ABSTRACT
Background
The exercise electrocardiogram (ECG) is a standard examination in patients with suspected coronary artery disease. However, despite a pathologic result, many patients undergoing diagnostic coronary angiography do not have any significant epicardial stenosis. In this study, we assessed the relation between a pathologic exercise ECG and coronary microvascular dysfunction in response to intracoronary acetylcholine (ACh) provocation in patients without any relevant epicardial stenosis.
Hypothesis
Coronary microvascular dysfunction is significantly more often in patients with angina, unobstructed coronary arteries and a pathologic exercise stress test compared to those without pathologic stress test.
Methods
This study recruited 137 consecutive patients with exertional angina pectoris who underwent diagnostic coronary angiography between September 2008 and April 2011 (68% women; mean age, 63 ± 10 years). In none of the patients was there a stenosis of >50%. All patients underwent an exercise ECG before angiography and intracoronary ACh provocation testing for assessment of coronary vasomotor responses directly after angiography.
Results
The exercise ECG showed an abnormal result in 69 patients (50%; ST‐segment depression ≥0.1 mV and/or reproduction of the patient's usual symptoms). The ACh test revealed a coronary vasomotor abnormality (reproduction of the patient's symptoms, ischemic ECG shifts ± diffuse distal vasoconstriction) in 87 patients (64%). Such a result was significantly more often found in patients with a pathologic exercise ECG (50/69 [72%] vs 19/69 [28%], P = 0.034). There were no other statistically significant differences between patients with and those without pathologic exercise ECG.
Conclusions
Coronary microvascular dysfunction is frequently found in patients with exertional angina pectoris and unobstructed coronary arteries. Such a finding is found significantly more often in presence of a pathologic exercise ECG.
Introduction
Patients with angina pectoris (AP) and unobstructed coronary arteries remain a diagnostic challenge in everyday clinical cardiology. Recently, we were able to show that up to 62% of these patients suffer from a coronary vasomotor disorder that can be unmasked by intracoronary acetylcholine (ACh) provocation testing.1 Thus, coronary vasomotor disorders represent a frequent condition in daily clinical routine. Nevertheless, they are often not considered or diagnosed. Studies have proposed that noninvasive measurements of microvascular function (eg, Endo‐PAT) correlate with ACh‐induced vasomotor disorders in Japanese patients,2 but this has so far not been shown in Caucasian patients. Moreover, an association between inflammatory markers and coronary microvascular dysfunction has been reported in this setting.3 However, there is currently no reliable noninvasive test available for the diagnosis of coronary microvascular dysfunction. In this study, we speculated that a pathologic exercise stress test in patients with unobstructed coronary arteries may be an indicator of coronary microvascular dysfunction rather than a false‐positive test. Consequently, we assessed the relation between a pathologic exercise stress test (exercise tolerance test [ETT]) ECG and coronary microvascular dysfunction in response to intracoronary ACh provocation testing in patients with AP despite unobstructed coronary arteries.
Methods
Patients
From September 2008 to June 2011, a total of 137 consecutive patients (44 men; mean age, 63 ± 11 years) who underwent diagnostic coronary angiography and were found to have unobstructed coronary arteries (no epicardial stenosis ≥50%) were included in the study. They had to fulfill the following inclusion criteria: exertional AP and ETT before coronary angiography (bicycle stress test). Intracoronary ACh provocation testing was performed directly after diagnostic coronary angiography. Subjects were excluded and the provocation test was not performed if patients had severe chronic obstructive pulmonary disease or impaired renal function (creatinine >2.0 mg/dL), or if spontaneous spasm was observed. The following information was recorded in every patient: cardiovascular risk factors including hypertension, diabetes, hypercholesterolemia, a history of smoking, and a positive family history for cardiovascular events (myocardial infarction or stroke in a parent or sibling); results of the ETT (a positive response was defined as transient ischemic ECG changes ≥0.1 mV in ≥2 contiguous leads, 80 ms after the J point, and/or reproduction of angina during the stress test).
Study Protocol
The study protocol complied with the Declaration of Helsinki and all patients gave written informed consent before angiography. All patients in the study underwent intracoronary provocation with ACh in accordance to a standardized protocol immediately after diagnostic angiography.1 Cardiovascular medications (β‐blockers, calcium channel blockers, and nitrates) were discontinued 48 hours before coronary angiography. Sublingual glyceryltrinitrate administration was permitted for the relief of chest pain at all times; however, none of the patients required this treatment <4 hours prior to angiography. Heart rate, blood pressure, and the 12‐lead ECG were continuously monitored during ACh testing. Ischemic ECG changes were defined as transient ST‐segment depression or elevation ≥0.1 mV in ≥2 contiguous leads.
Acetylcholine Testing
Incremental doses of 2 µg, 20 µg, 100 µg, and 200 µg of ACh were manually infused over a period of 3 minutes into the left coronary artery (LCA) via the angiographic catheter. In patients who remained asymptomatic and showed no diagnostic ST‐segment changes during LCA ACh infusion, 80 µg of ACh was injected into the right coronary artery (RCA).1
Transient atrioventricular block was frequently observed, mostly during provocation of the RCA. It almost always resolved within seconds after reducing the speed of the manual injection. Therefore, we did not test the RCA with a pacing catheter in the right ventricle avoiding potential complications. A bolus of glyceryltrinitrate 0.2 mg (Perlinganit; Schwarz Pharma, Monheim, Germany) was injected into the LCA or RCA to relieve angina and/or severe epicardial constriction. Nitrates were also infused routinely at the end of the ACh test into the RCA and LCA.
Acetylcholine Test Assessment
Angiographic responses during the ACh test were analyzed using computerized quantitative coronary angiography (QCA‐CMS, version 6.0; Medis, Leiden, the Netherlands). The ACh test was considered positive for coronary microvascular dysfunction when typical ischemic ST‐segment changes and AP developed in the absence of epicardial coronary constriction ≥75%‐diameter reduction.1 Additionally, epicardial vasoconstriction was measured, and ≥75%‐diameter reduction compared with the relaxed state following intracoronary nitroglycerine was recorded as significant. Patients who experienced no AP, constriction, or ST‐segment shifts were considered to have a negative ACh test response (normal coronary vasoreactivity).
Statistical Analysis
Data analysis was carried out using SPSS version 17.0 (SPSS Inc., Chicago, IL). Results are expressed as mean ± SD. The Student t test was used to compare continuous variables. For values without normal distribution, median and interquartile ranges are stated and the Mann‐Whitney U test was used for analysis. The Fisher exact test was used for categorical variables. Multiple logistic regression analysis was performed using forward variable selection based on likelihood ratios to identify predictors for a pathologic exercise stress test result. A 2‐tailed P value <0.05 was considered significant.
Results
Overall Results
The summary of the results of all patients is shown in Table 1. The ACh test was completed in all patients without any complications. The ACh test revealed coronary microvascular dysfunction in 87 patients (64%), of whom 33 showed distal and diffuse epicardial vasoconstriction at higher doses of ACh (Figure 1). In the remaining 50 patients, the ACh test was uneventful. The Fisher exact test revealed that patients with a pathologic ETT significantly more often had a pathologic ACh test result (69/50 compared with 68/37, P = 0.034; Figure 2A). Statistical comparison of patients with and those without a pathologic ETT revealed no significant differences regarding age, sex, left ventricular ejection fraction, and cardiovascular risk factors. However, when comparing patients with a pathologic ACh test with those without a pathologic ACh test, there were significantly more women with a positive family history for cardiovascular disease among the patients with a pathologic ACh test (P < 0.0001 and P = 0.035, respectively; Table 1 and Table 2).
Table 1.
Patient Characteristics
| All Patients, N = 137 | Uneventful ETT, n = 68 (50) | Pathologic ETT, n = 69 (50) | P Value | |
|---|---|---|---|---|
| Male sex | 44 (32) | 27 (40) | 17 (25) | 0.069 |
| Age, y | 63 ± 11 | 63 ± 11 | 63 ± 11 | 0.870 |
| LVEF, % | 73 ± 9 | 73 ± 8 | 73 ± 10 | 0.584 |
| ACh provocation testing abnormal | 87 (64) | 37 (54) | 50 (72) | 0.034 |
| Hypertension | 105 (77) | 53 (78) | 52 (75) | 0.840 |
| T2DM | 27 (20) | 17 (25) | 10 (14) | 0.138 |
| Hypercholesterolemia | 73 (53) | 36 (53) | 37 (54) | 0.999 |
| Current smoking | 38 (28) | 18 (26) | 20 (29) | 0.849 |
| Positive family history of CVD | 74 (54) | 33 (49) | 41 (59) | 0.232 |
Abbreviations: ACh, acetylcholine; CVD, cardiovascular disease; ETT, exercise tolerance (stress) test; LVEF, left ventricular ejection fraction; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Data are presented as mean ± SD or n (%).
Figure 1.
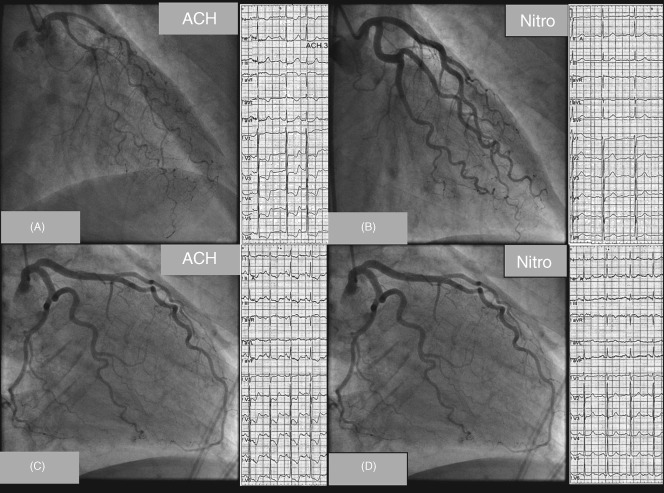
The upper panel shows LCA angiograms and ECGs of a patient with epicardial vasoconstriction following signs of coronary microvascular dysfunction. Note the diffuse but distally accentuated narrowing of the LAD during i.c. ACh infusion together with ischemic ECG shifts (A) and (B) resolution of both findings after nitroglycerine i.c. The lower panel shows an example of a patient with coronary microvascular dysfunction. (C) During ACh, the patient had reproduction of angina and ischemic ECG changes but no epicardial vasoconstriction. (D) After nitroglycerine i.c., chest pain and ECG changes resolved. Abbreviations: ACh, acetylcholine; ECG, electrocardiogram; i.c., intracoronary; LAD, left anterior descending artery; LCA, left coronary artery. With permission from Ong et al. (Ong P, Athanasiadis A, Mahrholdt H, Borgulya G, Sechtem U, Kaski JC (2012) Increased coronary vasoconstrictor response to acetylcholine in women with chest pain and normal coronary arteriograms (cardiac syndrome X). Clin Res Cardiol 101:673–681.)
Figure 2.
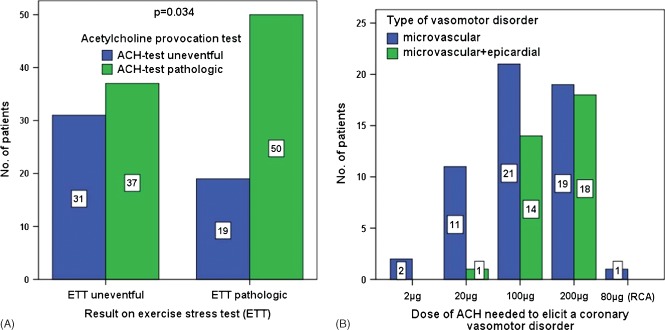
(A) Distribution of patients with pathologic vs uneventful ETT compared with pathologic vs uneventful ACh test, showing that patients with pathologic ETT significantly more often had a pathologic ACh test (P = 0.034). (B) Distribution of doses of ACh needed to elicit a coronary vasomotor disorder. Abbreviations: ACh, acetylcholine; ETT, exercise tolerance (stress) test.
Table 2.
Patient Characteristics According to ACh Test Result
| Uneventful ACh Test, n = 50 (36) | Pathologic ACh Test, n = 87 (64) | P Value | |
|---|---|---|---|
| Male sex | 28 (56) | 16 (18) | <0.0001 |
| Age, y | 63 ± 11 | 63 ± 11 | 0.870 |
| LVEF, % | 73 ± 8 | 73 ± 10 | 0.584 |
| ETT abnormal | 19 (38) | 50 (57) | 0.034 |
| Hypertension | 38 (76) | 67 (77) | 0.999 |
| T2DM | 12 (24) | 15 (17) | 0.376 |
| Hypercholesterolemia | 25 (50) | 48 (55) | 0.597 |
| Current smoking | 16 (32) | 22 (25) | 0.432 |
| Positive family history of CVD | 21 (42) | 53 (61) | 0.035 |
Abbreviations: ACh, acetylcholine; CVD, cardiovascular disease; ETT, exercise tolerance (stress) test; LVEF, left ventricular ejection fraction; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Data are presented as mean ± SD or n (%).
Multivariate logistic regression analysis incorporating all parameters from Table 1 revealed that the only significant predictor for a pathologic ETT was a pathologic ACh test (Table 3).
Table 3.
Multivariable Analysisa
| Pathologic ETT vs Uneventful ETT | OR, 95% CI, P Value |
|---|---|
| Coronary microvascular dysfunction on intracoronary ACh testing | 1.546, 1.076‐1.777, 0.029 |
Abbreviations: ACh, acetylcholine; CI, confidence interval; CVD, cardiovascular disease; DM, diabetes mellitus; ETT, exercise tolerance (stress) test; LVEF, left ventricular ejection fraction; OR, odds ratio.
The following univariate parameters were considered for the multivariable model: hypertension, DM, cigarette smoking, hypercholesterolemia, positive family history of CVD, sex, LVEF, age, ACh test result.
The doses of ACh needed to elicit coronary microvascular dysfunction are shown in Figure 2B. Patients without distal and diffuse epicardial vasoconstriction mainly reacted at doses between 2 µg and 100 µg, whereas those with concomitant epicardial constriction reacted mostly at 100 µg and 200 µg of ACh.
Discussion
The results of our study show that a pathologic ETT in patients with unobstructed coronary arteries is significantly more often associated with ACh‐induced coronary microvascular dysfunction compared to patients with an uneventful ETT. Thus, there is a substantial proportion of patients in whom the ETT may not be false‐positive, but rather is an indicator of a dysfunctional microcirculation. In this setting, the ACh test may allow diagnosis of microvascular dysfunction, similarly to invasive measurements of coronary flow reserve as previously shown by other investigators.4 Finally, coronary microvascular dysfunction may also be suspected in patients with a pathologic ETT and unobstructed coronary arteries in whom the ACh test may not be feasible, justifying a trial of treatment.
Traditionally, the clinical presentation with exertional AP, a pathologic ETT, and unobstructed coronary arteries has been called cardiac syndrome X, or microvascular angina.5, 6 Numerous studies have identified the coronary microcirculation as the main target in this condition, and it has been proposed that microvascular dysfunction can be due to chronic low‐grade inflammation caused by various stimuli.7, 8 Mechanistically, there are structural and functional alterations present in the coronary tree and the myocardium in this condition.9 From a functional point of view, impaired vasodilatation and/or enhanced vasoconstriction have been shown to play a pathogenetic role.4, 10 Furthermore, it has been proposed that coronary microvascular dysfunction can be patchily distributed along the microcirculation.11 Therefore, the amount and the distribution of microvascular dysfunction may be decisive for an ETT to become pathologic. However, the exact mechanisms underlying this heterogenic condition are still incompletely understood.
Various techniques have been applied to assess the coronary microcirculation.12, 13 Panting et al showed, for example, that patients with microvascular angina had reversible perfusion defects on cardiac stress magnetic resonance imaging (MRI) with adenosine.14 In addition, Yilmaz et al showed that perfusion defects on cardiac stress MRI are often present in patients with coronary vasomotor disorders assessed by ACh provocation testing.15 Another noninvasive approach is assessment of coronary flow reserve via transthoracic Doppler echocardiography (TTDE). Galiuto et al16 have shown that patients with microvascular angina frequently have impaired coronary flow reserved on TTDE. However, these noninvasive tests have limited diagnostic accuracy for identifying coronary microvascular dysfunction,17 underpinning the need for novel more sensitive noninvasive imaging techniques aimed at visualization of the coronary microcirculation in vivo.
Clinical Implications
Patients with a pathologic exercise stress test and unobstructed coronary arteries should further be investigated for coronary microvascular dysfunction using, for example, the ACh test. Identification of patients with a coronary microvascular disorder is important, as targeted treatment with angiotensin‐converting enzyme inhibitors, statins, and calcium antagonists may be able to improve symptoms18, 19, 20 and alter prognosis.21 Recently, it has been shown that even patients with unobstructed coronary arteries can have an elevated risk of coronary events22 and disability pension.23 Moreover, it has been shown that patients with microvascular dysfunction have more vulnerable plaque characteristic than those without.24 In addition, the patients can be reassured that a cause for their symptoms has been found and further unnecessary investigations can be avoided. Finally, in patients with a pathologic ETT and unobstructed coronary arteries in whom an ACh test is not possible, a trial of treatment for coronary microvascular dysfunction may be justified.
Study Limitations
Because the coronary microcirculation cannot be visualized in vivo at present, coronary microvascular dysfunction remains a diagnosis of exclusion. In this study, we did not use coronary flow reserve measurements to document microvascular dysfunction, as previously done by other investigators.4 This represents a limitation of this study, but this aspect is part of our ongoing work in this area. Instead, we used intracoronary ACh provocation testing with a definition for microvascular dysfunction previously proposed by our group.1
Conclusion
Acetylcholine‐induced coronary microvascular dysfunction is frequently found in patients with exertional AP and unobstructed coronary arteries. Such a finding is significantly more often found in presence of a pathologic exercise ECG.
Acknowledgments
The authors are grateful to nurses and technicians in the catheterization laboratories and to all staff members of the Department of Cardiology, Robert Bosch Hospital, Stuttgart, Germany, for their help and support during the study.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Ong P, Athanasiadis A, Borgulya G, et al. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries: the ACOVA Study (Abnormal Coronary Vasomotion in patients with stable angina and unobstructed coronary arteries). J Am Coll Cardiol. 2012;59:655–662. [DOI] [PubMed] [Google Scholar]
- 2. Matsuzawa Y, Sugiyama S, Sugamura K, et al. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010;55:1688–1696. [DOI] [PubMed] [Google Scholar]
- 3. Ong P, Sivanathan R, Borgulya G, et al. Obesity, inflammation and brachial artery flow‐mediated dilatation: therapeutic targets in patients with microvascular angina (cardiac syndrome X). Cardiovasc Drugs Ther. 2012;26:239–244. [DOI] [PubMed] [Google Scholar]
- 4. Reis SE, Holubkov R, Conrad Smith AJ, et al; WISE Investigators. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–741. [DOI] [PubMed] [Google Scholar]
- 5. Lanza GA. Cardiac syndrome X: a critical overview and future perspectives. Heart. 2007;93:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaski JC. Pathophysiology and management of patients with chest pain and normal coronary arteriograms (cardiac syndrome X). Circulation. 2004;109:568–572. [DOI] [PubMed] [Google Scholar]
- 7. Granger DN, Rodrigues SF, Yildirim A, et al. Microvascular responses to cardiovascular risk factors. Microcirculation. 2010;17:192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Recio‐Mayoral A, Mason JC, Kaski JC, et al. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. 2009;30:1837–1843. [DOI] [PubMed] [Google Scholar]
- 9. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 10. Ong P, Athanasiadis A, Mahrholdt H, et al. Increased coronary vasoconstrictor response to acetylcholine in women with chest pain and normal coronary arteriograms (cardiac syndrome X). Clin Res Cardiol. 2012;101:673–681. [DOI] [PubMed] [Google Scholar]
- 11. Maseri A, Crea F, Kaski JC, et al. Mechanisms of angina pectoris in syndrome X. J Am Coll Cardiol. 1991;17:499–506. [DOI] [PubMed] [Google Scholar]
- 12. Pries AR, Habazettl H, Ambrosio G, et al. A review of methods for assessment of coronary microvascular disease in both clinical and experimental settings. Cardiovasc Res. 2008;80:165–174. [DOI] [PubMed] [Google Scholar]
- 13. Lanza GA, Camici PG, Galiuto L, et al; Gruppo di Studio di Fisiopatologia Coronarica e Microcircolazione, Società Italiana di Cardiologia. Methods to investigate coronary microvascular function in clinical practice. J Cardiovasc Med. 2013;14:1–18. [DOI] [PubMed] [Google Scholar]
- 14. Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–1953. [DOI] [PubMed] [Google Scholar]
- 15. Yilmaz A, Athanasiadis A, Mahrholdt H, et al. Diagnostic value of perfusion cardiovascular magnetic resonance in patients with angina pectoris but normal coronary angiograms assessed by intracoronary acetylcholine testing. Heart. 2010;96:372–379. [DOI] [PubMed] [Google Scholar]
- 16. Galiuto L, Sestito A, Barchetta S, et al. Noninvasive evaluation of flow reserve in the left anterior descending coronary artery in patients with cardiac syndrome X. Am J Cardiol. 2007;99:1378–1383. [DOI] [PubMed] [Google Scholar]
- 17. Cassar A, Chareonthaitawee P, Rihal CS, et al. Lack of correlation between noninvasive stress tests and invasive coronary vasomotor dysfunction in patients with nonobstructive coronary artery disease. Circ Cardiovasc Interv. 2009;2:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pauly DF, Johnson BD, Anderson RD, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin‐converting enzyme inhibition is associated with improved microvascular function: a double‐blind randomized study from the National Heart, Lung and Blood Institute Women's Ischemia Syndrome Evaluation (WISE). Am Heart J. 2011;162:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kayikcioglu M, Payzin S, Yavuzgil O, et al. Benefits of statin treatment in cardiac syndrome X. Eur Heart J. 2003;24:1999–2005. [DOI] [PubMed] [Google Scholar]
- 20. Ozçelik F, Altun A, Ozbay G. Antianginal and anti‐ischemic effects of nisoldipine and ramipril in patients with syndrome X. Clin Cardiol. 1999;22:361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson BD, Shaw LJ, Pepine CJ, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH‐NHLBI‐sponsored Women's Ischemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27:1408–1415. [DOI] [PubMed] [Google Scholar]
- 22. Jespersen L, Hvelplund A, Abildstrøm SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 23. Jespersen L, Abildstrøm SZ, Hvelplund A, et al. Symptoms of angina pectoris increase the probability of disability pension and premature exit from the workforce even in the absence of obstructive coronary artery disease. Eur Heart J. 2013;34:3294–3303. [DOI] [PubMed] [Google Scholar]
- 24. Dhawan SS, Corban MT, Nanjundappa RA, et al. Coronary microvascular dysfunction is associated with higher frequency of thin‐cap fibroatheroma. Atherosclerosis. 2012;223:384–388. [DOI] [PubMed] [Google Scholar]


