Abstract
Background
Heart rate (HR) reduction in patients with systolic heart failure (HF) is a cornerstone of current therapy. The aim of this study was to evaluate the short‐term effect of the HR reduction with ivabradine on N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) in outpatients with systolic HF.
Hypothesis
Ivabradine improves survival and promotes left ventricle remodelling by reducing resting heart rate. Nt‐ProBNP absolute and trends predict prognosis. We hypothesized a possible association between heart rate decrease and Nt‐ProBNP values.
Methods
We included 25 outpatients with systolic HF on optimized medical therapy (80% on angiotensin‐converting enzyme inhibitors, 56% on spironolactone, and 88% on β‐blocker therapy), left ventricle ejection fraction <40%, and sinus rhythm and HR >70/bpm. After a 1 month running‐out period, to establish the clinical and NT‐proBNP stability, patients were started on ivabradine for 3 months.
Results
Ivabradine decreased NT‐proBNP (P = 0.002) from a median of 2850 pg/mL to 1802 pg/mL, corresponding to a median absolute and percent decrease of 964 pg/mL and 44.5%, respectively. The baseline HR correlated significantly with the baseline NT‐proBNP (rs = 0.411, P = 0.041). The absolute and percent HR decrease correlated with the absolute NT‐proBNP decrease (rs = 0.442, P = 0.027; rs = 0.395, P = 0.05). The greater the NT‐proBNP absolute decrease tertile, the greater the baseline HR (P = 0.023) and the absolute (P = 0.028) and percent (P = 0.064) HR variation.
Conclusions
In outpatients with systolic HF, the NT‐proBNP reduction obtained by short‐term ivabradine treatment correlates closely with the degree of HR reduction.
Introduction
Heart rate (HR) is a major determinant of myocardial oxygen demand, coronary blood flow, and myocardial performance affecting the prognosis of the general population1, 2 and of specific subgroups of patients, such as those with cardiovascular diseases,3 heart failure (HF),4 and multiorgan damage syndromes.5
The optimized medical therapy for systolic HF includes the introduction and uptitration of several drugs and cardiac resynchronization therapy for those with persistent symptoms.6 The left ventricle remodeling induced by angiotensin‐converting enzyme inhibitors, angiotensin receptors II blockers, and spironolactone is often accompanied by a decrease of natriuretic peptides levels,7, 8, 9, 10 whereas the β‐blockers have a variable effect.8, 11 The N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) absolute values and trends predict chronic HF prognosis.12
Patients with left ventricle systolic dysfunction have decreased stroke volume and may need to increase HR to increase cardiac output. The aggressive HR control could attenuate that response and may have negative inotropic properties. Ivabradine is a selective and specific inhibitor of the sinus node If current, resulting in a pure HR lowering effect. HR reduction with ivabradine improves left ventricle filling by the prolongation of the diastolic time and increases stroke volume. In patients with systolic HF and resting HR >70/min, it improves event‐free survival,13 quality of life,14 exercise capacity,15 and promotes left ventricle reverse remodeling at 8 months.16 However, it remains unclear what ivabradine's effect is on natriuretic peptides.17, 18
The objective of our study was to evaluate in stable outpatients with systolic HF the short‐term (3 months) effect of ivabradine on NT‐proBNP.
Methods
Population
Twenty‐five ambulatory, clinically stable outpatients with systolic chronic HF, followed in a specialized HF clinic in a tertiary teaching hospital, on optimized standard medical therapy were consecutively incorporated in the study between October 2010 and December 2010.
Eligibility Criteria
Patients with chronic HF, on optimized medical therapy according to European Society of Cardiology guidelines, left ventricle ejection fraction <40%, New York Heart Association (NYHA) class II‐III, and sinus rhythm and resting HR >70/min were eligible for inclusion in the study.
Exclusion Criteria
Patients were excluded who had acute decompensation (acute coronary syndromes and acute HF) and hemodynamically significant valve disease.
Study Design and Procedures
This was a 4‐month, open‐label, interventional, prospective study. The first month was a running‐out period to establish the patients' clinical and neurohormonal stability. During the next 3 months the patients were on ivabradine therapy.
At a scheduled clinic visit at the HF clinic, patients were screened for the eligibility and exclusion criteria. The NT‐proBNP (Elecsys 2010; Roche Diagnostics, Indianapolis, IN) was also determined. After a 1‐month running‐out period, without any modification of medical therapy, all screened patients were reevaluated to establish clinical and neurohormonal stability. Clinical stability was defined as: no weight change (<2 kg), same NYHA class, and no change of the diuretics dosage. Neurohormonal stability was defined as no significant change (<30%) of the NT‐proBNP. A sample of blood for standard laboratory testing and NT‐proBNP was obtained. Rhythm and resting HR was determined with an electrocardiogram. The ivabradine starting dose was 5 mg 3 times a day and the titration (up or lowering dose) was similar to the SHIFT (Systolic Heart failure treatment with the I f inhibitor ivabradine Trial) trial.13 Briefly, the starting dose of ivabradine was 5 mg twice daily. After 14 days, the dose was adjusted 7.5, 5, or 2.5 mg twice daily according to the resting HR and tolerability. At least monthly visits to the clinic were scheduled. At 3 months, a full clinical evaluation, including NYHA class assessment and laboratory workup including NT‐proBNP determination was performed. The clinical status variation was subjectively classified as worsen, unchanged, and improved by a patient‐reported self‐assessment. The protocol was approved by the institutional review board, and written consent was obtained from all patients.
Objective
The primary objective of our study was to evaluate the short‐term (3 months) effect of ivabradine on NT‐proBNP values in systolic HF outpatients. The secondary objective was to determine the relationship between baseline HR and its variation with ivabradine therapy and the NT‐proBNP and clinical status variation.
Statistics
Results are expressed as mean ± standard deviation, median (interquartile range [IQR]), and frequency (or rate). Parameter variations in relation to the baseline were evaluated with the paired Student t test and the Wilcoxon rank signed test (for NT‐proBNP). The relationship between parameters was determined with the Pearson correlation coefficient (or the Spearman correlation coefficient for nonparametric parameters). The variation in relation to the baseline value (for the HR and NT‐proBNP) was determined as a absolute (initial final) and relative (percent) variation. Resting HR (76 and 80 bpm), HR variation (4.7 and 14.6 bpm), and the NT‐proBNP variation (447 and 1876.3 pg/mL) were stratified according to the tertiles. The between groups comparison (NT‐proBNP tertiles in relation to the baseline HR and HR variation) was determined with the Kruskal‐Wallis 1‐way analysis of variance. All tests were 2‐tailed and performed using the Statistical Package for Social Sciences (SPSS 17) software (SPSS Inc., Chicago, IL).
Results
Baseline characteristics of the cohort are reported in Table 1. At baseline, resting HR was 79.2 ± 7.1 bpm, with 88% of the patients on β‐blockers and 24% on digoxin. The median ivabradine starting dose was 10 mg/day (8.7 ± 2.2 mg/d).
Table 1.
Clinical Characteristics, Concomitant Diseases, and Treatment at Baseline
| Demographics | |
|---|---|
| No. of Patients | 25 |
| Age, y | 63.8 ± 6.9 |
| Male/Female, % | 68/32 |
| Weight, kg | 75.9 ± 16.8 |
| NYHA class, II/III, % | 44/56 |
| LV ejection Fraction, % | 30.0 ± 8.0 |
| Concomitant diseases | |
| Diabetes mellitus, % | 40 |
| Hypertension, % | 80 |
| Dislipidemia, % | 28 |
| Ischemic heart disease, % | 44 |
| Etiology | |
| Idiopathic, % | 24 |
| Ischemic, % | 32 |
| Hypertensive, % | 20 |
| Postmyocarditis, % | 4 |
| Other, % | 20 |
| Treatment at inclusion | |
| Furosemide, %, mg/d | 92, 80 |
| Metolazone, % | 16 |
| Spironolactone, % | 56 |
| ACE inhibitor, % | 80 |
| Angiotensin receptor blocker, % | 24 |
| β‐blocker, % | 88 |
| 50% β‐blocker target dose, % | 45 |
| Digoxin, % | 24 |
| Statins, % | 56 |
| CRT, % | 28 |
Abbreviations: ACE, angiotensin‐converting enzyme; CRT, cardiac resynchronization therapy; LV, left ventricular, NYHA, New York Heart Association.
NT‐proBNP, HR, and Clinical Status Variation
As expected, there was no significant variation of the study parameters between the screening visit and baseline (Table 2). During the running‐out period, the median NT‐proBNP increase rate was 20.1%.
Table 2.
Cardiac Parameters and NT‐ProBNP Variation During the Study
| Screening | Baseline | 3 Months | P a | P b | |
|---|---|---|---|---|---|
| SBP, mm Hg | 117.8 ± 13.2 | 114.2 ± 26.7 | 125.0 ± 16.0 | 0.550 | 0.087 |
| DBP, mm Hg | 70.6 ± 11.8 | 70.8± 13.0 | 67.5 ± 13.9 | 0.728 | 0.199 |
| Resting HR, bpm | 77.9 ± 6.3 | 79.2 ± 7.1 | 69.2 ± 10.4 | 0.018 | <0.001 |
| NT‐proBNP, pg/mL | 1957.5 (915.5–4698.7) | 2850 (12.05–5831) | 1802 (632.5–3465.5) | 0.102 | 0.002 |
| Creatinine, mg/dl | 1.34 ± 0.49 | 1.32 ± 0.43 | 1.37 ± 0.49 | 0.285 | 0.428 |
| NYHA | 2.5 ± 0.5 | 2.5 ± 0.5 | 2.1 ± 0.3 | NS | <0.001 |
Abbreviations: DBP, diastolic blood pressure; HR, heart rate; NS nonsignificant; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide;
SBP, systolic blood pressure.
Date are presented as mean ± standard deviation, and for NT‐ProBNP as median (interquartile range).
P screening vs baseline.
P baseline vs 3 months.
At 3 months, in relation to the baseline, there was a significant decrease (P = 0.002) of NT‐proBNP, with a median absolute and percent decrease of 964 pg/mL and 44.5%, respectively. Resting HR decrease (P < 0.001) was 10.0 ± 10.4 bpm, corresponding to a 12.4 ± 12.5% decrease rate. NYHA class (P < 0.001) decreased significantly, 40% improved the NYHA and 56% the patient‐reported self‐assessment, whereas 40% experienced no change of the patient‐reported self‐assessment. During the 3 months there were no deaths or hospitalization. There was no significant change in blood pressure and renal function. One patient decreased the furosemide dosage and 2 stopped metolazone.
Not taking β‐blockers therapy was mainly associated with previous pulmonary disease (asthma or chronic obstructive pulmonary disease). Of the patients on β‐blockers (88%), only 1 achieved the target dose, and 45% achieved 50% of the target dose. The subgroup of patients achieving 50% of the β‐blockers target dose had no significant difference of the baseline HR, final HR, and baseline NT‐proBNP in relation to those on <50% target dose. Moreover, the percent variation of the HR (median, 16.4; IQR, 5.3–22.8% vs median, 11.9; IQR, 8.4–21.1; P = 0.746) and NT‐proBNP (median, 46.6; IQR, −17.6–76.4 vs median, 47.0; IQR, 23.1–54.3; P = 0.735) were similar.
HR Variation Relation With NT‐proBNP and Clinical Status
The baseline HR correlated significantly with baseline NT‐proBNP (rs = 0.411, P = 0.041). The absolute and percent HR decrease correlated with the absolute NT‐proBNP decrease (rs = 0.442, P = 0.027; rs = 0.395, P = 0.05).
The greater the NT‐proBNP absolute variation tertile (Figure 1), the greater the values of the baseline HR (P = 0.023) and of the absolute HR (P = 0.028) variation. The third tertile (NT‐proBNP absolute decrease >1876 pg/mL) had significantly greater (P < 0.05) values of the baseline HR and its absolute and percent decrease in relation to the other 2 tertiles. There were no changes in blood pressure.
Figure 1.
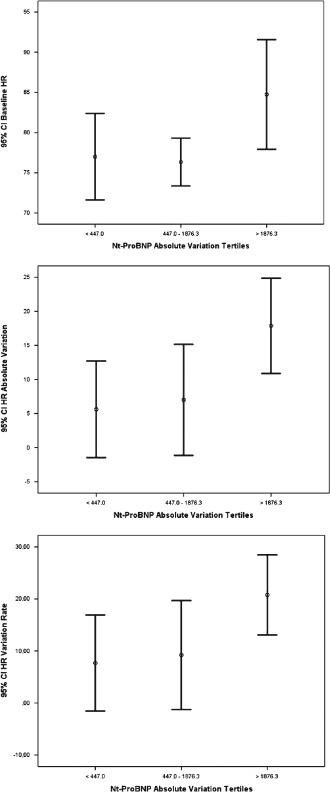
Shown are 95% confidence intervals (CI) for the baseline (P = 0.023), absolute (P = 0.028), and percent (P = 0.064) heart rate (HR) variation by the N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) absolute decrease tertiles.
The absolute and percent HR decrease correlated with the patient‐reported self‐improvement (rs = 0.451, P = 0.024; rs = 0.481, P = 0.015). The subgroup of patients who decreased the NYHA class had lower values of the final HR (75.6 ± 9.7 vs 64.0 ± 8.0, P = 0.004), and greater absolute (median, 4; IQR, 1–9 vs median, 14; IQR, 6–20; P = 0.044) and percent (median, 5; IQR, 1.3–12% vs median, 17; IQR, 8.5–22.8; P = 0.029) HR decrease.
Discussion
The present study shows that in outpatients with systolic HF on optimized medical therapy and resting HR >70/min, the expected HR reduction with ivabradine addition decreases significantly the NT‐proBNP after 3 months. Moreover, there was a direct relationship of the NT‐proBNP decrease with the HR decrease.
Elevated HR is an established marker for cardiovascular morbidity and mortality.19, 20, 21 HR reduction with β‐blockers22, 23 or with ivabradine13 improves left ventricle performance and has a positive left ventricle remodeling effect, reducing the risk of hospitalization and improving survival.
In the HF rat model, 90 days of ivabradine therapy reduces the left ventricle collagen accumulation and the left ventricle end‐systolic volume (LVESV), which is preserved even after ivabradine discontinuation.24 Possibly, the long‐term HR reduction might modify intrinsic myocardial structure contributing to a positive left ventricle remodeling. Ivabradine, by reducing resting HR, improves event‐free survival in patients with HF with or without adequate β‐blockage,13 along with reverse cardiac remodeling,16 improved quality of life,14 and improved exercise tolerance.15 In the BEAUTIFUL (MorBidity‐mortality EvAlUaTion of the If inhibitor ivabradine in patients with coronary disease and left ventricULar dysfunction) Echo substudy,17 including a subgroup of patients with left ventricle systolic dysfunction, there was a reduction of the LVESV (and not the end‐diastolic volume) with no changes in the NT‐proBNP levels. This could be related to the prolonged diastolic filling without reduction of the diastolic volume, which would contribute to increase the wall stress with consequent production of NT‐proBNP. On the contrary, in the SHIFT echocardiography substudy16 including patients with left ventricle systolic dysfunction and HF, there was a significant decrease on both systolic and diastolic volumes with improvement of the ejection fraction. This positive remodeling could be associated with the observed reduction of the collagen matrix in the animal model. One would expect in this clinical context a decrease of the NT‐proBNP with ivabradine HR reduction therapy in patients with systolic HF, although ivabradine will not impact blood pressure or fluid overload significantly. NT‐proBNP results (in the Echo/BNP substudy of SHIFT, N = 611, unpublished data) did not reach statistical significance25; however, in the subgroups of patients with nonischemic HF, reduction of BNP levels reached statistical significance in favor of ivabradine, as well in patients not taking at least half of the target dose of β‐blockers.25
NT‐proBNP is the inactive split product of the B‐type natriuretic peptide. It is a marker for the presence of left ventricle systolic dysfunction and a prognostic marker for morbidity and mortality in HF.26 NT‐proBNP levels often decline after initiation and uptitration of HF therapy such as vasodilators and aldosterone blockers.8, 9, 10 Persistently elevated (or rising) levels of NT‐proBNP are predictive of poor outcome.12 Several factors may influence the effect of drugs on the NT‐proBNP values: the baseline value, severity of HF, and age.8
Our cohort of patients includes ambulatory patients with left ventricle systolic dysfunction and severe chronic HF as expressed by NYHA class (56% class III), the NT‐proBNP baseline values, and high dosage of diuretics. It includes a great proportion of patients on β‐blocker therapy (88%); however, only 45% achieved 50% of the target dose, which is a little lower than in the SHIFT trial.4 To decrease the NT‐proBNP biological variability, there was a running‐out period of 1 month to establish the neurohormonal variability during clinical stability. The clinical stability was defined as no weight change (<2 kg), same NYHA class, and no diuretics dosage change. Only patients with NT‐proBNP variation <30% were admitted to the study. There is no universal value for the intraindividual variation of NT‐proBNP; nevertheless, it should be approximately 30%, with a change >23% likely to indicate significant change beyond background variation.27, 28 The median increase rate of the NT‐proBNP during the first month on no new therapy (run in) was 20%, which is within the accepted range of the study. On the other hand, NT‐proBNP variation was in the opposite direction after ivabradine addition. After 3 months of therapy with ivabradine, there was a median 44.5% decrease rate of the NT‐proBNP along with a significant clinical improvement (40% improved the NYHA class and 56% the patient‐reported self‐assessment). Moreover, 1 patient decreases the furosemide dosage and 2 stopped metolazone. This opposite directional variation of the NT‐proBNP during the running out period and the succeeding 3 months (on ivabradine), along with a completely different variation rate (20% increase vs 44.5% decrease) almost rules out NT‐proBNP spontaneous variability. The HR absolute decrease was similar to the 1‐year HR decrease of the SHIFT trial.4 The impact on the NYHA class was far greater than the 28% reported in SHIFT4; however, the patient‐reported improvement was lower. These findings were influenced by the open‐label nature of the present study.
Baseline HR was significantly associated with the baseline NT‐proBNP values. Moreover, the greater the tertile of the NT‐proBNP decrease the greater the values of the baseline HR and its variation. The NT‐proBNP decrease during the study was associated with HR variation. The patients with a possible worst prognosis and higher values of the baseline HR and NT‐proBNP would benefit the most with ivabradine, as they would have greater HR and NT‐proBNP reductions. Moreover, the HR and NT‐proBNP variation rates were not dependent on the achieved β‐blocker dose, despite having similar baseline HR and NT‐proBNP values. The magnitude of the HR reduction by β‐blocker plus ivabradine primarily determines subsequent effect on outcomes.29 The main mechanism for the clinical benefit and NT‐proBNP decrease, in the present study, was also HR reduction by ivabradine.
Thus, clinically stable outpatients with systolic HF on optimized medical therapy still with sinus HR >70 bpm, independent of the achieved β‐blocker dose, may benefit with ivabradine therapy, as there is a significant decrease of NT‐proBNP with its addition. Despite clinical stability, sinus HR>70 bpm might be a significant sign that the antiadrenergic treatment is not entirely successful, and more treatment is necessary. Ivabradine could be the answer.
Study Limitations
Limitations of our study are many and include the relatively small yet well‐controlled high‐risk population performed at a HF clinic in a tertiary teaching hospital. It is a single‐center trial, which limits generalization but allows stronger trial control and adherence to study protocol. It is an open‐label interventional study without a placebo control, using the patients as their own controls. The only clinical outcome was the NYHA functional class, which presents a great limitation as it is an open‐label, nonrandomized, nonblinded study and is without a placebo group. Nevertheless, the NYHA class and the clinical status variation would facilitate a link between the study's main objective and the patients' clinical status. Iabradine is already a recognized therapy in systolic HF and is recommended by the HF guidelines.6 Therefore, it would not be ethnical as a placebo‐controlled trial.
Conclusion
Our observations suggest that in outpatients with systolic HF, on optimized medical therapy and persistent resting HR >70/min, the NT‐proBNP reduction obtained with the ivabradine short‐term treatment associates closely with the grade of resting HR reduction.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Levy R, White P, Stroud W, et al. Studies of blood pressure in Army officers: 4. Transient tachycardia‐prognostic significance alone and in association with transient hypertension. JAMA. 1945;129:585–588. [Google Scholar]
- 2. Wilhelmsen L, Berglund G, Elmfeldt D, et al. The multifactor primary prevention trial in Goteborg, Sweden. Eur Heart J. 1986;7:279–288. [DOI] [PubMed] [Google Scholar]
- 3. Cooney MT, Vartiainen E, Laakitainen T, et al. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010;159:612–619. [DOI] [PubMed] [Google Scholar]
- 4. Bohm M, Swedberg K, Komajda M, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo‐controlled trial. Lancet. 2010;376:886–894. [DOI] [PubMed] [Google Scholar]
- 5. Hoke RS, Muller‐Werdan U , Lautenschlager C, et al. Heart rate as an independent risk factor in patients with multiple organ dysfunction: a prospective, observational study. Clin Res Cardiol. 2012;101:139–147. [DOI] [PubMed] [Google Scholar]
- 6. Lindenfeld J, Albert NM, Boehmer JP, et al. Executive Summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:475–539. [DOI] [PubMed] [Google Scholar]
- 7. Troughton RW, Richards AM, Yandle TG, et al. The effects of medications on circulating levels of cardiac natriuretic peptides. Ann Med. 2007;39:242–260. [DOI] [PubMed] [Google Scholar]
- 8. Rosenberg J, Gustafsson F, Remme WJ, et al. Effect of beta‐blockade and ACE inhibition on B‐type natriuretic peptides in stable patients with systolic heart failure. Cardiovasc Drugs Ther. 2008;22:305–311. [DOI] [PubMed] [Google Scholar]
- 9. White M, Rouleau JL, Afzal R, et al. Effects of enalapril, candersartran or both on neurohormonal activation and LV volumes and function in patients with heart failure not treated with beta‐blocker. Ther Adv Cardiovasc Dis. 2009;3:113–121. [DOI] [PubMed] [Google Scholar]
- 10. Berry C, Murphy NF, De Vito G, et al. Effects of aldosterone receptor blockage in patients with mild‐moderate heart failure taking a beta‐blocker. Eur J Heart Fail. 2007;9:429–434. [DOI] [PubMed] [Google Scholar]
- 11. Hartmann F, Packer M, Coats AJ, et al. Prognostic impact of plasma N‐terminal pro‐brain natriuretic peptide in severe chronic congestive heart failure: a substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation. 2004;110:1780–1786. [DOI] [PubMed] [Google Scholar]
- 12. Masson S, Latini R, Anand IS, et al. Prognostic value of changes in N‐terminal pro‐brain natriuretic peptide in Vaal‐HeFT (Valsartran Heart Failure Trial). J Am Coll Cardiol. 2008;52:997–1003. [DOI] [PubMed] [Google Scholar]
- 13. Swedberg K, Komadja M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomized placebo‐controlled study. Lancet. 2010;376:875–885. [DOI] [PubMed] [Google Scholar]
- 14. Ekman I, Chassany O, Komajda M, et al. Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure: results from the SHIFT study. Eur Heart J. 2011;32:2395–2404. [DOI] [PubMed] [Google Scholar]
- 15. Volterrani M, Cice G, Caminiti G, et al. Effect of carvedilol, ivabradine or their combination on exercise capacity in patients with heart failure (The CARVIVA HF trial). Int J Cardiol. 2011;151:218–224. [DOI] [PubMed] [Google Scholar]
- 16. Tardif JC, O'Meara E, Komajda M, et al. Effects of selective heart rate reduction with ivabradine on left ventricular remodeling and function: results from the SHIFT echocardiography substudy. Eur Heart J. 2011;32:2507–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ceconi C, Freedman SB, tardif JC, et al. Effect of heart rate reduction by ivabradine on left ventricular remodeling in the echocardiographic substudy of BEAUTIFUL. Int J Cardiol. 2010;146:408–414. [DOI] [PubMed] [Google Scholar]
- 18. Sarullo FM, Fazio G, Puccio D, et al. Impact of “off label” use of ivabradine on exercise capacity, gas exchange, functional class, quality of life, and neurohormonal modulation in patients with ischemic chronic heart failure. J Cardiovasc and Therapeut. 2010;15:349–355. [DOI] [PubMed] [Google Scholar]
- 19. Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. [DOI] [PubMed] [Google Scholar]
- 20. Bristow MR. Adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. [DOI] [PubMed] [Google Scholar]
- 21. Castagno D, Skali H, Takeuchi M, et al. CHARM Investigators. Association of heart rate and outcomes in a broad spectrum of patients with chronic heart failure. J Am Coll Cardiol. 2012;59:1785–1795. [DOI] [PubMed] [Google Scholar]
- 22. McAlister FA, Wiebe N, Ezekowitz JA, et al. Meta‐analysis: beta‐blocker dose, heart rate reduction, and death in patients with heart failure. Ann Inter Med. 2009;150:784–794. [DOI] [PubMed] [Google Scholar]
- 23. Triposkiadis F, Karayannis G, Giamouzis G, et al. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–1762. [DOI] [PubMed] [Google Scholar]
- 24. Mulder P, Barbier S, Chagraoui A, et al. Long‐term heart rate reduction induced by the selective I(f) current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation. 2004;109:1674–1679. [DOI] [PubMed] [Google Scholar]
- 25.European Medicines Agency. Committee for Medicinal Products for Human Use. EMA/194513/2012. Assessment report of Pocoralan. Procedure no.: EMEA/H/C/000597/II/0018. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Assessment_Report_‐_Variation/human/000597/WC500124542.pdf. Accessed February 27, 2013.
- 26. Maisel A, Mueller C, Adams K Jr, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824–839. [DOI] [PubMed] [Google Scholar]
- 27.Schou M, Gustafsson F, Kjaer A, et al. Long‐term clinical variation of NT‐proBNP in stable chronic heart failure patients. Eur Heart J. 2007;28:177–182. [DOI] [PubMed] [Google Scholar]
- 28. Schou M, Gustafsson F, Nielsen PH, et al. Unexplained week‐to‐week variation in BNP and NTproBNP is low in chronic heart failure patients during steady state. Eur J Heart Fail. 2007;9:68–74. [DOI] [PubMed] [Google Scholar]
- 29. Swedberg K, Komajda M, Bohm M, et al. SHIFT Investigators. Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta‐blocker dose? Findings from the SHIFT (Systolic Heart failure treatment with the I f inhibitor ivabradine Trial) study. J Am Coll Cardiol. 2012;59:1938–1945. [DOI] [PubMed] [Google Scholar]


