed in right hippocampal FC with the bilateral SMC (P < 0.001, GRF‐corrected, cluster size > 30; Fig. 2). A post hoc analysis demonstrated that the patients who received only first‐line immunotherapy showed significant reductions in right hippocampal FC with the mPFC, IPL and bilateral DLPFC and significant increases in right hippocampal FC with the bilateral SMC compared with that in both the HCs and patients with second‐line immunotherapy (Fig. 2). No significant differences were noted in right hippocampal FC in the patients with second‐line immunotherapy compared with the HCs (Fig. 2).
Figure 2.
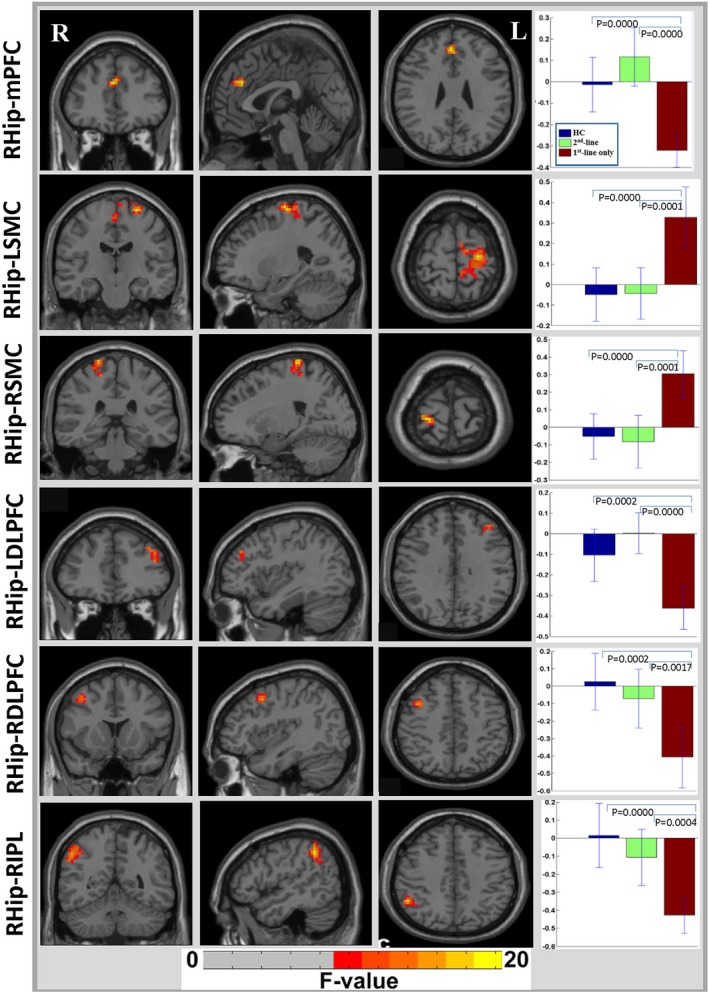
Significant resting‐state functional connectivity changes between the right hippocampus and cerebral cortex in patients with anti‐NMDA receptor encephalitis. Hip, hippocampus; L, left; R, right; mPFC, medial prefrontal cortex; SMC, sensorimotor cortex; SMA, supplementary motor area; DLPFC, dorsolateral prefrontal cortex; IPL, inferior‐parietal lobule; HC, healthy control group; first‐line only, the patients who received only first‐line immunotherapy; second‐line, the patients who received first‐line and second‐line immunotherapy.
A linear correlation analysis showed a significant positive correlation between bilateral hippocampal‐mPFC FC and CAVLT‐interference memory performance (left hippocampus, r = 0.453, P = 0.012; right hippocampus, r = 0.363, P = 0.04) and between right hippocampal‐RIPL FC and CAVLT‐interference memory performance (r = 0.41, P = 0.025) (Fig. 3). In addition, significant negative correlations were found between left hippocampal‐bilateral SMC FC and CAVLT‐Interference memory performance (left SMC, r = −0.57, P < 0.001; right SMC, r = −0.49, P = 0.0057) and between left hippocampal‐LSMC FC and CAVLT‐delayed memory performance (r = −0.53, P = 0.0026) (Fig. 3). No significant correlations between hippocampal‐mPFC FC and performance in the tests of working memory, semantic fluency, or block design were observed.
Figure 3.
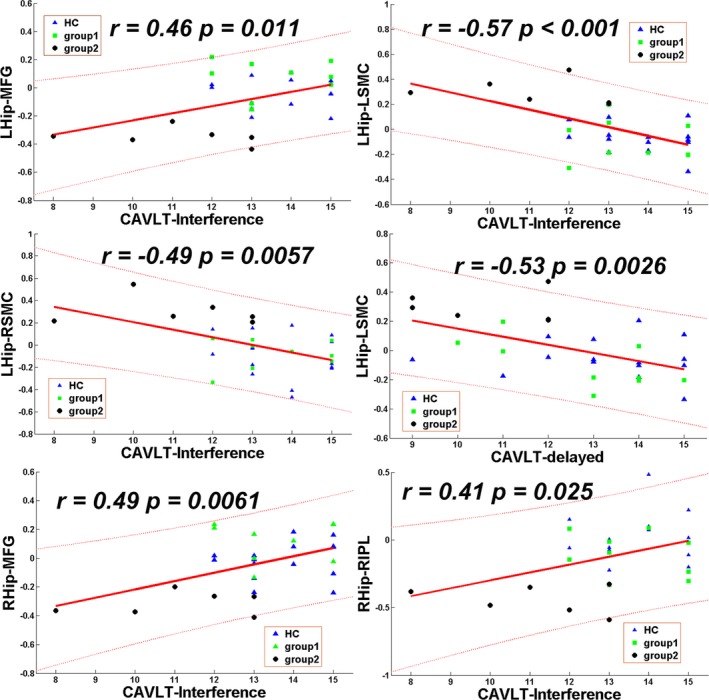
Significant correlations between hippocampal functional connectivity and individual episodic memory performance in patients with anti‐NMDA receptor encephalitis. Hip, hippocampus; L, left; R, right; mPFC, medial prefrontal cortex; SMC, sensorimotor cortex; IPL, inferior‐parietal lobule; CAVLT‐interference, Chinese auditory verbal learning test (immediate memory following interference); CAVLT‐delayed, Chinese auditory verbal learning test (delayed recall); HC, healthy control group; first‐line only, the patients who received only first‐line immunotherapy; second‐line, the patients who received first‐line and second‐line immunotherapy.
Discussion
Our study demonstrated that all patients received early immunotherapy and achieved good outcome (mRS, 0–2); however, in terms of verbal episodic memory, the patients who only received with first‐line immunotherapy showed significant impairments compared with both those who received second‐line immunotherapy and HCs, while no significant differences were noted in the patients with second‐line immunotherapy compared with the HCs. These results were consistent with that of a previous study6 but extended the findings to indicate that the early administration of intravenous second‐line immunotherapy may be associated with improved long‐term verbal episodic memory in patients with moderate‐to‐severe anti‐NMDA receptor encephalitis. However, other domains of cognition (SDMT, digit span test and block design test), which reflected frontal‐parietal function, did not return to normal levels in the patients with second‐line immunotherapy. This finding indicated that cognitive impairments of varying degrees may persist for a long time in critically ill patients despite aggressive treatment.
The clinical picture of the patients was typical, and a definite diagnosis of the patients was relatively timely in our study. Both first‐line and second‐line immunotherapy were applied in the early stage of the disease. The types, medication dosage, route of administration, and duration of immunotherapy were similar. There was no significant difference between the patients with first‐line immunotherapy only and those with second‐line immunotherapy with regard to the time of definite diagnosis, time of first‐line immunotherapy administration, potential predictive factors (EDB, seizures, ICU treatment and etiology), or time of follow‐up. Therefore, we believe that the verbal episodic memory improvements were not related to these factors.
The results of neuropsychological tests are not consistent with a previous study reporting that second‐line immunotherapy was less relevant to cognitive outcomes involving episodic memory.7 There are a number of confounding factors that may account for this discrepancy.6 Firstly, patients treated with second‐line immunotherapy were of greater severity than those who received only first‐line immunotherapy in the reported studies. In our study, we only included patients with moderate‐to‐severe anti‐NMDAR encephalitis. Secondly, patients in our study received intravenous second‐line immunosuppressants within 3 months of disease onset, whereas the timing varies in the literature. In most cases, second‐line immunotherapy was not initiated until first‐line therapy failed, which usually occurred at a relatively advanced stage.6 Other factors, such as the medication dosage, route of administration, duration of second‐line therapy, severity of the disease, and neuropsychological tests, may also influence cognitive performance.
Previous studies have reported that memory appears to be the most affected domain among all cognitive dysfunctions and was reported to highly correlate with an increased density of NMDA receptors in the hippocampus.12 Functional MRI studies have also demonstrated that reduced hippocampal FC,12, 13 hippocampal volumetrics, and microstructural integrity14 are associated with individual memory performance in patients with anti‐NMDA receptor encephalitis. In consistent with previous observations,12, 13 our study observed hippocampal FC with the mPFC decreased significantly and correlated with impaired memory performance in patients who received only first‐line immunotherapy. This finding was supported by the neuropsychological results that early intravenous second‐line immunotherapy may be associated with verbal episodic memory improvements.
Notably, a reduction in right hippocampal FC with the posterior DMN (IPL), but not the anterior DMN, was first observed in patients with anti‐NMDA receptor encephalitis.12, 13 Numerous studies support the notion that the IPL is part of the DMN and plays an important role in episodic memory.15 Normal anatomy and FC between the hippocampus and IPL are critically important for episodic memory processing.16 Therefore, reduced right hippocampal FC with the IPL may serve as a biomarker for anti‐NMDA receptor encephalitis and may be a primary factor in the episodic memory impairment associated with the disease.
Aside from the DMN, a reduction in right hippocampal FC with the bilateral DLPFC, as well as increases in bilateral hippocampal FC with the bilateral SMC and in left hippocampal FC with the SMA, were observed in the patients who received only first‐line immunotherapy. We also found that the alternations in left hippocampal FC with the bilateral SMC negatively correlated with memory performance. The hypothesis that the anterior DLPFC may be instrumental in implementing a top‐down inhibitory control signal that suppresses mnemonic processing has been supported in several studies.17 Moreover, increasing evidence supports SMC dysfunction in Alzheimer’s disease (AD),18 and decreased or rewired sensorimotor network connectivity in AD has been reported, even at an early stage, 19, 20 indicating that SMC connectivity may be associated with episodic memory processing. This contrary relationship between increased alternations in bilateral hippocampal FC with the SMC and reduced bilateral hippocampal FC with the DMN in the patients who received only first‐line immunotherapy may reflect an intrinsically inversely correlated relationship between the task‐negative DMN and task‐positive sensorimotor networks.21
This study had limitations that should be considered in interpreting our results. First, the study used a retrospective design rather than a prospective design. Hence, selection bias with treatment and bias related to time of treatment could not be avoided. A second limitation is the small sample size. Third, although all patients achieved a good overall outcome (mRS, 0–2), and there were no significant differences regarding memory performance between the patients with only 6–12 months of follow‐up and those with more than 1 year of follow‐up, the challenge regarding follow‐up variability should be noted. Fourth, because this study focused on patients with moderate‐to‐severe anti‐NMDAR encephalitis, it remains unclear whether the same association will apply to cognitive function in mild cases. Additional larger, longitudinal studies from different centers and multimodal functional MRI examinations are needed to determine the association between second‐line immunotherapy and cognitive function improvements.
In summary, the present study suggests that in the long term, the early administration of second‐line immunotherapy in patients with moderate‐to‐severe anti‐NMDA receptor encephalitis may be associated with more favorable overall verbal episodic memory outcomes. Early intervention with aggressive immunosuppressants may be justifiable in this population.
Author Contributions
Study concept and design: K. Wang, Z. Q. Chen and B. Y. Luo; data acquisition, analysis and interpretation: all authors; drafting of the manuscript: K. Wang and Z. Q. Chen; critical review of manuscript: all authors.
Conflict of Interest
None of the authors have potential conflict of interest to be disclosed.
Supporting information
Data S1 . Acquisition and initial image preprocessing of resting‐state fMRI data.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (81500905, 81201007) and Doctoral Fund of the Ministry of Education of China (20120101120070). We thank our patients and family members for their continuous effort and contribution to our clinical research.
Funding information
This work was supported by grants from National Natural Science Foundation of China (81500905, 81201007) and Doctoral Fund of the Ministry of Education of China (20120101120070).
Funding Statement
This work was funded by Doctoral Fund of the Ministry of Education of China grant 20120101120070; National Natural Science Foundation of China grants 81201007 and 81500905.
References
- 1. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti‐NMDA‐receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dalmau J, Lancaster E, Martinez‐Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti‐NMDAR encephalitis. Lancet Neurol 2011;10:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Irani SR, Bera K, Waters P, et al. N‐methyl‐D‐aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non‐paraneoplastic disorder of both sexes. Brain 2010;133(Pt 6):1655–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Viaccoz A, Desestret V, Ducray F, et al. Clinical specificities of adult male patients with NMDA receptor antibodies encephalitis. Neurology 2014;82:556–563. [DOI] [PubMed] [Google Scholar]
- 5. Florance NR, Davis RL, Lam C, et al. Anti‐N‐methyl‐D‐aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol 2009;66:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long‐term outcome in patients with anti‐NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McKeon GL, Robinson GA, Ryan AE, et al. Cognitive outcomes following anti‐N‐methyl‐D‐aspartate receptor encephalitis: a systematic review. J Clin Exp Neuropsychol 2018;40:234–252. [DOI] [PubMed] [Google Scholar]
- 8. Finke C, Kopp UA, Pruss H, et al. Cognitive deficits following anti‐NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry 2012;83:195–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keller S, Roitman P, Ben‐Hur T, et al. Anti‐NMDA receptor encephalitis presenting as an acute psychotic episode in a young woman: an underdiagnosed yet treatable disorder. Case Rep Psychiatry 2014;2014:868325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu F, Guo W, Fouche JP, et al. Multivariate classification of social anxiety disorder using whole brain functional connectivity. Brain Structure and Function 2015;220:101–115. [DOI] [PubMed] [Google Scholar]
- 12. Finke C, Kopp UA, Scheel M, et al. Functional and structural brain changes in anti‐N‐methyl‐D‐aspartate receptor encephalitis. Ann Neurol 2013;74:284–296. [DOI] [PubMed] [Google Scholar]
- 13. Peer M, Pruss H, Ben‐Dayan I, et al. Functional connectivity of large‐scale brain networks in patients with anti‐NMDA receptor encephalitis: an observational study. Lancet Psychiatry. 2017;4:768–774. [DOI] [PubMed] [Google Scholar]
- 14. Finke C, Kopp UA, Pajkert A, et al. Structural hippocampal damage following anti‐N‐methyl‐D‐aspartate receptor encephalitis. Biol Psychiat 2016;79:727–734. [DOI] [PubMed] [Google Scholar]
- 15. Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in cognitive sciences. 2005;9:445–453. [DOI] [PubMed] [Google Scholar]
- 16. Greicius MD, Srivastava G, Reiss AL, Menon V. Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 2004;101:4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson MC, Bunce JG, Barbas H. Prefrontal–hippocampal pathways underlying inhibitory control over memory. Neurobiol Learn Memory 2016;134:145–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Albers MW, Gilmore GC, Kaye J, et al. At the interface of sensory and motor dysfunctions and Alzheimer's disease. Alzheimers Dement 2015;11:70–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang J, Wang X, He Y, et al. Apolipoprotein E epsilon4 modulates functional brain connectome in Alzheimer's disease. Hum Brain Mapp 2015;36:1828–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Agosta F, Rocca MA, Pagani E, et al. Sensorimotor network rewiring in mild cognitive impairment and Alzheimer's disease. Hum Brain Mapp 2010;31:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 2005;102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 . Acquisition and initial image preprocessing of resting‐state fMRI data.


