Abstract
Background
Three‐quarters of rehospitalizations ($44 billion yearly estimated cost) may be avoidable. A screening tool for the detection of potential readmission may facilitate more efficient case management.
Hypothesis
An elevated red blood cell distribution width (RDW) is an independent predictor of hospital readmission in patients with unstable angina (UA) or non–ST‐elevation myocardial infarction (NSTEMI).
Methods
The study is a retrospective observational cohort analysis of adults admitted in 2007 with UA or NSTEMI. Data were gathered by review of inpatient medical records. The rate of 30‐day nonelective readmission and time to nonelective readmission were recorded until November 1, 2011, and compared by RDW group using the 95th percentile (16.3%) as a cutoff.
Results
The median follow‐up time of the 503 subjects (average age, 65 ± 13 years; 56% male) was 3.8 years (interquartile range: 0.3–4.3 years). Those readmitted within 30 days were older, had more comorbidities and higher RDW and creatinine levels, and were more likely to have had an intervention. At 3.8 years of follow‐up, subjects with high RDW (>16.3%) were more likely to be readmitted compared to those with normal RDW (≤16.3%) (72.28% vs 59.95%, P = 0.003). In multivariable analyses, high RDW was a statistically significant predictor of readmission in general (hazard ratio: 1.35 (95% confidence interval [CI]:1.02‐1.79), P = 0.033) but not of 30‐day rehospitalization (odds ratio: 1.34 (95% CI: 0.78‐2.31), P = 0.292). Its area under the receiver operating characteristic curve was 0.54 (sensitivity 23% and specificity 85%).
Conclusions
An elevated RDW is an independent predictor of hospital readmission in patients with UA or NSTEMI.
Introduction
Hospital readmissions are recognized as both a significant contributor to healthcare costs and a putative indicator of healthcare quality. For Medicare recipients, 19.6% are readmitted within 30 days, accounting for $15 billion in spending. Three‐quarters of rehospitalizations ($44 billion yearly estimated cost) may be avoidable.1, 2, 3 Age, gender, race, socioeconomic status, type of insurance, disease burden, weight, nutritional and functional status, length of stay, chronic diseases, and medication use and compliance contribute to the potential for readmission.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 A screening tool for the detection of potential cases would likely make further case management more efficient, motivating efforts to develop reliable prediction models for risk of readmission.19, 20, 21, 22
Red blood cell distribution width (RDW) is a measure of heterogeneity in erythrocytes size used in the differential diagnosis of microcytic anemia. Higher levels of RDW have been found to be associated with increased mortality among patients with heart failure, myocardial infarction, coronary artery disease (CAD), or in those undergoing angiography.23, 24, 25, 26 A high RDW was also found to be an independent predictor of mortality and associated with elevated cardiovascular biomarkers and cardiac enzymes.27, 28, 29, 30, 31 The role of RDW, a readily available routine laboratory value, has not been investigated as a rehospitalization predictive factor and is the subject of this report. The hypothesis of this study is that an elevated RDW is an independent predictor of hospital readmission in patients with unstable angina (UA) or non–ST‐elevation myocardial infarction (NSTEMI).
Methods
Study Population
The present retrospective, observational, cohort study was conducted at Beth Israel Medical Center, a tertiary care center in New York City. Its patient population was previously described.32 It included all patients age 18 years or older who were admitted in 2007 to the telemetry floors with a diagnosis of UA or NSTEMI and who underwent a coronary angiography during that admission. Patients who needed critical care, presented with ST‐elevation myocardial infarction (STEMI), were admitted to nontelemetry floors, or did not require (or did not undergo) coronary angiography were excluded.
Definitions
Patients in this study presented to the medical center with chief complaints of chest pain, shortness of breath, or decreased exercise tolerance, for which they were admitted to the telemetry floors and underwent coronary angiography regardless of the presence or absence of electrocardiogram (ECG) changes. They were divided into 2 groups: UA if they did not have detectable cardiac biomarkers (troponin I <0.03 ng/mL) and NSTEMI if they had a troponin I level ≥0.03 ng/mL without ECG changes qualifying for STEMI. The end points were the following nonelective readmissions: any 30‐day readmission, 30‐day cardiac readmission (chief complaints of chest pain, shortness of breath or decreased exercise tolerance, admitting diagnoses of acute coronary syndromes [ACS] or ischemic heart disease, telemetry floor or coronary care unit disposition), any readmission after 30 days, and cardiac readmission after 30 days. RDW level was considered high if >95th percentile of the institution's laboratory (16.3%), and normal if ≤16.3%. This grouping was based on the calibration and the reported normal range of the institution's laboratory, which uses the COULTER LH 750 Hematology Analyzer (Beckman Coulter, Inc., Brea, CA).
Data Collection
From a retrospective chart review of all patients admitted to the tertiary care center from January 1, 2007 through December 31, 2007, data were collected on the subjects' demographics (age, gender, and race), insurance coverage (Medicaid, Medicare, other), clinical history (heart failure, CAD, hypertension [HTN], diabetes, dyslipidemia, smoking, family history of CAD [FHx], and previous coronary artery bypass graft [CABG] and stenting), laboratory measurements (white blood cell count, hemoglobin, RDW, platelets, and creatinine), maximal troponin I level, diagnosis (UA or NSTEMI), maximal temperature (°F), length of stay, and treatment modality (medical therapy, percutaneous coronary intervention [PCI], or CABG). Longitudinal follow‐up ended on November 1, 2011, and the outcomes were recorded from internal electronic databases.
Ethics
The study was approved by the Beth Israel Medical Center Institutional Review Board. The requirement for obtaining an informed consent was waived. There were no conflicts of interest.
Statistical Analysis
Data were analyzed using Stata version 11.2 (StataCorp, College Station, TX). Categorical data were analyzed using χ2 or Fisher exact tests. Continuous variables whose distribution followed the normality assumptions were analyzed using the Student t test and analysis of variance (ANOVA). Variables whose distribution did not approximate normality were analyzed using the nonparametric Wilcoxon rank sum and Kruskal‐Wallis tests. Multivariable logistic regression analyses were used to determine the independent relation of elevated RDW and any 30‐day readmission and 30‐day cardiac readmission, respectively. Cox proportional hazards models were used to determine the independent relation of elevated RDW and any readmission, and cardiac readmission, respectively. A receiver operating characteristic (ROC) analysis was performed to assess the sensitivity of the prediction variables and the final model. The multivariate models were adjusted for all the variables that were of statistical significance in the univariate and bivariate analyses. Assessment for confounders and effect modifiers was performed and none were detected. Regression diagnostics and assessment of the fit of the models were conducted via the Hosmer‐Lemeshow goodness of fit test, which showed that they fit well. All tests were 2‐tailed, and P values <0.05 were considered statistically significant.
Results
Among the 1631 patients who underwent cardiac catheterization in 2007, 536 patients fulfilled the selection criteria. Thirty‐three had incomplete or missing data and were excluded from the analysis. Baseline characteristics of the remaining 503 subjects are listed in Table 1. The median follow‐up time was 3.8 years (interquartile range: 0.3–4.3 years). On average, the study population was 65 years of age and had a high prevalence of comorbidities. Fifty‐six percent (n = 280) were male. The study population showed racial diversity (with relative [35%] Hispanic preponderance). Twenty‐one percent (n = 107) were readmitted within 30 days, 77 of them with a cardiac chief complaint. During the follow‐up period, 63% (n = 319) were readmitted, 255 of them for cardiac chief complaints.
Table 1.
Baseline Characteristics of Study Subjectsa
| Characteristics | Value |
|---|---|
| Follow‐up time, y | 3.8 (0.3–4.3) |
| Sociodemographics | |
| Age, y | 65 ± 13 |
| Male | 280 (56%) |
| Race | |
| Caucasian | 106 (21%) |
| Black | 63 (13%) |
| Hispanic | 178 (35%) |
| Asian | 40 (8%) |
| Other | 116 (23%) |
| Insurance | |
| Medicaid | 60 (12%) |
| Medicare | 175 (35%) |
| Other | 268 (53%) |
| Laboratory measurements | |
| WBC (1000/μL) | 8 ± 3 |
| Hemoglobin (g/dL) | 13 ± 2 |
| RDW (%) | 15 ± 3 |
| Platelets (1000/μL) | 244 ± 87 |
| Tn max (ng/mL) | 0.01 (0.00–0.12) |
| Creatinine (mg/dL) | 1 (0.8–1.3) |
| Medical history | |
| Heart failure | 173 (34%) |
| CAD | 287 (57%) |
| HTN | 392 (78%) |
| Diabetes | 218 (43%) |
| Dyslipidemia | 228 (45%) |
| Smoking | 155 (31%) |
| FHx | 106 (21%) |
| Prior interventions | |
| Previous CABG | 72 (14%) |
| Previous stenting | 139 (28%) |
| Diagnosis | |
| UA | 406 (81%) |
| NSTEMI | 97 (19%) |
| Clinical measurements | |
| T max, °F | 99.3 ± 1.0 |
| Length of stay, d | 6 (4–10) |
| Treatment | |
| Medical therapy only | 270 (53%) |
| PCI | 180 (36%) |
| CABG | 53 (11%) |
| Readmission | |
| 30‐day readmission | 107 (21%) |
| 30‐day cardiac readmission | 77 (15%) |
| Any readmission | 319 (63%) |
| Cardiac readmission | 255 (51%) |
Abbreviations: CABG, coronary artery bypass graft; CAD, coronary artery disease; FHx, family history of coronary artery disease; HTN, hypertension; NSTEMI, non–ST‐elevation myocardial infarction; PCI, percutaneous coronary intervention; RDW, red blood cell distribution width; T max, maximal temperature; Tn max, maximal troponin I; UA, unstable angina; WBC, white blood cell count.
N = 503. Continuous variables are reported as mean ± standard deviation if normally distributed, median (range) if not. Categorical variables are reported as frequency (percentage).
The bivariate analyses by outcome are listed in Table 2. Age, gender, race, type of insurance, RDW, creatinine level, length of stay, and treatment modality were all statistically significantly associated with the outcomes of interest. At 3.8 years of follow‐up, subjects with high RDW (>16.3%) were more likely to be readmitted compared to those with normal RDW (≤16.3%, 72.28% vs 59.95%, P = 0.003) (Figure 1). In logistic regression models for 30‐day readmissions, male gender (odds ratio [OR]: 1.72, 95% confidence interval [CI]: 1.09‐2.73), diabetes (OR: 1.61, 95% CI: 1.04‐2.49), maximal temperature (OR: 1.30, 95% CI: 1.06‐1.60), age (OR: 1.02, 95% CI: 1.00‐1.04), and dyslipidemia (OR: 1.87, 95% CI: 1.14‐3.05) but not high RDW (OR: 1.34, 95% CI: 0.78‐2.31) (Table 3) were found to be statistically significantly associated with 30‐day rehospitalization. Of note, few patients had folate, vitamin B12, iron, ferritin, total iron binding capacity, and ejection fraction measurements performed for these variables to be properly tested in our analyses.
Table 2.
Characteristics of the Study Population by outcomes*
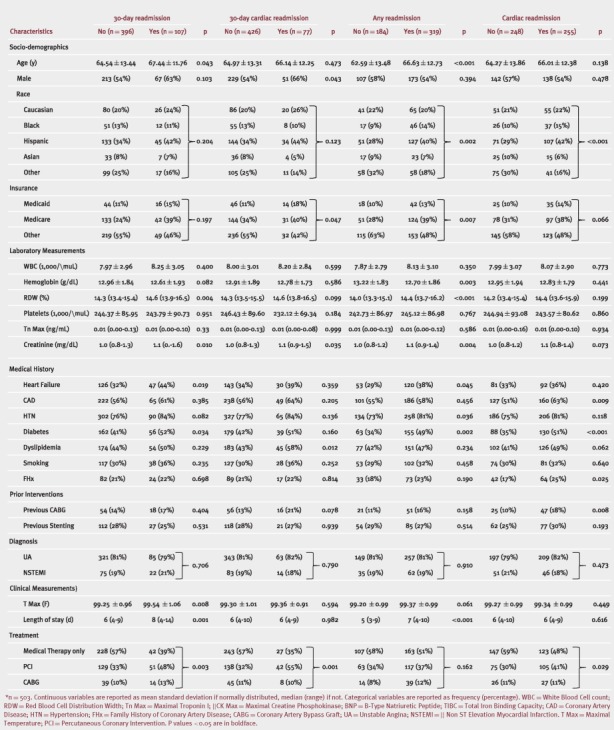
Figure 1.
Time to readmission by red blood cell distribution width (RDW) group.
Table 3.
Multivariable Logistic Regression Models and Cox Proportional Hazards for High Red Blood Cell Distribution Width
| OR (95% CI) | P | |
|---|---|---|
| Outcome = 30‐day readmission | ||
| Unadjusted model | 1.57 (0.95‐2.59) | 0.078 |
| Adjusted modela | 1.34 (0.78‐2.31) | 0.292 |
| Outcome = 30‐day cardiac readmission | ||
| Unadjusted model | 1.49 (0.85‐2.63) | 0.163 |
| Adjusted modelb | 1.70 (0.92‐3.15) | 0.091 |
| HR (95% CI) | P | |
| Outcome = any readmission | ||
| Unadjusted model | 1.48 (1.14‐1.92) | 0.003 |
| Adjusted modelc | 1.35 (1.02‐1.79) | 0.033 |
| Outcome = cardiac readmission | ||
| Unadjusted model | 1.10 (0.81‐1.49) | 0.527 |
| Adjusted modeld | 1.20 (0.88‐1.63) | 0.248 |
Abbreviations: CI, confidence interval; HR, hazard ratio; OR, odds ratio.
Adjusted for creatinine, heart failure, diabetes, maximum temperature, treatment modality, and length of stay.
Adjusted for sex, type of insurance, creatinine, dyslipidemia, and treatment modality.
Adjusted for age, sex, race, type of insurance, creatinine, heart failure, hypertension, diabetes, and length of stay.
Adjusted for race, coronary artery disease, diabetes, previous coronary artery bypass graft, and treatment modality.
In Cox proportional hazards models, high RDW (hazard ratio [HR]: 1.41, 95% CI: 1.08‐1.83), diabetes (HR: 1.53, 95% CI: 1.23‐1.90), creatinine level >1 mg/dL (HR: 1.44, 95% CI: 1.15‐1.81), family history of coronary artery disease (FHx) (HR: 1.39, 95% CI: 1.06‐1.82), and age (HR: 1.01, 95% CI: 1.00‐1.02) were statistically significant predictors of readmission. In a multivariable Cox proportional hazards model adjusted for statistically significant variables, high RDW was a statistically significant predictor of readmission (HR: 1.35, 95% CI: 1.02‐1.79, P = 0.033) (Table 3). In further analyses, the sensitivity and specificity of high RDW at predicting readmission were 23% and 85%, respectively, with an area under the ROC curve of 0.54. In comparison the C‐statistic of the full predictive model (high RDW, high creatinine, diabetes, age, and FHx) was 0.66 (Figure 2). Of note, the area under the ROC curve for the predictive model was 0.65 without the high RDW variable, but the difference did not achieve statistical significance (P = 0.256).
Figure 2.
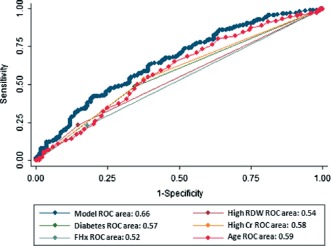
Areas under the receiver operating characteristic (ROC) curve. Abbreviations: Cr, creatinine; FHx, family history of coronary artery disease; RDW, red blood cell distribution width.
Discussion
The rate of hospital readmission is considered an important measure for assessing the performance of the healthcare system. Detecting a high probability for readmission could identify opportunities for targeted improvement efforts in patient care and follow‐up.33 RDW, an index in the variability of the size of circulating red cells (anisocytosis), is routinely reported by automated laboratory equipment used to perform complete blood counts. Although its use had been generally limited to the evaluation of microcytic anemia, mounting evidence suggests additional roles for this measurement. The present study demonstrated an independent association between high RDW and rate of readmission in patients presenting with UA or NSTEMI. This association was found to be independent of multiple potential confounding factors, such as age, sex, race, insurance type, length of stay, or comorbidities.
In previous reports age, gender, race, socioeconomic status, type of insurance, disease burden, weight, nutritional and functional status, length of stay, chronic diseases, and medication use and compliance have been identified as contributors to the likelihood of rehospitalization.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Some of these factors have been incorporated in various prediction models that have been developed. In their study on cardiac‐related emergency readmissions, Wallman et al. developed a prediction model with a C‐statistic of 0.75 that contains 11 variables: number of previous admissions, anemia, ACS, congestive heart failure, diabetes, renal disease, residence out of area, no procedure applied during hospitalization, major or minor therapeutic procedure applied during hospitalization, and the existence of HTN.19
Comorbidities and age are the cornerstones of the prediction model for pneumonia patients developed by Capelastegui et al.20 In this small study (81 patients), the area under the curve [AUC] for the model was 0.77. In the study by Hasan et al.,21 the number of hospital admissions in the preceding year, the Charlson Comorbidity Index, marital status, and having a regular physician provided a model with AUC of 0.65. Of the reported prediction models in the literature, the predicting emergency admissions over the next year (PEONY) algorithm,22 with a C‐statistic of 0.8, seems to have the best discrimination of high risk for readmission. It includes age, gender, socioeconomic status, medication, the number of previous admissions, and previous total bed days. However, it has not been tested on independent datasets to assess its validity.
The present study on RDW in patients with UA or NSTEMI extends our understanding of risk factors for rehospitalization and supports the current focus on readmission in patients with acute myocardial infarction and heart failure, and PCI.34, 35 The relation of an elevated RDW to readmission in general (HR: 1.35, P = 0.033, and C‐statistic 0.54) as per the present study is similar to the reported results in the literature, where AUCs of predictive models (including the one from this analysis) vary between 0.65 and 0.8.
Although RDW is an emerging biomarker, and despite the cumulative literature about the association of RDW and prognosis, the underlying biological mechanism for this relation remains unknown. One conceivable pathway is that there are subclinical disease processes that cause a subtle dysregulation of erythrocyte homeostasis that is expressed in RDW.
Several study limitations should be considered in the interpretation of the results. The present study is a retrospective analysis and has all the inherent limitations of a retrospective study. For example, data on medication and compliance, previous admissions, and factors required for disease burden scoring (propensity or Charlson) such as ejection fraction measurements or chronic lung disease were not consistently available in the medical records to allow for proper analysis. As a single‐center study, it may reflect a single standard of practice, thus limiting external validity. However, the study population was diverse from a racial perspective, and the clinical characteristics of patients admitted to the hospital did not differ from those in other studies suggesting reasonable generalizability. The baseline characteristics between the outcome groups were similar, but there were certain differences that were adjusted for. Despite accounting for multiple variables and assessing for potential confounding factors and effect modifiers, residual confounding variables could lead to the observed results. As with all analyses of observational data, this study cannot distinguish causality from association. The cutoff value for high RDW in absolute numbers differed from that of bigger studies, such as the National Health and Nutrition Examination Survey III,23 but it was consistent with the laboratory's range. Finally, this study does not provide a mechanism for the association between RDW and the observed outcome.
Conclusion
This study demonstrated that an elevated RDW is an independent predictor of hospital readmission in patients with UA or NSTEMI. Its role in prediction models deserves further investigation. RDW may be considered an inexpensive, readily available, additional tool for detecting patients who may require more elaborate case management with the aim of preventing readmission.
Acknowledgments
The author thanks Thomas Killip, MD, FACC, for his contribution to this article.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 2. Weissman JS, Ayanian JZ, Chasan‐Taber S, et al. Hospital readmissions and quality of care. Med Care. 1999;37:490–501. [DOI] [PubMed] [Google Scholar]
- 3. Hackbarth G, Chernew M, Armstrong S, et al. A path to bundled payment around a rehospitalization. In: Report to the Congress: reforming the delivery system. Washington, DC: Medicare Payment Advisory Commission, June 2008:83–103. http://www.medpac.gov/documents/Jun08_EntireReport.pdf (accessed 10/10/2012).
- 4. Rodriguez F, Joynt KE, Lopez L, et al. Readmission rates for Hispanic Medicare beneficiaries with heart failure and acute myocardial infarction. Am Heart J. 2011;162:254–226. [DOI] [PubMed] [Google Scholar]
- 5. Friedmann JM, Jensen GL, Smiciklas‐Wright H, et al. Predicting early nonelective hospital readmission in nutritionally compromised older adults. Am J Clin Nutr. 1997;65:1714–1720. [DOI] [PubMed] [Google Scholar]
- 6. Allaudeen N, Vidyarthi A, Maselli J, et al. Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6:54–60. [DOI] [PubMed] [Google Scholar]
- 7. Mudge AM, Kasper K, Clair A, et al. Recurrent readmissions in medical patients: a prospective study. J Hosp Med. 2011;6:61–67. [DOI] [PubMed] [Google Scholar]
- 8. Garcia‐Perez L, Linertova R, Lorenzo‐Riera A, et al. Risk factors for hospital readmissions in elderly patients: a systematic review. QJM. 2011;104:639–651. [DOI] [PubMed] [Google Scholar]
- 9. Philbin EF, Dec GW, Jenkins PL, et al. Socioeconomic status as an independent risk factor for hospital readmission for heart failure. Am J Cardiol. 2001;87:1367–1371. [DOI] [PubMed] [Google Scholar]
- 10. Hess G, Preblick R, Hill J, et al. Effects of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker therapy after hospital discharge on subsequent rehospitalization for acute myocardial infarction and heart failure. Congest Heart Fail. 2009;15:170–175. [DOI] [PubMed] [Google Scholar]
- 11. Howie‐Esquivel J, Dracup K. Effect of gender, ethnicity, pulmonary disease, and symptom stability on rehospitalization in patients with heart failure. Am J Cardiol. 2007;100:1139–1144. [DOI] [PubMed] [Google Scholar]
- 12. Foraker RE, Rose KM, Suchindran CM, et al. Socioeconomic status, Medicaid coverage, clinical comorbidity, and rehospitalization or death after an incident heart failure hospitalization: Atherosclerosis Risk in Communities cohort (1987 to 2004). Circ Heart Fail. 2011;4:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tuppin P, Neumann A, Danchin N, et al. Evidence‐based pharmacotherapy after myocardial infarction in France: adherence‐associated factors and relationship with 30‐month mortality and rehospitalization. Arch Cardiovasc Dis. 2010;103:363–375. [DOI] [PubMed] [Google Scholar]
- 14. van Diepen S, Bakal JA, McAlister FA, et al. Mortality and readmission of patients with heart failure, atrial fibrillation, or coronary artery disease undergoing noncardiac surgery: an analysis of 38 047 patients. Circulation. 2011;124:289–296. [DOI] [PubMed] [Google Scholar]
- 15. Kociol RD, Lopes RD, Clare R, et al. International variation in and factors associated with hospital readmission after myocardial infarction. JAMA. 2012;307:66–74. [DOI] [PubMed] [Google Scholar]
- 16. Hannan EL, Zhong Y, Lahey SJ, et al. 30‐day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569–576. [DOI] [PubMed] [Google Scholar]
- 17. Khumri TM, Reid KJ, Kosiborod M, et al. Usefulness of left ventricular diastolic dysfunction as a predictor of one‐year rehospitalization in survivors of acute myocardial infarction. Am J Cardiol. 2009;103:17–21. [DOI] [PubMed] [Google Scholar]
- 18. Khawaja FJ, Shah ND, Lennon RJ, et al. Factors associated with 30‐day readmission rates after percutaneous coronary intervention. Arch Intern Med. 2012;172:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wallmann R, Llorca J, Gomez‐Acebo I, et al. Prediction of 30‐day cardiac‐related‐emergency‐readmissions using simple administrative hospital data [published online ahead of print July 20, 2011]. Int J Cardiol. doi:10.1016/j.ijcard.2011.06.119. [DOI] [PubMed]
- 20. Capelastegui A, Espana Yandiola PP, et al. Predictors of short‐term rehospitalization following discharge of patients hospitalized with community‐acquired pneumonia. Chest. 2009;136:1079–1085. [DOI] [PubMed] [Google Scholar]
- 21. Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Donnan PT, Dorward DW, Mutch B, et al. Development and validation of a model for predicting emergency admissions over the next year (PEONY): a UK historical cohort study. Arch Intern Med. 2008;168:1416–1422. [DOI] [PubMed] [Google Scholar]
- 23. Perlstein TS, Weuve J, Pfeffer MA, et al. Red blood cell distribution width and mortality risk in a community‐based prospective cohort. Arch Intern Med. 2009;169:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel KV, Semba RD, Ferrucci L, et al. Red cell distribution width and mortality in older adults: a meta‐analysis. J Gerontol A Biol Sci Med Sci. 2010;65:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tonelli M, Sacks F, Arnold M, et al.; Cholesterol and Recurrent Events (CARE) Trial Investigators. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117:163–168. [DOI] [PubMed] [Google Scholar]
- 26. Zalawadiya SK, Veeranna V, Niraj A, et al. Red cell distribution width and risk of coronary heart disease events. Am J Cardiol. 2010;106:988–993. [DOI] [PubMed] [Google Scholar]
- 27. Allen LA, Felker GM, Mehra MR, et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010;16:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dabbah S, Hammerman H, Markiewicz W, et al. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am J Cardiol. 2010;105:312–317. [DOI] [PubMed] [Google Scholar]
- 29. Poludasu S, Marmur JD, Weedon J, et al. Red cell distribution width (RDW) as a predictor of long‐term mortality in patients undergoing percutaneous coronary intervention. Thromb Haemost. 2009;102:581–587. [DOI] [PubMed] [Google Scholar]
- 30. Cavusoglu E, Chopra V, Gupta A, et al. Relation between red blood cell distribution width (RDW) and all‐cause mortality at two years in an unselected population referred for coronary angiography. Int J Cardiol. 2010;141:141–146. [DOI] [PubMed] [Google Scholar]
- 31. Lippi G, Filippozzi L, Montagnana M, et al. Clinical usefulness of measuring red blood cell distribution width on admission in patients with acute coronary syndromes. Clin Chem Lab Med 2009;47:353–357. [DOI] [PubMed] [Google Scholar]
- 32. Ephrem G, Kanei Y. Elevated red blood cell distribution width is associated with higher recourse to coronary artery bypass graft. Cardiology. 2012;123:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Podulka J, Barrett M, Jiang HJ, et al. 30‐day readmissions following hospitalizations for chronic vs. acute conditions, 2008. HCUP Statistical Brief #127. February 2012. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb127.pdf. (accessed on 10/10/2012). [PubMed]
- 34. Krumholz HM, Normand SL. Public reporting of 30‐day mortality for patients hospitalized with acute myocardial infarction and heart failure. Circulation. 2008;118:1394–1397. [DOI] [PubMed] [Google Scholar]
- 35. Kereiakes DJ. Return to sender hospital readmission after percutaneous coronary intervention. J Am Coll Cardiol. 2009;54:908–910. [DOI] [PubMed] [Google Scholar]


