Abstract
Background
Concerns about an inhibitory effect of proton pump inhibitors (PPIs) on clopidogrel metabolism have been raised. Because the pharmacological effect of clopidogrel is dependent on genetically determined activity of the hepatic cytochrome P450 isoenzymes system, it is important to examine the interaction between different PPIs and high on‐treatment platelet reactivity (HPR) after controlling for genetic variability. The aim of the study was to assess the effect of 2 PPIs and a histamine‐2 (H2) receptor‐blocker on platelet reactivity in a crossover trial where each patient was alternately treated with each drug.
Hypothesis
Omeprazole reduces HPR more than other PPI or H2 blockers.
Methods
Patients treated with aspirin and clopidogrel for at least 1 month were assigned to 3 consecutive 1‐month treatment periods during which they were treated with each of the 3 study medications twice daily: omeprazole 20 mg, famotidine 40 mg, and pantoprazole 20 mg. At the end of each treatment phase, platelet function was evaluated with the Verify Now system using 2 cutoff values (>208 P2Y12 reaction units [PRUs] and >230 PRUs) for the definition of HPR.
Results
Patients with HPR were older than those without HPR (62 ± 10 vs 55 ± 8 years, respectively, P = 0.03). HPR was more prevalent during omeprazole therapy compared to famotidine or pantoprazole (48%, 33%, and 31%, respectively, for the 208 PRU cutoff, P= 0.04; and 37%, 17%, and 23%, respectively, for the 230 PRU cutoff, P= 0.003).
Conclusions
After eliminating the effects of interindividual variability in clopidogrel metabolism, omeprazole therapy was associated with substantially more HPR than famotidine or pantoprazole.
Introduction
Increased high on‐treatment platelet reactivity (HPR)1 is associated with an increased risk of cardiovascular events.2, 3 Clopidogrel is a prodrug activated by hepatic cytochrome P450 (CYP450) isoenzymes, namely CYP2C19 and CYP3A4. The CYP2C19 gene polymorphism is associated with higher platelet aggregability, greater clopidogrel resistance, and an increased risk for adverse clinical outcomes.4, 5, 6 In patients with coronary artery disease (CAD) treated with dual antiplatelet therapy with aspirin and clopidogrel, concomitant treatment with proton pump inhibitors (PPIs) significantly reduces the risk of upper gastrointestinal (GI) bleeding.7, 8 However, retrospective study data suggest that concomitant therapy with PPIs and clopidogrel is associated with increased mortality when compared to clopidogrel therapy without PPIs.9 A reduction in the clinical efficacy of clopidogrel due to PPI therapy was supported by experimental data demonstrating inhibition of the antiplatelet effect of clopidogrel by PPIs.2 The postulated mechanism underlying this interaction is competitive hepatic metabolism of clopidogrel and various PPIs by the CYP450 system, leading to reduced levels of the active clopidogrel metabolite.10 Loss‐of‐function polymorphisms in the CYP450 genes may also reduce clopidogrel metabolism and are therefore likely to intensify interactions between drugs metabolized by the CYP450 system.6, 11, 12, 13 The inhibitory effect of omeprazole on the antiaggregation effects of clopidogrel is most pronounced among the various PPIs studied.14 A recent retrospective study showed that only omeprazole was related with adverse outcome in patients with acute coronary syndrome.15
In a recent randomized trial, omeprazole therapy did not increase the rates of cardiovascular events in patients receiving concomitant therapy with clopidogrel. That trial was stopped prematurely, however, due to funding problems.8
Because the effect of clopidogrel is largely determined by genetically determined activity of the CYP450 system, the true impact of PPI therapy on HPR can only be defined after controlling for interindividual genetic variability.
In the present study, we assessed the effects of 2 different PPIs and 1 histamine‐2 (H2) blocker on platelet reactivity in patients with CAD who were treated with aspirin and clopidogrel in a crossover trial where each patient was treated with each of the 3 drugs in sequence.
Methods
Study Design and Patient Selection
This is a single‐center, prospective, randomized, single‐blinded, crossover study conducted in the Cardiology Department of The Tel Aviv Medical Center. Subjects (males and females 18 years of age or older) who were being treated with clopidogrel (75 mg once daily) and aspirin (100 mg once daily) for at least 1 month were eligible for enrollment. All had undergone percutaneous coronary intervention (PCI) with implantation of 1 or more drug‐eluting stent for the treatment of stable or unstable CAD. Excluded from the trial were patients with known hypersensitivity to any of the study drugs, a platelet count <50,000/μL, heart failure (New York Heart Association class ≥3), left ventricular ejection fraction <25%, acute myocardial infarction within the 30 days preceding enrollment, serum creatinine >2.5 mg/dL, a history of a bleeding diathesis or GI hemorrhage, or hepatic disease.
All enrolled patients were assigned to 3 consecutive treatment periods, each of 4 to 6 weeks'duration enabling a washout phase between each treatment period. The therapeutic protocol was twice daily omeprazole 20 mg, famotidine 40 mg, or pantoprazole 20 mg. The drug doses were chosen to maximize the potential differences between the groups. The sequence of the 3 treatment phases was by randomized assignment (closed envelope). There were no significant differences in the order of therapies due to our randomization process. Platelet resistance was evaluated on the completion of each of the 3 treatment phases. The duration was chosen to reach steady state drug levels after the previous drug had washed out of the system. Each patient received a letter with the results of his/her platelet function with a recommendation on further treatment.
The trial was approved by the local institutional ethics committee. All patients provided written informed consent. The trial was registered as a clinical trial at clinicaltrials.gov, identifier: NCT00950339.
The sequence of administration was as follows: (1) 1 month of PPI treatment (omeprazole, 20 mg twice daily), (2) 1 month of H2 blocker treatment (famotidine, 40 mg twice daily), and (3) 1 month of PPI treatment (pantoprazole, 20 mg twice daily). At the end of each phase, a blood sample was taken from each patient for platelet reactivity and clopidogrel resistance evaluation. Adherence was evaluated by counting the pills left in the packets of the prior phase. Adherence to study protocol exceeded 87%, with no differences in compliance between the 3 studied drugs (P= 0.80).
Platelet Reactivity
Platelet function was evaluated using a validated method: the VerifyNow System (Accumetrics Inc., San Diego, CA), which is a point‐of‐care turbidimetry‐based optical detection system that measures platelet‐induced aggregation. Platelet function was measured with the VerifyNow P2Y12 test at baseline and again at the end of each phase of treatment. This test has been described elsewhere in detail.16 In brief, this assessment measures adenosine phosphate‐induced platelet agglutination as an increase in light transmittance and utilizes a proprietary algorithm to report values in P2Y12 reaction units (PRUs) and percent inhibition. A higher PRU count reflects greater P2Y12‐mediated platelet reactivity. Whole blood was obtained by venipuncture into 1.8‐mL draw plastic Vacuette tubes (Greiner Vacuette North America, Inc., Monroe, NC) containing 3.2% sodium citrate with a 21‐gauge needle. Before blood sampling, patients were after an overnight fast. The last tablet taken was 10 hours before. The tubes were inverted 4 times and incubated at room temperature for 10 minutes, after which they were inverted an additional 4 times and placed into the verifyNow system device. The platelet reactivity analysis was performed by personnel unaware of the patients'clinical data and the study medications.
Clopidogrel Resistance
Various definitions for clopidogrel resistance have been used in different studies. In the inclusion criteria for the Gauging Responsiveness With a VerifyNow Assay—Impact on Thrombosis and Safety (GRAVITAS) study, the investigators used PRUs ≥230 as the definition for clopidogrel resistance.3 In a post hoc GRAVITAS analysis, they showed that platelet activity <208 PRUs remained independently associated with the primary end point at 60 days (hazard ratio [HR]: 0.23, 95% confidence interval [CI]: 0.05‐0.98, P= 0.047) and tended to be associated with the primary end point at 6 months (adjusted HR: 0.54, 95% CI: 0.28‐1.04, P=0.065).3 We therefore defined resistance using both cutoffs in the present study, once with a PRUs limit of 208 and again with a PRUs limit of 230.
Statistics
Calculation of sample size for the present study was based on previous experience.10 We estimated a increase of 20% in platelet reactivity. Therefore, to achieve an α of 0.05 and a power of 0.8, we estimated that we needed 50 patients. Continuous variables are displayed as mean ± standard deviation and were compared using the Student t test, whereas categorical data were compared using the χ2 or Fisher exact test. Platelet function test results were compared using the repeated measures test in the generalized linear model module. A 2‐tailed P < 0.05 was considered statistically significant. All analyses were performed with SPSS 19.0 software (IBM, Armonk, NY).
Figure 1.
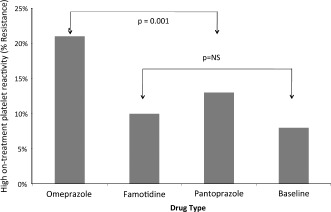
Percentage of resistance among the 3 study medications according to the 230 platelet reactivity unit cutoff. The overall difference between them is highly significant (P= 0.001). There was a nonsignificant (NS) difference between baseline, famotidine, and pantoprazole (depicted in graph).
Results
Between September 2009 and December 2011, 62 patients fulfilled study entry criteria and were recruited into the study. Nine of them withdrew consent, and 1 developed peptic complaints prior to study initiation and was excluded from further participation, leaving a total of 52 patients whose data were included in the final analysis. Baseline patient characteristics stratified by HPR status during omeprazole therapy are presented in Table 1 to assess clinical variables that might hint to patients at risk for HPR. Other than the more advanced age of patients with HPR compared to those without HPR, the baseline characteristics were well balanced in both groups. Platelet function test results at the end of each of the treatment periods are presented in Table 2. Regardless of the cutoff used, HPR was significantly more frequent during omeprazole therapy than at baseline or with famotidine or pantoprazole therapy (21% versus, 8%, 10%, and 13%, respectively, for the 230 PRU cutoff, P < 0.001; and 28% versus 9%, 19%, and 18%, respectively, for the 208 PRU cutoff, P < 0.001). The rates of HPR did not differ significantly between the famotidine and pantoprazole treatment periods (P= 0.99). Patients who were resistant on pantoprazole also exhibited resistance to famotidine. No major bleeding or adverse cardiovascular events occurred during the study. In addition, there were no peptic complaints during the study for any of the participants.
Table 1.
Clinical Characteristics of the Study Participants
| Clopidogrel‐Resistant Patients on Omeprazole (n= 19) | Clopidogrel‐Sensitive Patients on Omeprazole (n= 33) | P Value | |
|---|---|---|---|
| Age, y | 62 ± 10 (range, 46–87) | 55 ± 8 (range, 34–77) | 0.03 |
| Male gender | 16 (84%) | 30 (91%) | 0.116 |
| Current smokers | 3 (16%) | 7 (21%) | 0.56 |
| Past smokers | 9 (47%) | 13 (39%) | 0.71 |
| Hypertension | 16 (84%) | 19 (58%) | 0.41 |
| Diabetes mellitus | 7 (37%) | 6 (18%) | 0.18 |
| Dyslipidemia | 17 (90%) | 25 (76%) | 0.41 |
| Prior stroke | 2 (10%) | 0 (0%) | 0.07 |
| Medications | |||
| ACE inhibitors | 12 (63%) | 21 (64%) | 0.74 |
| Aspirin | 19 (100%) | 33 (100%) | 1 |
| β‐Blockers | 12(63%) | 22(67%) | 0.57 |
| Clopidogrel | 19 (100%) | 33 (100%) | 1 |
| Statins | 17 (90%) | 31 (94%) | 0.61 |
| LDL‐C (mg/dL) | 82±19 | 78±20 | 0.65 |
| HbA1c (%) | 6.2±1.2 | 5.6±1.2 | 0.78 |
Abbreviations: ACE, angiotensin‐converting enzyme; HbA1c, glycated hemoglobin; LDL‐C, low‐density lipoprotein cholesterol.
Table 2.
Aggregation Values According to Antacid Medications
| Variable | Baseline | Omeprazole | Famotidine | Pantoprazole | P Value |
|---|---|---|---|---|---|
| PRUs | 196 ± 19 | 217 ± 12 | 179 ± 11 | 187 ± 12 | 0.026 |
| Resistant patients to >230 PRUs | 8% | 21% | 10% | 13% | <0.001 |
| Resistant patients to >208 PRUs | 9% | 28% | 19% | 18% | <0.001 |
Abbreviations: PRUs, P2Y12 reaction units.
Values are mean ± standard error.
Discussion
The present prospective crossover study shows, for the first time, that after controlling for genetic variability in clopidogrel metabolism (a potential confounder in previous trials12, 17), omeprazole use was associated with a doubling of the rates of HPR compared with the other 2 tested antacid regimens. This was shown using the VerifyNow system, which has become the most popular of platelet function testing in recent years due to its ease of use and reliability, and was a consistent finding independent of the cutoff used to define platelet resistance.3 The only clinical marker we found related to increased platelet resistance was older age.18 This could be attributed to altered metabolism. However, the exact mechanism is not clear.
It is expected that clopidogrel is to remain the most widespread antiplatelet agent used in clinical practice despite the emergence of new alternative antiplatelet medications, given its proven clinical efficacy and the availability of cheap generic formulations. Therefore, evaluation of possible drug interactions with clopidogrel remains relevant and important. The evidence in the literature regarding the potential clinical significance of the interaction between PPIs and clopidogrel is controversial.3, 9, 19 Treating patients according to platelet function tests did not prove beneficial.19, 20 The conflicting results might be related to the fact that many of the studies were observational and not prospective, and thus potentially misleading. On the other hand, 1 study that was randomized did not take into consideration the genetic polymorphisms of the patients and was stopped prematurely. Genetic polymorphisms could affect the response to clopidogrel and might increase the likelihood of drug interactions mediated by cytochrome P450. Testing for CYP polymorphisms is expensive, and that probably precludes its extended use.
Our study results show that treatment with omeprazole carries a significantly higher probability for HPR in a real‐world clinical setting among patients who had not undergone genetic testing.
In the Clopidogrel and the Optimization of Gastrointestinal Events trial, 3873 patients on dual antiplatelet therapy were randomized to receive omeprazole or placebo.8 The cohort of patients in that study was much larger than in any previous randomized trial. The results showed that omeprazole treatment was associated with a significant reduction in clinically manifested GI bleeding events, including overt bleeding, compared with placebo (2.9% vs 1.1%, respectively, P < 0.001), with no increase in cardiovascular events (P= 0.96). That study was unfortunately stopped prematurely due to financial considerations. In addition, they used a special formulation that included clopidogrel and omeprazole in 1 tablet that is no longer available. The randomization in that trial was clinically based, and patient genotypes were not taken into account.
A different conclusion was reached in the study by Ho et al.9 In this retrospective analysis of 8205 patients, concomitant use of clopidogrel and PPIs was associated with an increased risk of adverse outcomes (all‐cause mortality or rehospitalization for myocardial infarction or unstable angina) compared with the use of clopidogrel without PPIs )29.8% vs 20.8%, respectively), with an adjusted odds ratio of 1.25 (95% CI: 1.11‐1.41). That study included a myriad of antacid regimens, and it had been suggested that patients receiving the antacid had more comorbid conditions.9 Goodman et al21 published a substudy of the Platelet Inhibition and Patient Outcomes (PLATO) trial that evaluated the effect of PPI on clopidogrel resistance. They found that the use of a PPI was independently associated with a higher rate of cardiovascular events in patients with acute coronary syndrome receiving clopidogrel. However, a similar association was observed between cardiovascular events and PPI use during ticagrelor treatment and with other non‐PPI gastrointestinal treatment. Therefore, in the PLATO trial, the association between PPI use and adverse events may be due to confounding, as PPI use was a marker for, rather than a cause for, higher rates of cardiovascular events. However, another recent article showed that only omeprazole was related with adverse outcome in patients with acute coronary syndrome.15
In spite of the small number of patients in the present study, it might nevertheless shed some light on the discrepancy between the results of the different clinical studies cited.
Famotidine is an H2 blocker that does not metabolize by using the P450 system. Pantoprazole is metabolized by multiple CYPs in the P450 system (ie, CYP2C19 and CYP3A4). Omeprazole is metabolized by CYP2C19 alone. These differences in pharmacology and metabolism might explain the differences between these medications in our results.
Clopidogrel resistance is estimated to be present in at least 20% to 30% of patients undergoing PCI.22 Our study demonstrated that omeprazole, but not pantoprazole or famotidine, is associated with increased platelet reactivity. Our finding is in agreement with a randomized study of 104 patients who were given a higher maintenance dose of 150 mg clopidogrel after coronary stenting; 44% of the clopidogrel recipients in the omeprazole group were nonresponders compared with 23% in the pantoprazole group (P= 0.04).23 However, that study used the vasodilator‐stimulated phosphoprotein (VASP), and the differences could be explained by genetic differences between the groups.
Recently, Yano et al24 recruited 130 acute coronary syndrome (ACS) patients and randomized them to receive a Japanese standard dose of omeprazole, 10 mg daily, or famotidine, 20 mg daily, for at least 4 weeks. There was no significant difference in the platelet reactivity index (PRI) measured with vasodilator‐stimulated phosphoprotein phosphorylation assay between the omeprazole group (n= 65) and famotidine group (n= 65). However, it should be noted that the dose of omeprazole was low, the cohort consisted of ACS patients, and last, the trial was randomized and not a crossover. Therefore, genetic polymorphisms might still play a role.
Another study on stable CAD patients25 consisted of a crossover design between 2 PPI drug regimens without an H2 blocker. In addition, the study cohort was substantially smaller than ours. However, they did demonstrate the adverse interaction between omeprazole and clopidogrel. Therefore, we feel that our study adds new and important data to this controversial topic.
Our results are in accordance with the current guidelines that support administering PPIs to patients who are being treated with dual antiplatelet therapy, but recommend refraining from omeprazole.26, 27 In addition, we show that 2 commonly used antacid medications are good alternatives to omeprazole, because they do not negatively affect clopidogrel resistance as much. Pantoprazole is an effective PPI; however, its main disadvantage is its high cost. Famotidine, on the other hand, is inexpensive, but it is less effective in preventing acid secretion because it is an H2 blocker. Therefore, an antacid medication should be chosen according to the risk of developing peptic bleeding and dyspepsia. Those risk factors include advanced age, concomitant use of warfarin, steroids, nonsteroidal anti‐inflammatory drugs, or an existing Helicobacter pylori infection. Patients who are free of risk factors for GI bleeding receive little if any absolute risk reduction from a PPI, and the risk/benefit balance would seem to favor the use of antiplatelet therapy without concomitant PPIs.26 Therefore, low‐risk patients could suffice with H2 blockers, whereas high‐risk patients should be recommended another PPI such as pantoprazole.26
Study Limitations
The main limitation of this study is that it is not powered for clinical end points. This was a single‐center study, and only 1 method was used for assessing platelet function. Future randomized studies that are powered for clinical end points will be needed to substantiate our findings.
Conclusion
After eliminating the effects of interindividual variability in clopidogrel metabolism, omeprazole was associated with substantially more clopidogrel resistance compared to other antacid regimens.
Acknowledgments
The authors thank Esther Eshkol for editorial assistance. The authors would like to thank the great work by Noa Menashe, Miri Revivo and Avital Levitov.
Trial registration: clinicaltrials.gov, identifier: NCT00950339.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Bonello L, Tantry US, Marcucci R, et al. Working Group on High On‐Treatment Platelet Reactivity. Consensus and future directions on the definition of high on‐treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–933. [DOI] [PubMed] [Google Scholar]
- 2. Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109:3171–3175. [DOI] [PubMed] [Google Scholar]
- 3. Price MJ, Angiolillo DJ, Teirstein PS, et al. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time‐dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 Assay: Impact on Thrombosis and Safety (GRAVITAS) trial. Circulation. 2011;124:1132–1137. [DOI] [PubMed] [Google Scholar]
- 4. Trenk D, Hochholzer W, Fromm MF, et al. Cytochrome p450 2c19 681g>a polymorphism and high on‐clopidogrel platelet reactivity associated with adverse 1‐year clinical outcome of elective percutaneous coronary intervention with drug‐eluting or bare‐metal stents. J Am Coll Cardiol. 2008;51:1925–1934. [DOI] [PubMed] [Google Scholar]
- 5. Sibbing D, Stegherr J, Latz W, et al. Cytochrome P450 2C19 loss‐of‐function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur Heart J. 2009;30:916–922. [DOI] [PubMed] [Google Scholar]
- 6. Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309–317. [DOI] [PubMed] [Google Scholar]
- 7. Aihara H, Sato A, Takeyasu N, et al. ICAS Registry Investigators. Effect of individual proton pump inhibitors on cardiovascular events in patients treated with clopidogrel following coronary stenting: results from the Ibaraki Cardiac Assessment Study Registry Catheter Cardiovasc Interv. 2012;80:556–563. [DOI] [PubMed] [Google Scholar]
- 8. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909–1917. [DOI] [PubMed] [Google Scholar]
- 9. Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301:937–944. [DOI] [PubMed] [Google Scholar]
- 10. Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double‐blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol. 2008;51:256–260. [DOI] [PubMed] [Google Scholar]
- 11. Bhatt DL. Tailoring antiplatelet therapy based on pharmacogenomics: how well do the data fit? JAMA. 2009;302:896–897. [DOI] [PubMed] [Google Scholar]
- 12. Mega JL, Close SL, Wiviott SD, et al. Cytochrome p‐450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. [DOI] [PubMed] [Google Scholar]
- 13. Simon T, Verstuyft C, Mary‐Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–375. [DOI] [PubMed] [Google Scholar]
- 14. Siller‐Matula JM, Spiel AO, Lang IM, et al. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J. 2009;157:148.e1–e5. [DOI] [PubMed] [Google Scholar]
- 15. Lin CF, Shen LJ, Wu FL, et al. Cardiovascular outcomes associated with concomitant use of clopidogrel and proton pump inhibitors in patients of acute coronary syndrome in Taiwan. Br J Clin Pharmacol. 2012;74:824–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Price MJ. Bedside evaluation of thienopyridine antiplatelet therapy. Circulation. 2009;119:2625‐2632. [DOI] [PubMed] [Google Scholar]
- 17. Lee JM, Park S, Shin DJ, et al. Relation of genetic polymorphisms in the cytochrome p450 gene with clopidogrel resistance after drug‐eluting stent implantation in Koreans. Am J Cardiol. 2009;104:46–51. [DOI] [PubMed] [Google Scholar]
- 18. Cuisset T, Quilici J, Grosdidier C, et al. Comparison of platelet reactivity and clopidogrel response in patients ≤75 years versus >75 years undergoing percutaneous coronary intervention for non‐ST‐segment elevation acute coronary syndrome. Am J Cardiol. 2011;108:1411–1416. [DOI] [PubMed] [Google Scholar]
- 19. Price MJ, Berger PB, Teirstein PS, et al. Standard‐ vs high‐dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097–1105. [DOI] [PubMed] [Google Scholar]
- 20. Reny JL, Berdague P, Poncet A, et al. Antiplatelet Drug Resistances and Ischemic Events (ADRIE) Study Group. Antiplatelet drug response status does not predict recurrent ischemic events in stable cardiovascular patients: Results of the Antiplatelet Drug Resistances and Ischemic Events study Circulation. 2012;125:3201–3210. [DOI] [PubMed] [Google Scholar]
- 21. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the Platelet Inhibition and Patient Outcomes trial. Circulation. 2012;125:978–986. [DOI] [PubMed] [Google Scholar]
- 22. Tentzeris I, Siller‐Matula J, Farhan S, et al. Platelet function variability and non‐genetic causes. Thromb Haemost. 2011;105(suppl 1):S60–S66. [DOI] [PubMed] [Google Scholar]
- 23. Cuisset T, Frere C, Quilici J, et al. Comparison of omeprazole and pantoprazole influence on a high 150‐mg clopidogrel maintenance dose the PACA (Proton Pump Inhibitors And Clopidogrel Association) prospective randomized study. J Am Coll Cardiol. 2009;54:1149–1153. [DOI] [PubMed] [Google Scholar]
- 24. Yano H, Tsukahara K, Morita S, et al. Influence of omeprazole and famotidine on the antiplatelet effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes: a prospective, randomized, multicenter study. Circ J. 2012;76:2673–2680. [DOI] [PubMed] [Google Scholar]
- 25. Yamane K, Kato Y, Tazaki J, et al. Effects of PPIs and an H2 blocker on the antiplatelet function of clopidogrel in Japanese patients under dual antiplatelet therapy. J Atheroscler Thromb. 2012;19:559–569. [DOI] [PubMed] [Google Scholar]
- 26. Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;56:2051–2066. [DOI] [PubMed] [Google Scholar]
- 27. Hamm CW, Bassand JP, Agewall S, et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: The Task Force For The Management Of Acute Coronary Syndromes (ACS) in Patients Presenting Without Persistent ST‐Segment Elevation of the European Society Of Cardiology (ESC). Eur Heart J. 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]


