ABSTRACT
Background
Stress cardiomyopathy manifests as reversible left ventricular apical ballooning in the absence of epicardial coronary obstruction. Transient microcirculatory dysfunction has been proposed as a potential putative mechanism. This study aimed to understand the natural history of this dysfunction using readily available noninvasive methods.
Hypothesis
Stress cardiomyopathy presents with profound microvascular dysfunction that improves quickly over a period of 3 to 4 weeks.
Methods
Nine consecutive patients with Takotsubo cardiomyopathy were followed serially with myocardial perfusion echocardiograms at 24 hours, within 1 week, and 3 to 6 months after index admission.
Results
The mean left ventricular ejection fraction (LVEF) steadily improved from 38% at baseline to 48% within 1 week to 67% by the end of 3 to 6 months follow‐up. The number of wall segments with reduced or absent perfusion decreased from 4.1 at baseline to 2 at 1 week. By 3 to 6 months, perfusion had returned to normal in all but 1 segment in 1 patient. At 1 week, the relative improvement in mean LVEF was 26%, whereas perfusion had improved by nearly 50%, suggesting a fairly pronounced improvement in microcirculatory function prior to recovery of wall motion.
Conclusions
Patients with Takotsubo cardiomyopathy present with significant acute microcirculatory dysfunction that recovers quickly prior to the recovery of regional wall motion abnormalities.
Introduction
Stress cardiomyopathy (SCM), also known as Takotsubo cardiomyopathy (TC), is an enigmatic syndrome characterized by reversible left ventricular apical ballooning in the absence of epicardial coronary obstruction, triggered frequently by physical or emotional stress, mimicking an acute coronary syndrome.1, 2, 3, 4, 5, 6, 7 Several theories have been postulated regarding its pathophysiologic etiology including transient microcirculatory dysfunction.8 Acute disruption of microcirculation as a key pathogenic mechanism for SCM was suggested by poor thrombolysis in myocardial infarction (TIMI) frame counts and TIMI flow grade, as well as myocardial perfusion echocardiography (MPE) in the majority of SCM patients undergoing coronary angiography at the Mayo Clinic.9, 10, 11 More recently, evaluation of microcirculatory function in SCM was studied by using the index of microcirculatory resistance (IMR), which is an invasive index of the status of the microvasculature.12 Little information is, however, available regarding the time course of this dysfunction. The aim of the current study was to assess and describe the time course of improvement in the microcirculatory dysfunction using serial noninvasive MPE.
Methods
Nine consecutive patients meeting the published criteria for SCM by the Mayo Clinic (Table 1) were identified from January 2003 to July 2004 in our institution and studied prospectively. Written informed consent was obtained for enrollment into the study and for the performance of contrast echocardiography.
Table 1.
Published Criteria for Takotsubo Cardiomyopathy (Mayo Clinic)
| The Mayo Clinic Criteria |
|---|
| Transient akinesis or dyskinesis of the left ventricular apical and midventricular segments with regional wall motion abnormalities extending beyond a single epicardial vascular distribution |
| Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture |
| New ECG abnormalities (either ST‐segment elevation or T‐wave inversion) |
| Absence of: |
| Recent significant head trauma |
| Intracranial bleeding |
| Pheochromocytoma |
| Obstructive epicardial coronary artery disease |
| Myocarditis |
| Hypertrophic cardiomyopathy |
Abbreviations: ECG, electrocardiograph.
All patients underwent serial standard contrast transthoracic echocardiographic studies at 3 time points: within 24 hours of admission, within the first week, and at 3 to 6 months outpatient follow‐up. MPE was performed at each setting using commercially available contrast agents (Definity; Lantheus Medical Imaging, N. Billerica, MA) and ultrasound systems (Acuson Sequoia; Seimens Corp., Munich, Germany). An intermittent bolus dose of activated contrast agent was given via a peripheral intravenous cannula to achieve adequate myocardial opacification of the left ventricle (LV) cavity. Images were acquired using the destruction‐replenishment technique (ie, continuous imaging at low power using a mechanical index [MI] of 0.1 with intermittent pulse disruption of microbubbles using high power [MI >1]). Images were captured sequentially in the apical 4, 3, and 2 chambers for a total of 8 cardiac cycles to evaluate the replenishment phase and were digitized and stored in electronic format.
Regionality and severity of wall motion abnormalities (WMAs) were defined using the 17 segment model as per the American Society of Echocardiography guidelines.13 Left ventricular ejection fraction (LVEF) was calculated by using the modified Simpson's rule. Presence of perfusion abnormality was determined by visually estimating the number of cardiac cycles required to refill each myocardial segment following bubble disruption. Myocardial segmental opacification within ≤4 cycles was classified as preserved microcirculation. A binary score of preserved or absent (or reduced) perfusion (>4 cycles) was assigned for each segment. All echocardiographic analysis was performed by a single experienced echocardiographer (S.W.Z.) who was blinded to the time course of the echocardiographic acquisition.
Results
Clinical characteristics of patients are listed in Table 2. All patients were postmenopausal women with a mean age of 66.5 ± 9 years. Cardiac risk factors included hypertension (44%), hypercholesterolemia (33%), active smoking (77%), and none of the patients had diabetes. All but 3 patients gave a history of a prior acute emotional stressor, and those 3 presented with a preceding acute physical stress. All patients underwent coronary angiography, and none had evidence of obstructive coronary artery disease. There were no recurrences during the 6‐month follow‐up period. The mean LVEF steadily improved from 38% ± 9% at admission to 48% ± 9% within 1 week, to normal (67% ± 9%) by the end of follow‐up (Figure 1). The number of wall segments with absent perfusion decreased from 4 ± 1.7 at baseline to 2 ± 1.6 at 1 week (Figure 2). By 3 to 6 months, perfusion had returned to normal in all patients except 1. There was 1 segment that required 5 cardiac cycles to perfuse, thus meeting the study criteria of absent/decreased perfusion. By 1 week, the relative improvement in mean LVEF was 26%, whereas myocardial perfusion had improved by nearly 50% (Figure 1).
Table 2.
Clinical Characteristics of Patients
| Patients Characteristics | All Patients, n = 9 |
|---|---|
| Age, y, mean ± SD | 66.5 ± 9 |
| Female, no. (%) | 9 (100) |
| Risk factors, no. (%) | |
| Hypertension | 4 (44) |
| Diabetes | 0 (0) |
| Hypercholesterolemia | 3 (33) |
| Smoking | 7 (78) |
| Alcohol abuse | 0 (0) |
| Family history of CAD | 4 (44) |
| Hospital presentation, no. (%) | |
| Preceding emotional stressor | 6 (67) |
| Preceding physical stressor | 3 (33) |
| Chest pain | 8 (89) |
| ST‐segment elevation | 2 (22) |
| Q waves | 1 (11) |
| Positive biomarkers | 8 (89) |
| Heart failure | 3 (33) |
| ICU admission | 4 (44) |
| Ventilator | 1 (11) |
| CAD on angiography, no. (%) | 0 (0) |
| Recurrence at 6‐month follow‐up, no. (%) | 0 (0) |
Abbreviations: CAD, coronary artery disease; ICU, intensive care unit; SD, standard deviation.
Figure 1.
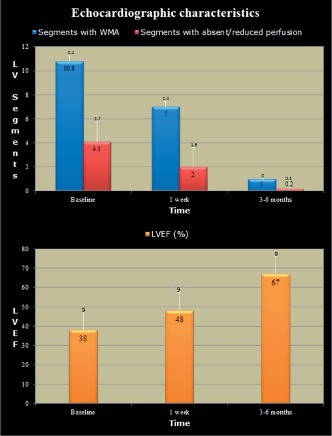
Echocardiographic characteristics of 9 patients presenting with Takotsubo cardiomyopathy undergoing serial myocardial perfusion echocardiographic studies. Upper panel demonstrates the improvement in left ventricle (LV) segments with wall motion abnormalities (WMA) and impaired perfusion over time. The lower panel demonstrates improvement in global LV function over the same time duration. Abbreviations: LVEF, left ventricular ejection fraction.
Figure 2.
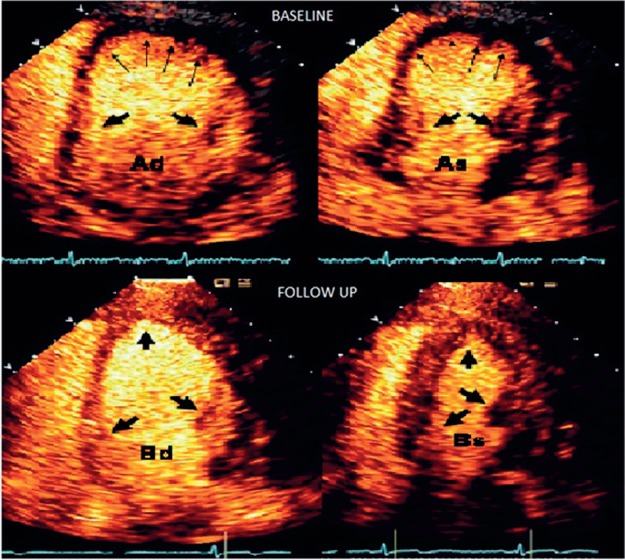
Perfusion echocardiogram at admission (A) and 6‐month follow‐up (B) in diastole (d) and systole (s). Panel Ad reveals thinning and lack of contrast uptake in the apex (thin arrows) with good uptake at the base (fat arrows). Panel As shows good thickening of the basal segments with ballooning of apex (thin arrows). Panel Bd and Bs show normal perfusion and wall motion on follow‐up.
Discussion
TC is a well‐recognized entity that was first described in the Japanese population.1, 14 It often presents in a dramatic fashion, resulting in acute severe left ventricular dysfunction with near total reversal within a few days to weeks. Its recognition and differentiation from acute coronary syndrome thus becomes paramount. Although several patterns of LV contraction abnormalities have been identified, a distinctive pattern of left ventricular apical ballooning is most frequently noted in TC.15, 16 The link between emotional or physical stress and reversible LV dysfunction in the absence of epicardial coronary disease has not been well established. Theories of epicardial vessel spasm with excess catecholamine surge17 or rupture of vulnerable plaque with spontaneous recanalization18, 19 have not been convincing. Additionally, pretreatment with β‐blockers does not appear to affect the severity of TC.20 Researchers have therefore turned their attention to microcirculation as a potential culprit in the pathogenesis of this cardiomyopathy. Investigators at the Mayo Clinic demonstrated that the majority of patients with TC had abnormal TIMI frame counts despite normal epicardial vessels.9 In an analogous study,12 the invasive IMR was calculated both in patients with TC and ST‐segment myocardial infarction at the time of coronary angiography. In both cohorts, the IMR was elevated, suggesting microcirculatory dysfunction; however, the difference in the IMR between the 2 groups was not statistically significant. These finding suggest that although microcirculatory insult occurs in both groups, this phenomenon is transient in patients with TC given the complete recovery of LV function. We conducted our study to assess the recovery of microcirculation over time.
Echocardiographic contrast bubbles have similar rheology as red blood cells. In the absence of significant epicardial stenosis the degree of myocardial uptake of contrast agents directly correlates with the integrity of the microcirculation. This modality can be utilized to assess microcirculation reliably and has good reproducibility. In our cohort, baseline myocardial contrast echocardiography (MCE) demonstrated nearly uniform absence of perfusion in the apical segments with reduced perfusion in the midventricular segments. Although initially the apex is ballooned due to acute positive remodeling, the typical ballooned appearance is lost within days in the majority of patients. Improvement in myocardial perfusion appeared to precede correction of regional WMAs, suggesting that acute transient microvascular dysfunction is present, whereas improvement in regional wall motion ensues only after the microcirculation begins to recover. Within several months, both myocardial perfusion and wall motion had normalized. Rapid and complete restoration of the microcirculation would be less likely if microcirculatory function was secondary to epicardial coronary artery occlusion or vasospasm causing disruption of the microcirculatory architecture.
Our study has several limitations. The exact mechanism of transient microcirculatory dysfunction is not addressed by our study. MCE was analyzed visually and can be subjective. Segments with decreased perfusion were not quantitatively assessed, as quantification of decreased perfusion is somewhat imprecise. All echocardiographic analysis was performed by a single experienced reader, reducing interobserver bias, who was blinded to the time course of the echocardiographic acquisition. Use of a semiquantitative binary scale of absent or preserved function allows for a simplified method of utilizing contrast echocardiography at the bedside, thus reducing subjective bias. Our study is unique in that it looked at serial progression of recovery of microperfusion and wall motion, which lends insight into the natural history of LV functional recovery. We have also demonstrated the feasibility of using MCE as a semiquantitative tool to evaluate microcirculatory function over time.
Conclusion
Patients with TC present with significant acute microcirculatory dysfunction that recovers quickly preceding recovery of WMAs. MCE is a useful noninvasive tool to assess tissue perfusion and can be used easily at the bedside given commercially available technologies. Further studies are needed to completely elucidate the underlying pathogenic mechanism of TC.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Tsuchihashi K, Ueshima K, Uchida T, et al. Angina Pectoris‐Myocardial Infarction Investigations in Japan. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. J Am Coll Cardiol. 2001;38:11–18. [DOI] [PubMed] [Google Scholar]
- 2. Witzke C, Lowe HC, Waldman H, et al. Transient left ventricular apical ballooning. Circulation. 2003;108:2014. [DOI] [PubMed] [Google Scholar]
- 3. Desmet WJ, Adriaenssens BJ, Dens JA. Apical ballooning of the left ventricle: first series in white patients. Heart. 2003;89:1027–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472–479. [DOI] [PubMed] [Google Scholar]
- 5. William DG. Recognition of the apical ballooning syndrome in the United States. Circulation. 2005;111:388–390. [DOI] [PubMed] [Google Scholar]
- 6. Azzarelli S, Galassi AR, Amico F, et al. Clinical features of transient left ventricular apical ballooning. Am J Cardiol. 2006;98:1273–1276. [DOI] [PubMed] [Google Scholar]
- 7. Bybee KA, Prasad A. Stress‐related cardiomyopathy syndrome. Circulation. 2008;118:397–409. [DOI] [PubMed] [Google Scholar]
- 8. Kume T, Akasaka T, Kawamoto T, et al. Assessment of coronary microcirculation in patients with takotsubo‐like left ventricular dysfunction. Circ J. 2005;69:934–939. [DOI] [PubMed] [Google Scholar]
- 9. Bybee KA, Prasad A, Barsness GW, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94:343–346. [DOI] [PubMed] [Google Scholar]
- 10. Elesber A, Lerman A, Bybee KA, et al. Myocardial perfusion in apical ballooning syndrome: correlate of myocardial injury. Am Heart J. 2006;152:469e9–e13. [DOI] [PubMed] [Google Scholar]
- 11. Abdelmoneim SS, Mankad SV, Bernier M, et al. Microvascular function in Takotsubo cardiomyopathy with contrast echocardiography: prospective evaluation and review of literature. J Am Soc Echocardiogr. 2009;22:1249–1255. [DOI] [PubMed] [Google Scholar]
- 12. Kim HS, Tremmel JA, Nam CW, et al. Quantitative comparison of microcirculatory dysfunction in patients with stress cardiomyopathy and ST‐segment elevation myocardial infarction. J Am Coll Cardiol. 2011;58:2430–2431. [DOI] [PubMed] [Google Scholar]
- 13. Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. [DOI] [PubMed] [Google Scholar]
- 14. Dote K, Sato H, Tateishi H, et al. Myocardial stunning due to simultaneous multi‐vessel spasms: a review of five cases. J Cardiol. 1991;21:203–214. [PubMed] [Google Scholar]
- 15. Song BG, Chun WJ, Park YH, et al. The clinical characteristics, laboratory parameters, electrocardiographic, and echocardiographic findings of reverse or inverted takotsubo cardiomyopathy: comparison with mid or apical variant. Clin Cardiol. 2011;34:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paixao AR, Balaji N, Ryzhikov A, et al. Left ventricular contraction patterns in patients with suspected acute coronary syndrome and normal coronary angiograms: a new look at the takotsubo syndrome. Clin Cardiol. 2011;34:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kurisu S, Sato H, Kawagoe T, et al. Takotsubo like left ventricular dysfunction with ST‐segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J. 2002;143:448–455. [DOI] [PubMed] [Google Scholar]
- 18. Ibanez B, Choi BG, Navarro F, et al. Takotsubo syndrome: a form of spontaneous aborted myocardial infarction? Eur Heart J. 2006;27:1509–1510. [DOI] [PubMed] [Google Scholar]
- 19. Ibanez B, Navarro F, Cordoba M, et al. Takotsubo transient left ventricular apical ballooning: is intravascular ultrasound the key to resolve the enigma? Heart. 2005;91:102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palla A, Dande A, Petrinin J, et al. Pretreatment with low‐dose B‐Adrenergic antagonist therapy does not affect the severity of Takotsubo Cardiomyopathy. Clin Cardiol. 2012;35:478–481. [DOI] [PMC free article] [PubMed] [Google Scholar]


