ABSTRACT
Although physical fitness is a powerful prognostic marker in clinical medicine, most cardiovascular population‐based studies do not have a direct measurement of cardiorespiratory fitness. In line with the call from the National Heart Lung and Blood Institute for innovative, low‐cost, epidemiologic studies leveraging electronic medical record (EMR) data, we describe the rationale and design of the Henry Ford ExercIse Testing Project (The FIT Project). The FIT Project is unique in its combined use of directly measured clinical exercise data retrospective collection of medical history and medication treatment data at the time of the stress test, retrospective supplementation of supporting clinical data using the EMR and administrative databases and epidemiologic follow‐up for cardiovascular events and total mortality via linkage with claims files and the death registry. The FIT Project population consists of 69 885 consecutive physician‐referred patients (mean age, 54 ± 10 years; 54% males) who underwent Bruce protocol treadmill stress testing at Henry Ford Affiliated Hospitals between 1991 and 2009. Patients were followed for the primary outcomes of death, myocardial infarction, and need for coronary revascularization. The median estimated peak metabolic equivalent (MET) level was 10, with 17% of the patients having a severely reduced fitness level (METs < 6). At the end of the follow‐up duration, 15.9%, 5.6%, and 6.7% of the patients suffered all‐cause mortality, myocardial infarction, or revascularization procedures, respectively. The FIT Project is the largest study of physical fitness to date. With its use of modern electronic clinical epidemiologic techniques, it is poised to answer many clinically relevant questions related to exercise capacity and prognosis.
Background
Cardiorespiratory fitness is an important marker of cardiovascular heath in the general adult population.1, 2, 3, 4 Reduced fitness is a risk factor for all‐cause mortality and cardiovascular disease (CVD), independent of body fatness.5, 6 In contrast, an increase of 1 metabolic equivalent (MET) is associated with 13% decrease in cardiovascular mortality in a recent meta‐analysis.7
Multiple studies have demonstrated the important role of cardiorespiratory fitness in predicting outcomes.4 However, most of these studies are limited by intermediate follow‐up duration (usually <10 years), small to moderate sample size (usually 700–25,000 participants; just 3 studies have a sample size more than 10 000), and limited ethnic diversity. For example, the Cooper Center Longitudinal Study (CCLS) was a study of predominantly white participants with high socioeconomic status compared with the general population.8, 9, 10 In addition, many of the prior studies included subjects with lower risk‐factor burden compared to the general population. (The prevalence of diabetes mellitus [DM] in the CCLS and Aerobics Center Longitudinal Study11 were 4% and 5%, respectively.) As a result, the generalizability of fitness data to the patient population encountered in daily clinical practice is limited, and the differential prognostic value of fitness across different age groups, gender, and races is not well described.12, 13, 14, 15, 16, 17
Given its importance in predicting health outcomes, it is appropriate to determine the prognostic value of cardiorespiratory fitness in a larger study with longer follow‐up duration. However, with the current limitation in research funding, this would be a very costly project.18 Recently, the American Heart Association (AHA) called for a national registry to assess the population's cardiorespiratory fitness.19 In addition, the National Institutes of Health and National Heart, Lung, and Blood Institute advised investigators to utilize new innovative tools that could decrease the cost of new registries and randomized controlled trials.20 One potential way to accomplish this is to use the “big data” present in electronic medical records (EMRs) and administrative and insurance claims files to develop rich, real‐world, low‐cost, outcome‐driven registries.21, 22, 23, 24 Such registries can strengthen the available clinical evidence, while minimizing cost, and can be replicated across the world.
To this end, we herein describe the methods of the Henry Ford Exercise Testing Project (The FIT Project), a large, single‐center, retrospective study aiming to determine the long‐term association between cardiorespiratory fitness and clinical events in an ethnically diverse cohort.
Methods
Study Design
The FIT project is an investigator‐initiated retrospective cohort study leveraging modern data sources. The FIT Project is unique in its combined use of directly measured exercise data and estimates of physical fitness, retrospective collection of medical history and medication treatment data taken at the time of the stress test, retrospective supplementation of supporting clinical data using the EMR and administrative databases and epidemiologic follow‐up for total mortality and select nonfatal outcomes via linkage with the death registry and medical claims files, respectively.
The primary purpose of the FIT Project is to study the long‐term prognostic implications of cardiorespiratory fitness in an ethnically diverse cohort. However, beyond this primary goal, the FIT Project will also be used to answer a variety of questions unrelated to fitness where large sample size and long‐term follow‐up are required. The purpose of this article is to provide an overview on the design, procedures, and methods used in the FIT Project.
Study Setting and Population
The FIT Project population consists of 69 885 consecutive patients who underwent physician‐referred treadmill stress testing at Henry Ford Health System‐affiliated hospitals and ambulatory care centers in metropolitan Detroit, Michigan between 1991 and 2009. These medical centers are part of a large, vertically integrated organization that provides healthcare and offers a managed care insurance plan. Treadmill, medical history, and medication data were collected by exercise physiologists and nurses, and entered at the time of testing into a common clinical reporting tool used to generate clinical reports and to directly populate the system's EMR. Supporting clinical data and follow‐up for cardiovascular outcomes were derived from the EMR and administrative databases shared in common across system‐affiliated subsidiaries. The FIT Project was approved by the Henry Ford Health System institutional review board.
Treadmill Testing
All patients underwent routine, clinically referred, symptom‐limited maximal treadmill stress testing following the standard Bruce protocol.25 For individuals with repeat stress testing, only the results of the first test were included in the database. Patients <18 years old at the time of stress testing or patients undergoing pharmacological stress testing, modified Bruce, and other non‐Bruce protocol tests were not included in the database.
In accordance with AHA/American College of Cardiology (ACC) guidelines,26 tests could be terminated at the discretion of the supervising clinician for potentially life‐threatening reasons, which included significant arrhythmias, abnormal hemodynamic responses, diagnostic ST‐segment changes, exercise‐limiting symptoms such as chest pain or shortness of breath, or if the patient was unable to continue. Otherwise, patients were allowed to reach their peak attainable workload independent of heart rate achieved.
Resting heart rate and blood pressure were manually assessed immediately before to each test. As a general guide, target heart rate was calculated as 85% of the age‐predicted maximal heart rate determined by the formula 220 − age. Failure to achieve this heart rate was referred to as chronotropic incompetence. In addition to continuous heart rate monitoring, blood pressure was measured every 3 minutes during the test. The highest recorded heart rate and blood pressure were considered the peak heart rate and peak blood pressure. The treadmill speed was set initially at 2.7 km/h, then increased to 4.0, 5.4, 6.7, 8.0, 8.8 km/h on minutes 3, 6, 9, 12, and 15, respectively. In the first 3 minutes the grade was set at 10%, followed by a 2% increase every 3 minutes. The patient exercised for 3 minutes in each stage.25 If necessary to complete the test, patients were allowed to hold on to the support rail for balance. Exercise workload, expressed in estimated METs, was calculated by the Quinton treadmill controller based on achieved speed, and elevation METs results27 were categorized into 4 groups: <6, 6 to 10, 10 to 12, >12 METs.
Medical History and Medication Use
A medical history including age, gender, race, indication for stress test, risk factor burden, past medical history, and active medication use was obtained by a nurse and/or exercise physiologist immediately prior to the stress test. Race was defined exclusively by self‐report. Obesity was defined by self‐report and/or assessment by the clinician. Current smoking was defined as self‐reported active smoking at the time of the stress test. Family history of coronary artery disease was defined as compatible history in a first‐degree relative. Indication for stress testing was extracted from the stress test requisition form provided by the referring physician, and subsequently categorized into common indications.
Other risk factors were gathered by self‐report at the time of the test, then supplemented by a retrospective search of the EMR and administrative databases. A database‐verified diagnosis was considered present when the appropriate International Classification of Diseases, 9th Revision (ICD‐9) code was present on ≥3 separate encounters within the health system. DM was defined as a prior diagnosis of diabetes, use of hypoglycemic medications including insulin, or a database‐verified diagnosis of diabetes. Hypertension was defined as a prior diagnosis of hypertension, use of antihypertensive medications, or a database‐verified diagnosis of diabetes. The blood pressure at the time of the test was not used to diagnose hypertension. Dyslipidemia was defined by prior diagnosis of any major lipid abnormality, use of lipid‐lowering medications, or a database‐verified diagnosis of hypercholesterolemia or dyslipidemia. Known coronary artery disease was defined as prior myocardial infarction, coronary angioplasty, coronary artery bypass surgery, or prior documented obstructive coronary artery disease (CAD) on a prior angiogram. Prior congestive heart failure was defined as prior clinical diagnosis of systolic or diastolic heart failure. Prior atrial fibrillation was defined as prior clinical diagnosis of at least paroxysmal atrial fibrillation. Risk factors were considered absent when they were not reported as present at the time of stress testing or did not meet criteria for a database‐verified diagnosis.
Medication use was gathered by self‐report at the time of the test and categorized into common indications (eg, antihypertensive, lipid‐lowering). Use of inhalers was considered to be a marker for chronic lung disease. In cases of missing data, medication use was supplemented and verified by a retrospective search of the EMR, as well as pharmacy claims files for patients enrolled in the system's integrated health plan.
Laboratory Testing
Laboratory results were identified (as available) through a retrospective search of the EMR and laboratory databases. For each patient and for each laboratory, the test performed closest to the date of the stress test was selected for inclusion.
The Framingham Risk Score (FRS) was calculated using the 1998 FRS equation.28 In cases where recent cholesterol measurements within 90 days of the stress test were unavailable, presence or absence of dyslipidemia was used to classify patients into normal or abnormal total cholesterol and high‐density lipoprotein categories (200–240 mg/dL and 35–44 mg/dL for history of dyslipidemia, respectively). We also calculated the global risk predication model recently recommended by the ACC/AHA29 and the estimated glomerular filtration rates using the Levey modification of diet in renal disease formula.30
Follow‐up and Mortality Ascertainment
Mortality ascertainment was conducted in April 2013 using an algorithm for searching the Social Security Death Index (SSDI) Death Master File (DMF) making use of social security number, first name, last name, and date of birth. Ascertainment was conducted following federal law changes in 2011 limiting reporting of certain deaths by state agencies. A complete algorithmic search of the SSDI DMF could be completed in over 99.5% of patients.
Patients were also followed for nondeath outcomes up until May 2010. To limit bias associated with loss to follow‐up or follow‐up outside the health system, for nondeath outcomes patients were censored at their last contact with the integrated Henry Ford Health System group practice when ongoing coverage with the health plan could no longer be confirmed.
Myocardial infarction and revascularization were ascertained through linkage with administrative claims files from services delivered by the system‐affiliated group practice and/or reimbursed by the system's health plan. Linkage was performed using appropriate ICD‐9 (410.xx) and Current Procedural Terminology codes for percutaneous coronary intervention (92920–92944), and coronary artery bypass grafting surgery.
New diagnoses of hypertension, diabetes, atrial fibrillation, and atrial flutter were ascertained by EMR search and by linkage with claims files from services delivered by the system‐affiliated group practice and/or reimbursed by the system's health plan. Linkage was performed using appropriate ICD‐9 codes (hypertension [401.XX], diabetes [250.XX], atrial fibrillation or flutter [427.3X]). A new diagnosis was considered present when the appropriate ICD‐9 code was present on ≥3 separate follow‐up encounters.
Statistical Analysis
For analyses planned from the FIT Project, an a priori statistical analysis plan (SAP) will be reviewed and approved by an internal FIT Project 3‐member committee prior to analysis. We will make a copy of the SAP available on a soon‐to‐be‐developed FIT Project website. In general, analyses from the FIT Project will make use of standard survival analysis techniques using time‐to‐event data.
For the descriptive analysis in this article, categorical data are presented as percent frequencies and compared between groups by χ2 or Fisher exact test. Continuous variables are presented as mean ± 1 standard deviation and compared using the Student t test. Non‐normally distributed variables are presented as median and 25th to 75th interquartile range and were compared using nonparametric testing. Unadjusted Kaplan‐Meier survival analysis was also performed and compared via log‐rank testing.
Results
The baseline characteristics of the FIT Project are shown in Table 1. The FIT Project is a predominantly low‐ to intermediate‐risk cohort as per the FRS, whereas it is closer to high risk using the new ACC/AHA global CVD risk calculator. The median age was 54 ± 10 years with male predominance (54%).
Table 1.
Baseline Characteristics of the Study Cohort (N = 69 885)
| Characteristic | Value |
|---|---|
| Age, y, median (IQR) | 53.9 (18.0) |
| Race (%) | |
| White | 44 597 (63.8) |
| Black | 20 605 (29.5) |
| Asian | 659 (0.9) |
| Hispanic | 451 (0.6) |
| Native American | 354 (0.5) |
| Other | 3219 (4.6) |
| Gender (%) | |
| Female | 32 155 (46) |
| Male | 37 730 (54) |
| Weight, lb, median (IQR), N = 66 005 | 182 (52) |
| Weight, lb, males, median (IQR), N = 35 637 | 195 (47) |
| Weight, lb, females, median (IQR), N = 30 368 | 165 (53) |
| BMI, kg/m2, median (IQR), N = 19 662 | 28.5 (7.3) |
| Diabetes, n (%) | 13 823 (19.8) |
| Hypertension n (%) | 46 060 (65.9) |
| Hyperlipidemia (%) | 31 126 (44.5) |
| History of smoking, n (%) | 29 004 (41.5) |
| Family history of coronary heart disease, n (%) | 34 997 (50.1) |
| Prior congestive heart failure, n (%) | 1579 (2.3) |
| Prior coronary artery disease, n (%) | 10 190 (14.6) |
| Prior myocardial infraction, n (%) | 8064 (11.5) |
| Prior coronary artery angioplasty, n (%) | 3186 (4.6) |
| Prior coronary artery bypass surgery, n (%) | 2665 (3.8) |
| Aspirin use, n (%) | 15 534 (22.2) |
| Statin use, n (%) | 14 719 (21.1) |
| β‐Blocker use, n (%) | 15 441 (22.1) |
| ACEI/ARB use, n (%) | 15 493 (22.1) |
| Framingham Risk Score, n = 58 363 | 8.8% (4.7–15.7) |
| 2013 AHA/ACC CVD Risk Score, n = 58 363 | 7.1% (2.5–16.9) |
Abbreviations: ACC, American College of Cardiology; ACEI, angiotensin‐converting enzyme inhibitor; AHA, American Heart Association; ARB, angiotensin receptor blocker; BMI, body mass index; CVD, cardiovascular disease; IQR, interquartile range.
Data on stress test performance are shown in Table 2. Nearly 47%, 9%, and 7% of the patients underwent stress testing for evaluation of chest pain, shortness of breath, or risk stratification of patients with known CAD, respectively. A complete list of indications for the stress test is shown in Table 2. Most patients (74%) achieved their target heart rate, and 26% of the study cohort had chronotropic incompetence. The median MET level achieved was 10. The distribution of achieved METs during exercise testing is shown in Figure 1.
Table 2.
Stress Testing Indication and Results (N = 69 885)
| Stress Variable | Value |
|---|---|
| Indication for stress testing | |
| Chest pain | 33 099 (47.7%) |
| Rule out ischemia | 7486 (10.7%) |
| Shortness of breath | 6365 (9.1%) |
| Risk stratification for known coronary disease | 4679 (6.7%) |
| Risk factors only | 3642 (5.2%) |
| Prior abnormal test (prior stress test) | 3061 (4.4%) |
| Palpitation | 2151 (3.1%) |
| Dizziness | 1231 (1.8%) |
| Research screening | 1587 (2.3%) |
| Preoperation | 1478 (2.1%) |
| Other | 5106 (7%) |
| Resting heart rate, bpm, median (IQR) | 72 (17) |
| Resting SBP, mm Hg, median (IQR) | 130 (24) |
| Resting DBP, mm Hg, median (IQR) | 80 (14) |
| Peak heart rate, bpm, median (IQR) | 151 (28) bpm |
| Peak SBP, mm Hg, median (IQR) | 178 (36) |
| Peak DBP, mm Hg, median (IQR) | 82 (14) |
| Peak rate pressure product, median (IQR) | 26 660 (7720) bpm |
| Percent achieving predicted maximal HR, median (IQR) | 91 (11) |
| METs achieved, median (IQR) | 10 (3) |
| Achieved 85% of predicted maximal HR, n (%) | 51 870 (74.2) |
| Achieved 100% of predicted maximal HR, n (%) | 9664 (13.8) |
| Normal resting ECG, n (%), n = 63 582 | 31 547 (50) |
Abbreviations: DBP, diastolic blood pressure; ECG, electrocardiography; HR, heart rate; IQR, interquartile range; METs, metabolic equivalents; SBP, systolic blood pressure.
Figure 1.
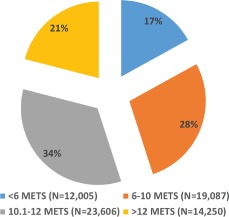
Distribution of fitness level achieved among the Henry Ford Exercise Testing Project cohort (N = 68 947). Nine hundred eight‐four patients had missing values for the metabolic equivalents (METS).
The mean follow‐up duration was 11.1 ± 4.7 years for the occurrence of all‐cause mortality. Figure 2 shows the follow‐up durations of the entire study group for all‐cause mortality. At the end of the follow‐up period, 11 085 (15.9%) patients died. An unadjusted Kaplan‐Meier curve (by fitness category) is shown in Figure 3. In addition, there were a total of 3879 (5.6%) new myocardial infarctions and 4688 (6.7%) new revascularization procedures. Among those free of these diseases at baseline, there were 8974 (37.7%) new cases of hypertension, 9473 (16.9%) new cases of diabetes, 5112 (7.5%) new cases of atrial fibrillation, and 1352 (1.9%) cases of new atrial flutter.
Figure 2.
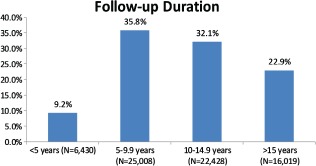
Percentage of patients in the Henry Ford Exercise Testing Project cohort sorted by duration of follow‐up. More than half of the patients had more than 10 years of follow‐up for all‐cause mortality.
Figure 3.
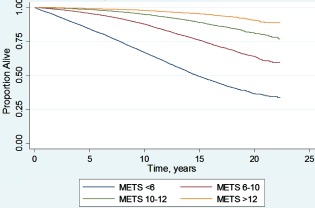
Unadjusted Kaplan‐Meier survival of the entire Henry Ford Exercise Testing Project cohort. There is a graded decrease in survival with decreasing functional capacity (P < 0.001). Abbreviations: METS, metabolic equivalents.
Discussion
Serving as an example of a successful, large, low‐cost research study using modern electronic epidemiologic techniques, in this article, we have described the rationale and methods of the largest database involving cardiorespiratory fitness and long‐term follow‐up for clinical end points.
We seek to demonstrate that it is possible to utilize different electronic data sources (EMRs, administrative claims data, laboratory data, pharmacy records, and insurance claims data) to create a large research database within a healthcare system providing a well‐established bioinformatics infrastructure. Such an effort could be replicated in other areas that engage in a high volume of clinical procedure like coronary angiography, echocardiography, and pharmacological stress testing. This strategy is increasingly feasible as structured electronic reporting of frequently performed cardiac procedures becomes common practice.21, 22, 23, 24 Combining clinical databases with administrative and claims data allows for the creation of large registry‐type databases that can help address important clinical questions. Such projects require time investment from experienced data programmers who understand the back end structure of these corporate data stores. In addition, these programmers need to have an advanced understanding of the clinical and statistical associations being investigated to correctly combine several discrete datasets into 1 accurate research database.
Given the large sample size, long duration of follow‐up, and diverse patient population, The FIT Project will be able to answer important questions that describe the association between cardiorespiratory fitness and clinical outcomes. The interaction between age, gender, and racial differences in the prognostic value of cardiorespiratory fitness will also be examined. Finally, the interaction between various parameters measured during exercise and other high‐risk prognostic markers (diabetes, renal failure, known CAD, metabolic syndrome, heart failure) will be studied. The incremental value of exercise testing on top of the FRS or the new 2013 ACC/AHA global CVD score will be investigated. We also hope to publish a new risk score for translating routine exercise treadmill data into overall prognosis. Finally, we hope to reproduce or perhaps challenge existing definitions of target heart (for example by gender or in the presence of β‐blockers) and chronotropic incompetence (as a function of age).
Study Strength and Limitations
The FIT project has multiple strengths. It is the largest epidemiologic database of objectively assessed clinical exercise data to date. It is a clinical database, which has distinct advantages over volunteer study populations. Our database also has significant racial diversity, with nearly 30% of our cohort being black. In addition, the duration of follow‐up extends to 22 years for all‐cause mortality, with more than 50% of patients followed for at least 10 years (Figure 2). Using the SSDI allows us to capture vital status of nearly all our patients.
However, our project has several limitations. Our study reports the experience of a single center with its practice patterns and mode of operation. Although the population studied is diverse, it may not be representative of the entire population of the United States. Most of our patients underwent a clinically indicated stress test to assess symptoms or to address other clinical questions. Thus, the findings may not be generalizable to asymptomatic and otherwise healthy individuals. In addition, the exclusive reliance on the Bruce protocol may have resulted in a possible bias. Subjects who are older, more often overweight, or even those who are especially fit may all be tested using different protocols, indicating a possible narrowing of the study population.
Holding onto the railing for support during the test may have resulted in overestimation of the fitness level, especially with the unavailability of metabolic testing. Although body weight was available for 66 005 patients, height was recorded in the EMR for just 20 833 patients, allowing for the calculation of body mass index in approximately 25% of the entire cohort. The ejection fraction were available for only a fraction of patients. Importantly, electrocardiography results (ie, ST‐segment changes) from the stress test were not available in our project; therefore, the Duke Treadmill Score could not be calculated. For outcomes other than all‐cause mortality, we relied on 3 citations in the EMR and claims data to confirm the diagnosis. These events were not adjudicated by an independent panel of clinical experts.
Conclusion
We have described the methods and rationale of the FIT Project. This project is in the unique position to answer many clinically relevant questions related to cardiorespiratory fitness and long‐term outcomes.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Blair SN, Kohl HW III, Paffenbarger RS Jr, et al. Physical fitness and all‐cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. [DOI] [PubMed] [Google Scholar]
- 2. Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. [DOI] [PubMed] [Google Scholar]
- 3. Blair SN, Haskell WL. Objectively measured physical activity and mortality in older adults. JAMA. 2006;296:216–218. [DOI] [PubMed] [Google Scholar]
- 4. Hsu S, Ton VK, Dominique Ashen M, et al. A clinician's guide to the ABCs of cardiovascular disease prevention: the Johns Hopkins Ciccarone Center for the Prevention of Heart Disease and American College of Cardiology Cardiosource Approach to the Million Hearts Initiative. Clin Cardiol. 2013;36:383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee DC, Sui X, Church TS, et al. Changes in fitness and fatness on the development of cardiovascular disease risk factors hypertension, metabolic syndrome, and hypercholesterolemia. J Am Coll Cardiol. 2012;59:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kokkinos P, Myers J, Kokkinos JP, et al. Exercise capacity and mortality in black and white men. Circulation. 2008;117:614–622. [DOI] [PubMed] [Google Scholar]
- 7. Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all‐cause mortality and cardiovascular events in healthy men and women: a meta‐analysis. JAMA. 2009;301:2024–2035. [DOI] [PubMed] [Google Scholar]
- 8. Vigen R, Ayers C, Willis B, et al. Association of cardiorespiratory fitness with total, cardiovascular, and noncardiovascular mortality across 3 decades of follow‐up in men and women. Circ Cardiovasc Qual Outcomes. 2012;5:358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berry JD, Willis B, Gupta S, et al. Lifetime risks for cardiovascular disease mortality by cardiorespiratory fitness levels measured at ages 45, 55, and 65 years in men. The Cooper Center Longitudinal Study. J Am Coll Cardiol. 2011;57:1604–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta S, Rohatgi A, Ayers CR, et al. Cardiorespiratory fitness and classification of risk of cardiovascular disease mortality. Circulation. 2011;123:1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee DC, Sui X, Artero EG, et al. Long‐term effects of changes in cardiorespiratory fitness and body mass index on all‐cause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study. Circulation. 2011;124:2483–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Orakzai RH, Orakzai SH, Nasir K, et al. Association of increased cardiorespiratory fitness with low risk for clustering of metabolic syndrome components in asymptomatic men. Arch Med Res. 2006;37:522–528. [DOI] [PubMed] [Google Scholar]
- 13. Gatterer H, Ulmer H, Dzien A, et al. High cardiorespiratory fitness is more beneficial in pre‐diabetic men than women. Clinics (Sao Paulo). 2011;66:747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol. 2007;165:1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Swift DL, Johannsen NM, Lavie CJ, et al. Racial differences in the response of cardiorespiratory fitness to aerobic exercise training in Caucasian and African American postmenopausal women. J Appl Physiol ( 1985. ) 2013;114:1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ramaraj R. Cardiorespiratory fitness in elderly. Eur J Cardiovasc Prev Rehabil. 2008;15:234. [DOI] [PubMed] [Google Scholar]
- 17. Bentley D, Khan S, Oh P, et al. Physical activity behavior two to six years following cardiac rehabilitation: a socioecological analysis. Clin Cardiol. 2013;36:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matheson GO, Klugl M, Engebretsen L, et al. Prevention and management of non‐communicable disease: the IOC Consensus Statement, Lausanne 2013. Sports Med. 2013;43:1075–1088. [DOI] [PubMed] [Google Scholar]
- 19. Kaminsky LA, Arena R, Beckie TM, et al. The importance of cardiorespiratory fitness in the United States: the need for a national registry: a policy statement from the American Heart Association. Circulation. 2013;127:652–662. [DOI] [PubMed] [Google Scholar]
- 20. Lauer MS. Time for a creative transformation of epidemiology in the United States. JAMA. 2012;308:1804–1805. [DOI] [PubMed] [Google Scholar]
- 21. Service RF. Biology's dry future. Science. 2013;342:186–189. [DOI] [PubMed] [Google Scholar]
- 22. Kaiser J. Epidemiology. Budget woes threaten long‐term heart studies. Science. 2013;341:701. [DOI] [PubMed] [Google Scholar]
- 23. Murdoch TB, Detsky AS. The inevitable application of big data to health care. JAMA. 2013;309:1351–1352. [DOI] [PubMed] [Google Scholar]
- 24. Lynch C. Big data: how do your data grow? Nature. 2008;455:28–29. [DOI] [PubMed] [Google Scholar]
- 25. Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–562. [DOI] [PubMed] [Google Scholar]
- 26. Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol. 2002;40:1531–1540. [DOI] [PubMed] [Google Scholar]
- 27. American Heart Association. Committee on Exercise . Exercise Testing and Training of Apparently Healthy Individuals: A Handbook for Physicians. Dallas, TX: American Heart Association; 1972. [Google Scholar]
- 28. Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 29. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013. ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published online ahead of print November 12, 2013]. Circulation. doi: 10.1161/01.cir.0000437738.63853.7a.
- 30. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–S266. [PubMed] [Google Scholar]


