Abstract
Although atherosclerotic cardiovascular disease (CVD) is the most common cause of morbidity and mortality in the world, the long disease latency affords ample opportunity for preventive care. Indeed, lifelong exposure to atherogenic apoliprotein B‐containing lipoproteins has consistently been shown to increase the cumulative risk of suffering a CVD event, including myocardial infarction, stroke, and symptomatic peripheral arterial disease. Over the past 25 years, lipid‐lowering therapies have been developed that are proven to not only lower cholesterol, but also to decrease adverse CVD events and CVD mortality. This review will highlight several key clinical trials encompassing several classes of lipid‐lowering medications that have provided clinicians with an evidence‐based framework for managing their patients' cardiovascular risk.
Introduction
Atherosclerotic cardiovascular disease (CVD) is the most common cause of morbidity and mortality in the world, accounting for 17.3 million deaths per year, with a projected increase to 23.6 million deaths by 2030.1 According to the World Health Organization, up to 80% of CVD is preventable.1 Risk of CVD can be reduced by preventing or treating modifiable risk factors, such as dyslipidemia, smoking, hypertension, diabetes mellitus (DM), obesity, unhealthy diet, and sedentary lifestyle. These factors account for >90% of the population‐attributable risk of CVD.2 Primary prevention remains the cornerstone in combating this epidemic worldwide. The use of lipid‐lowering agents in patients without established CVD has become one of the most important interventions.3
Primary prevention is working. Compared with 1980, there were 341 745 fewer deaths in 2000 from coronary heart disease (CHD) in the United States, with 44% of that decrease secondary to changes in modifiable risk factors. Approximately 24% of that reduction was directly related to decreased total cholesterol (TC).4 More recently, an analysis in Ontario, Canada, found a 35% decrease in CHD mortality from 1994 to 2005, with 48% of the decrease attributable to risk‐factor modification, including control of dyslipidemia and hypertension.5
Indeed, the Johns Hopkins Precursor Study showed that elevated cholesterol in early adulthood was associated with CVD later in life, suggesting a critical role for early risk‐factor modification in preventing future disease.6 Moreover, individuals with a nonsense mutation in the PSCK9 gene (which causes an increase in low‐density lipoprotein cholesterol [LDL‐C] receptors and thus lower serum LDL‐C levels) had 28% lower LDL‐C levels, and CHD was reduced by 88%.7 This observation is compatible with an emerging criteria in preventive cardiology: The earlier lipids are lowered, the better.
The success of large randomized controlled trials testing risk‐reduction strategies in patients with risk factors but without overt CVD (primary prevention) has helped usher in the field of preventive cardiology. This article will focus on the evidence for the role of lipid‐lowering agents for primary prevention of CVD and provide the clinician with an individualized prevention strategy that can be implemented in the clinical setting.
Assessing Cardiovascular Disease Risk
Landmark trials in preventive cardiology have utilized specific eligibility criteria to target individuals at risk for developing a future cardiovascular event. Among those without CVD, it is important to identify low‐risk, moderate‐risk, and high‐risk individuals to tailor therapy. There are a number of established risk scores to determine an individual's 10‐year risk of having a cardiovascular event.8 Although not one has been used for entry criteria in an outcome‐driven randomized control clinical trial (RCT), risk scoring provides a starting point for the primary prevention of CVD. Here we focus on 3 common risk assessments: the Framingham Risk Score (FRS) for hard CHD events, the D'Agostino Score for total CVD events, and the Reynolds Risk Score (RRS; Table 1).3, 9, 10, 11
Table 1.
Risk Scores for Predicting CVD Risk
| Risk Score | Components | Predicts | Interpretation | Disadvantages |
|---|---|---|---|---|
| Framingham Risk Score (FRS) | Age, gender, total cholesterol, HDL‐C, smoking, SBP | 10‐y risk of MI or CHD‐related death | Low risk: <10%; moderate risk: 10%–20%; high risk: >20% | Does not predict the risk of developing other cardiovascular events (stroke, PAD, and HF); does not incorporate FH; can over/underestimate risk in non‐US populations |
| D'Agostino Score (revised FRS) | Same as FRS | 10‐y risk of CHD, PAD, and HF | Low risk: <10%; moderate risk: 10%–20%; high risk: >20% | Does not include biomarker data |
| Reynolds Risk Score (RRS) | Same as FRS + FH of early MI + hs‐CRP | 10‐year risk of MI, coronary revascularization, cardiovascular death, stroke | Low risk: <10%; moderate risk: 10%–20%; high risk: >20% |
Abbreviations: CHD, coronary heart disease; CVD, cardiovascular disease; FH, family history; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; hs‐CRP, high‐sensitivity C‐reactive protein; MI, myocardial infarction; PAD, peripheral arterial disease; SBP, systolic blood pressure.
The FRS remains the standard for estimating risk and is used as part of the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guidelines for managing dyslipidemia. The FRS predicts myocardial infarction (MI)‐ or CHD‐related death by assessing age, total cholesterol, high‐density lipoprotein cholesterol (HDL‐C), systolic blood pressure (SBP), and smoking status. Those with a <10% 10‐year risk are deemed low risk; 10% to 20%, moderate risk; and >20%, high risk.
A limitation of the FRS is that it does not predict the risk of developing other cardiovascular events, including stroke, peripheral arterial disease (PAD), and heart failure, all of which contribute significantly to the overall CVD morbidity and mortality throughout the world. Further, it often underestimates the risk of total CVD events, especially in women. This issue was partially addressed by D'Agostino and colleagues, who developed a more comprehensive FRS that included a model for 10‐year risk prediction of CHD, stroke, PAD, and heart failure that can be used easily in an office setting.11 For example, a 50‐year‐old woman with a total cholesterol of 200 mg/dL, HDL‐C of 40 mg/dL, untreated hypertension with a SBP of 140 mm Hg, and a smoking history would have a 5% risk (low risk) of an event over the course of 10 years as estimated by the traditional FRS, but the comprehensive FRS would increase her risk to 15% (moderate risk).
The RRS, developed as an alternative to the FRS, adds family history (MI in a parent < age 60 years) along with high‐sensitivity CRP (hs‐CRP) to traditional CVD risk factors.10 Use of the RRS in the Women's Health Study reclassified 40% of women from low risk based on the FRS to intermediate risk.9 In a direct comparison of the RRS with the FRS in the Women's Health Initiative Observational Cohort involving a multiethnic population with clinical CVD, the RRS was a better discriminator in assessing CVD risk, especially among African American and Caucasian women.12
Cholesterol
Elevated circulating cholesterol–containing apolipoprotein B lipoproteins play a critical role in atherogenesis and are essential in the development of coronary plaque. The biological process of atherosclerosis is initially clinically silent, beginning with lipoprotein retention in the arterial wall triggering a localized inflammatory response and, in some cases, a potentially catastrophic manifestation of newly diagnosed CVD such as MI, stroke, or sudden cardiac death.13, 14 Based on many landmark trials, the standard therapy for lowering culprit lipoprotein is 3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase inhibitors, or statins (Table 2).
Table 2.
Summary of Landmark Statin Primary Prevention Clinical Trials
| Trial | Drug | Study Population | Duration of Follow‐up, y | Baseline LDL‐C, mg/dL | % Change in LDL‐C vs Control | Results | NNT |
|---|---|---|---|---|---|---|---|
| WOSCOPS | Pravachol 40 mg/d vs placebo | 6595; men only, hyperlipidemia | 4.9 | 192 | −26% | TC 20%, MI/CHD death 31%, death 22% | 42 |
| AFCAPS/TexCAPS | Lovastatin 20–40 mg/d vs placebo | 6605; men 84.9%, women 15.1%; hyperlipidemia | 5.2 | 150 | −27% | MI/UA/sudden cardiac death 38%, event rate in women | 50 |
| MEGA | Pravastatin 10–20 mg/d vs diet | 7832; men 31.5%, women 68.5%; hyperlipidemia | 5.3 | 156.3 | −15% | TC 11%, MI/UA/sudden cardiac death/coronary revascularization 33% | 119 |
| ASCOT‐LLA | Atorvastatin 10 mg/d vs placebo | 10 305; men 81.2%, women 18.8%; hypertension with >3 CVD risk factors | 3.3 | 131.2 | −35% | Nonfatal MI, CHD‐related death 36% | 99 |
| JUPITER | Rosuvastatin 20 mg/d vs placebo | 17 802; men 61.8%, women 38.2%; healthy people with CRP >2.0 mg/L, LDL <130 mg/dL | 1.9 | 108 | −50% | hs‐CRP 37%, MI/stroke/arterial revascularization/UA/CV death 44% | 25 at 5 y |
Abbreviations: AFCAPS/TexCAPS, Air Force/Texas Coronary Atherosclerosis Prevention Study; ASCOT‐LLA, Anglo‐Scandinavian Cardiac Outcomes Trial–Lipid‐Lowering Arm; CHD, cardiovascular heart disease; CRP, C‐reactive protein; CV, cardiovascular; CVD, cardiovascular disease; JUPITER, Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin; LDL‐C, low‐density lipoprotein cholesterol; MEGA, Primary Prevention of Cardiovascular Disease with Pravastatin in Japan; MI, myocardial infarction; NNT, number needed to treat; TC, total cholesterol; UA, unstable angina; WOSCOPS, West of Scotland Coronary Prevention Study.
Statins
The 1995 West of Scotland Coronary Prevention Study (WOSCOPS) was an early statin trial in 6595 hyperlipidemic men age 45 to 64 years with 92% of participants free of known CVD at study entry. Average baseline TC was 272 mg/dL, and participants were randomized to pravastatin 40 mg/day vs placebo with a primary endpoint of nonfatal MI and death from CHD. After an average follow‐up of 4.9 years, the pravastatin arm had 20% and 26% decreases in TC and LDL‐C, respectively.15
The primary endpoint was reached in 248 participants in the placebo arm and 174 in the pravastatin arm (relative risk [RR] reduction 31% with pravastatin therapy, 95% confidence interval [CI]: 17%–43%, P < 0.001). The RR reduction in all‐cause mortality was 22% with pravastatin (106 events in the pravastatin arm, 135 in the placebo group; 95% CI: 0%–40%, P < 0.051).15 The benefits of pravastatin for primary prevention persisted in long‐term analysis: Men treated for 5 years with pravastatin had an 18% RR reduction in nonfatal MI and death from CHD after 10 years of follow‐up.16
In 1998, the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) enrolled 5608 men and 997 women without clinical CVD in a randomized, double‐blind trial of lovastatin 20 to 40 mg/day vs placebo. Prior to drug therapy, participants had a mean TC of 221 mg/dL, LDL‐C of 150 mg/dL, HDL‐C of 36 mg/dL in men and 40 mg/dL in women, and triglycerides of 158 mg/dL. The primary endpoint was the first major coronary event, defined as fatal or nonfatal MI, unstable angina (UA), or sudden cardiac death. During an average follow‐up of 5.2 years, there were 183 major coronary events in the placebo arm vs 116 in the lovastatin arm (RR: 0.62, 95% CI: 0.50–0.79, P < 0.001). Subgroup analysis found a corresponding benefit in women, making this the first major trial to demonstrate a role of for primary‐prevention statin therapy in men and women.17
The Primary Prevention of Cardiovascular Disease with Pravastatin in Japan (MEGA) trial was the first prospective, blinded RCT to evaluate the benefit of statins in an Asian population with overall low risk for CVD. The study enrolled 3966 participants to a heart healthy diet and 3866 participants to pravastatin 10 to 20 mg/day and diet (68% of the total study population were women). The primary endpoint for the study was first occurrence of CHD (nonfatal and fatal MI, sudden cardiac death, UA, coronary revascularization).
After an average follow‐up of 5.3 years, there was a 33% relative reduction in CHD events in the pravastatin arm vs control (66 events vs 101 events; hazard ratio [HR]: 0.67, 95% CI: 0.49–0.91, P = 0.01). The number needed to treat at 5.3 years to prevent 1 CHD event was 119. Despite the moderate decrease in TC and LDL‐C with the low‐dose pravastatin (−11% and −18%, respectively) in the MEGA trial (Figure 1), the risk reduction in CHD events was similar to other primary‐prevention trials. There was no significant benefit for pravastatin therapy among women despite a 29% reduction in the primary endpoint, most likely due to a low event rate.18
Figure 1.

LDL‐C reduction in landmark statin primary prevention trials. Abbreviations: AFCAPS/TexCAPS, Air Force/Texas Coronary Atherosclerosis Prevention Study; ASCOT‐LLA, Anglo‐Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm; JUPITER, Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin; LDL‐C, low‐density lipoprotein cholesterol; LDL‐P, placebo arm low‐density lipoprotein cholesterol; LDL‐S, statin arm low‐density lipoprotein cholesterol; MEGA, Primary Prevention of Cardiovascular Disease with Pravastatin in Japan; WOSCOPS, West of Scotland Coronary Prevention Study.
The Anglo‐Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT‐LLA) randomized 10 305 participants with hypertension, 3 other CVD risk factors, and nonfasting TC <6.5 mmol/L (approximately 250 mg/dL) to atorvastatin 10 mg/day or placebo. Participants were followed for an average of 3.3 years, with a primary endpoint of nonfatal MI or CHD‐related death. There was a 36% reduction in the primary endpoint in the atorvastatin arm compared with placebo (100 vs 154 events, respectively; HR: 0.64, 95% CI: 0.50–0.83, P = 0.005). Significant reductions in secondary endpoints of stroke, total CVD events, and total coronary events were also noted among patients randomized to atorvastatin. A clear benefit with atorvastatin therapy was seen as early as 1 year after enrollment, thus resulting in early trial termination for efficacy as assessed by the trial safety and monitoring board.19
The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) randomized 17 092 nondiabetic men (age >50 years) and women (age >60 years) without CVD, LDL‐C <130 mg/dL, and hs‐CRP ≥2.0 mg/L to rosuvastatin 20 mg/day or placebo. There was a 50% decrease in LDL‐C, a 37% decrease in hs‐CRP, and a 44% decrease in the composite primary endpoint of MI, stroke, arterial revascularization, hospitalization for UA, or death from cardiovascular causes. The 4‐year number needed to treat to prevent 1 primary endpoint was 31.20 The JUPITER trial provided further evidence that statins prevent CVD even in individuals with lower cholesterol levels.
Statins and Mortality Benefit
There have been 3 recent meta‐analyses looking into the role of statins in the primary prevention of all‐cause mortality and CHD outcomes. Brugts and colleagues reviewed 10 RCTs involving 70 388 participants with a mean follow‐up duration of 4.1 years. The average age of the study population was 63 years, and 23% had documented DM. There was a 12% decrease in the odds of all‐cause mortality (OR: 0.88, 95% CI: 0.81–0.96). Approximately 6% of the study participants had baseline CHD. However, after excluding those studies with participants with CHD there was still a significant decrease in all‐cause mortality (odds ratio [OR]: 0.87; 95% CI: 0.78–0.97).21
Ray and colleagues in 2010 investigated 11 RCTs involving 65 229 participants for an average follow‐up of 3.7 years. There was a 9% reduction in all‐cause mortality (RR: 0.91, 95% CI: 0.86–1.00) that was borderline statistically significant, but the point estimate was similar to other primary‐prevention meta‐analyses.22
The recently released updated 2013 Cochrane review of statins for primary prevention showed among 18 RCTs (19 trial arms) with 56 934 participants there was a 14% decrease in total mortality (OR: 0.86, 95% CI: 0.79–0.94). There was a 25% decrease in combined fatal and nonfatal CVD events (RR: 0.75, 95% CI: 0.70–0.81). A reduction in revascularization rates was also seen (RR: 0.62, 95% CI: 0.54–0.72). Further, this review provided evidence regarding the safety of statins. There were no differences in total adverse events, myalgias, rhabdomyolysis, elevation in liver enzymes, or cancer. There was a small but significant increase in DM (OR: 1.18, 95% CI: 1.01–1.39), which was driven by the JUPITER study.23 However, in a secondary analysis of this RCT, Ridker and colleagues showed that among patients with ≥1 risk factor for DM, there were 134 fewer vascular events or deaths for every 54 incident cases of DM among rosuvastatin‐treated participants (Table 3).24
Table 3.
Statin Side Effect Profile
| Type of Event | No. of Studies | Participants on Study Drug (Placebo) | No. With Adverse Event (Placebo) | RR or OR (95% CI) |
|---|---|---|---|---|
| Total adverse events | 12 | 20 718 (19 998) | 5748 (5090) | RR 1.00 (0.97–1.03) |
| Stopped treatment | 9 | 11 054 (10 588) | 940 (973) | OR 0.86 (0.65–1.12) |
| Myalgia | 9 | 19 396 (18 542) | 1847 (1704) | RR 1.03 (0.97–1.09) |
| Rhabdomyolysis | 6 | 19 410 (19 058) | 3 (3) | RR 1.00 (0.23–4.38) |
| DM | 2 | 12 205 (12 202) | 342 (290) | OR 1.18 (1.01–1.39) |
| Elevated liver enzymes | 10 | 20 420 (19 674) | 476 (472) | RR 1.16 (0.87–1.54) |
| Cancer | 11 | 19 789 (18 950) | 1180 (1075) | RR 1.01 (0.93–1.10) |
Abbreviations: CI, confidence interval; DM, diabetes mellitus; OR, odds ratio; RR, relative risk.
Data are from the 2013 Cochrane meta‐analysis.23
The overall safety of statins was further demonstrated by a meta‐analysis of adverse effects in 72 RCTs of statins including nearly 160 000 subjects, which showed no significant increase in the incidence of cancer, rhabdomyolysis, or creatine kinase elevations. There was an increase in the incidence of DM (OR: 1.09, 95% CI: 1.02‐1.16) and elevated transaminases (OR: 1.31, 95% CI: 1.04‐1.66 and OR: 1.28, 95% CI: 1.11‐1.48 for aspartate aminotransferase and alanine aminotransferase, respectively). The latter increases were reversible and did not lead to any serious liver injury or death.25 Overall, the benefits of statin therapy far outweigh the risk of adverse effects with appropriate clinical monitoring.
Nonstatin Lipid‐Lowering Medications
Niacin
Niacin (vitamin B3) affects circulating cholesterol by raising HDL‐C and lowering triglyceride levels and LDL‐C. Previous RCTs used niacin combined with either statins (Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 2 [ARBITER 2] trial, Oxford Niaspan study) or ezetimibe (ARBITER 6–HDL and LDL Treatment Strategies in Atherosclerosis [HALTS] trial) to determine if there were significant differences in surrogate endpoints for CVD, namely carotid intima‐media thickness (cIMT) measured by ultrasound or magnetic resonance imaging. There was significant improvement in cIMT thickness progression in participants receiving niacin; however, a significant number of individuals had an adverse reaction to niacin (69% reported flushing in the ARBITER‐2 trial). There are no RCTs investigating the role of niacin in the primary prevention of CVD.26, 27, 28
Fibrates
Fibrates such as fenofibrate and gemfibrozil reduce LDL‐C and triglycerides and raise HDL‐C. The Helsinki Heart Study randomized 4081 men age 40 to 55 years without clinical CVD to gemfibrozil or placebo. After 5 years of follow‐up, the fibrate drug arm demonstrated significantly increased levels of HDL‐C and a reduction in LDL‐C. There was a 34% reduction in CHD in the gemfibrozil arm, but no difference in mortality.29 The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial randomized 5518 high‐risk participants with type 2 DM to a combination of fenofibrate‐simvastatin vs simvastatin alone. After a 4.7‐year follow‐up, there was no difference in the primary endpoint of fatal CVD events, nonfatal MI, or nonfatal stroke.30
Given the lack of a demonstrable mortality benefit, fibrates should not be considered a first‐line treatment for primary prevention in adults with triglycerides <500 mg/dL, but they may be an alternative for individuals who are unable to tolerate statins.
Fish Oil
Retrospective cohort analyses, including data from the Nurses' Health Study and Health Professionals Follow‐up Study, have had mixed signals in regard to fish‐oil consumption and CVD events.31, 32 The Japan EPA (eicosapentaenoic acid) Lipids Intervention Study (JELIS) randomized 18 645 patients with a TC ≥251 mg/dL to either 1800 mg of EPA + statin (pravastatin 10 mg or simvastatin 5 mg) or statin alone. The primary combined endpoint of sudden cardiac death, fatal or nonfatal MI, UA, or revascularization was reduced by 19% in the EPA + statin arm after 4.6 years of follow‐up. When a subgroup analysis was performed, the primary outcome was not met in the primary‐prevention arm.33 Routine use of fish oil as monotherapy for primary prevention of CVD is not recommended.
Ezetimibe
Ezetimibe decreases LDL‐C by inhibiting absorption of cholesterol in the intestine. The Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial randomized 725 participants with heterozygous familial hypercholesterolemia to ezetimibe/simvastatin or to simvastatin monotherapy. There was no difference in the primary endpoint of change in cIMT.34 The Study of Heart and Renal Protection (SHARP) trial randomized 9270 participants with chronic kidney disease to a combination of simvastatin and ezetimibe or placebo. The primary composite outcome of nonfatal MI, cardiac death, stroke, or arterial revascularization was significantly reduced in the drug arm (526 events in the drug arm vs 619 in the in the placebo group for a RR reduction of 0.83; 95% CI: 0.74–0.94, P = 0.0021).35 Although there may be some benefit to adding ezetimibe to statin therapy in select patients, addition of ezetimibe to statin therapy has not been shown to be superior to statin monotherapy.
Novel Therapeutic Agents Under Development
In addition to the currently approved therapies listed above, there are a number of nonstatin lipid‐lowering medications currently in various phases of development with a potential target for primary prevention in patients who are statin‐intolerant or as adjunctive medications in those who are unable to reach their lipid goals on statins.
The forerunners in this category include the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. PCSK9 is responsible for targeting the LDL‐receptor (LDL‐R) protein for catalytic degradation, therefore hindering the ability to scavenge more free LDL from the serum. In the presence of PCSK9 inhibitors, the LDL‐R is able to return to the cell surface and remove more circulating LDL‐C from the blood, effectively lowering the concentration of circulating LDL‐C. Multiple agents are being developed to target PCSK9, including fully human monoclonal antibodies (REGN727/SAR236553, Regeneron Pharmaceuticals; AMG145, Amgen Pharmaceuticals; RN316 (PF‐04950615), Pfizer; RG7652, Roche).36 Inhibition of PCSK9 has demonstrated consistent results in many primary‐prevention populations: as monotherapy in the MENDEL trial (LDL‐C lowered by 48%–51% and Lp(a) lowered by 30%) and in statin‐intolerant patients in the Goal Achievement After Utilizing an Anti‐PCSK9 Antibody in Statin‐Intolerant Subjects (GAUSS) trial (LDL‐C lowered by 41%–51%, and up to 63% with ezetemibe).37 It remains to be seen whether they will improve outcomes in large phase III outcomes trials.
Diet and Exercise
Obesity is closely associated with CVD.38 From 1980 to 2000, the average body mass index (BMI) in the United States increased from 25.6 to 28.2. This increase in BMI was estimated to have directly contributed to 25 905 deaths over that time period.4 Lifestyle modification including a heart‐healthy diet, weight loss, and regular aerobic exercise remains the centerpiece for the primary prevention of CVD. In the Italian Diabetes and Exercise Study (IDES), 606 participants with type 2 DM were randomized to a supervised intense aerobic exercise regimen vs counseling alone. The exercise group had significant improvements in systolic and diastolic blood pressure, HDL‐C, LDL‐C, waist circumference, and glycated hemoglobin.39 The American Heart Association has accordingly recommended an ideal level of physical activity to be >150 minutes/week of moderate‐intensity activity or >75 minutes/week of vigorous activity.
The Prevención con Dieta Mediterránea (PREDIMED) study is a multicenter RCT of 772 high‐risk primary‐prevention patients randomized to a Mediterranean diet (rich in olive oil, fruit, vegetables, nuts, and fish, with minimal red meat and sweets) vs a low‐fat diet. After 3 months, significant decreases in CVD risk factors were recorded, including in plasma glucose, systolic blood pressure, and the TC/HDL‐C ratio.40 The PREDIMED investigators conducted another multicenter RCT randomizing high‐risk patients to either a Mediterranean diet with supplemental extra‐virgin olive oil or nuts vs a control group with only dietary instruction. This prospective study was stopped after 4.8 years when a threshold benefit in the intervention groups was met. Major CVD events were significantly reduced in both Mediterranean diet arms.41
Other heart‐healthy diets, including the Dietary Appr‐oaches to Stop Hypertension (DASH) and Optimal Macronutrient Intake Trial for Heart Health (OMNIHeart) diets, have been shown to decrease CVD risk factors such as hypertension, impaired fasting glucose, and cholesterol.42, 43 The OmniHeart trial showed that substitution of saturated fats with protein decreased LDL‐C by 3.3 mg/dL, increased HDL‐C by 1.3 mg/dL, and decreased triglycerides by 15.7 mg/dL (P = 0.01, P = 0.02, P = 0.001, respectively). Data from the Coronary Heart Disease Policy Model predict that a population‐wide decrease in sodium intake of 1200 mg per day would decrease the annual number of CHD events by 60 000 to 120 000 and overall mortality by 44 000 to 92 000 per year.44
Clinical Recommendations
When evaluating a patient for primary prevention of CVD, the first step is assessment of risk. The NCEP ATP III guidelines recommend use of the FRS for hard CHD. Due to the aforementioned limitations in such an approach, we suggest also using either the RRS or the D'Agostino Risk Profile to more accurately assess total 10‐year CVD risk and to avoid underestimation of individuals at higher risk.
For patients with an estimated 10‐year risk of CVD <5%, the focus should be on advocating lifestyle modification, with a heart‐healthy diet and regular aerobic exercise as the focus of therapy. As in patients of all risk levels, strategies for weight loss should be discussed if the patient is overweight, with a goal BMI of <25, and CVD risk factors such as hypertension should be controlled and smoking cessation emphasized. Based on the 2012 Cholesterol Treatment Trialists' meta‐analysis, discussion regarding starting statin therapy for those with elevated cholesterol levels should be initiated. Low‐risk participants without vascular disease had a 39% RR reduction in major vascular events at 5 years when treated with statin therapy compared with control (RR: 0.61, 95% CI: 0.45–0.81); however, the absolute short‐term benefits are less than those in patients at higher risk levels.45
In patients with a moderate risk profile (10‐year risk of 5%–20%), discussion should be especially prioritized regarding initiation of statin therapy with a goal to be on the highest tolerated dose for a goal total cholesterol <200 mg/dL, LDL‐C <100 mg/dL, and triglycerides <150 mg/dL. The LDL‐C goal of <100 mg/dL is more aggressive than guidelines currently suggest because primary‐prevention trials, particularly ASCOT‐LLA and JUPITER, have shown that a decrease in LDL‐C at any level is associated with improved CVD outcomes (Figure 2).17, 19, 20, 46 Further, as with all risk groups, lifestyle modification and controlling other CVD risk factors for ideal cardiovascular health are fundamental.
Figure 2.
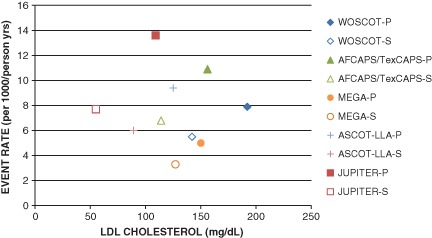
Event rate in placebo vs statin arms in landmark statin primary prevention trials. Abbreviations: AFCAPS/TexCAPS‐P/AFCAPS/TexCAPS‐S, Air Force/Texas Coronary Atherosclerosis Prevention Study Placebo/Statin; ASCOT‐LLA‐P/ASCOT‐LLA‐S, Anglo‐Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm Placebo/Statin; JUPITER‐P/JUPITER‐S, Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin Placebo/Statin; LDL‐C, low‐density lipoprotein cholesterol; LDL‐P, placebo arm low‐density lipoprotein cholesterol; LDL‐S, statin arm low‐density lipoprotein cholesterol; MEGA‐P/MEGA‐S, Primary Prevention of Cardiovascular Disease with Pravastatin in Japan Placebo/Statin; WOSCOPS‐P/WOSCOPS‐S, West of Scotland Coronary Prevention Study Placebo/Statin.
There are some at‐risk patients who may be hesitant to start lipid‐lowering medications for primary prevention. In such cases, noninvasive imaging of coronary artery calcium (CAC) can provide further risk stratification. Detrano and colleagues showed that a CAC score of 101 to 300 in a multiethnic population was associated with an HR of 7.7 for having a coronary event.47 In those meeting JUPITER entry criteria, Blaha and colleagues found that 74% of all coronary events were in the 25% of individuals with CAC scores >100, suggesting that CAC could be used to target subgroups of patients who are expected to derive the most, and the least, absolute benefit from statin treatment.48
The prospective St. Francis Heart Study followed 4903 asymptomatic participants who underwent CAC for 4.3 years and found that CAC predicted CVD events independent of CRP and traditional risk factors, and it was superior to FRS in predicting events. Further, a CAC score >100 was associated with an increased RR of 9.6 for all CVD events.49
For adults with a 10‐year CVD risk score >20%, who have established coronary artery disease or an equivalent risk condition (DM, PAD, abdominal aortic aneurysm), statin therapy is clearly indicated along with lifestyle changes. Given its benefit in decreasing not only cardiovascular events but also mortality, statins comprise first‐line pharmacotherapy in treating dyslipidemia. Although some controversy has been raised regarding the potential for adverse effects with treatment of lower‐risk individuals, statins have been shown to be generally quite safe and efficacious across a wide range of patient profiles, and the American Heart Association and American College of Cardiology echo their priority in use.50 Further, as suggested by Martin and colleagues, chronic kidney disease ≥ stage 2 should be considered a CHD equivalent, and patients with this condition may also benefit from aggressive lipid control.51, 52, 53
Obtaining ideal cardiovascular health begins with lifestyle modifications, including cessation of smoking, heart‐healthy diet, and daily aerobic activity. Based on landmark trials, statins play a crucial role in modifying dyslipidemia and preventing CVD.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Mendis S, Puska P, Norrving B; World Health Organization . Global Atlas on Cardiovascular Disease Prevention and Control: Policies, Strategies, and Interventions Geneva: World Health Organization; 2011. [Google Scholar]
- 2. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 3. Expert Panel on Detection , Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 4. Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. [DOI] [PubMed] [Google Scholar]
- 5. Wijeysundera HC, Machado M, Farahati F, et al. Association of temporal trends in risk factors and treatment uptake with coronary heart disease mortality, 1994–2005. JAMA. 2010;303:1841–1847. [DOI] [PubMed] [Google Scholar]
- 6. Pearson TA, LaCroix AZ, Mead LA, et al. The prediction of midlife coronary heart disease and hypertension in young adults: the Johns Hopkins multiple risk equations. Am J Prev Med. 1990;6(2 suppl):23–28. [PubMed] [Google Scholar]
- 7. Cohen JC, Boerwinkle E, Mosley TH, et al. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. [DOI] [PubMed] [Google Scholar]
- 8. Cooney MT, Dudina AL, Graham IM. Value and limitations of existing scores for the assessment of cardiovascular risk: a review for clinicians. J Am Coll Cardiol. 2009;54:1209–1227. [DOI] [PubMed] [Google Scholar]
- 9. Ridker PM, Paynter NP, Rifai N, et al. C‐reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ridker PM, Buring JE, Rifai N, et al. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. [DOI] [PubMed] [Google Scholar]
- 11. D'Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 12. Cook NR, Paynter NP, Eaton CB, et al. Comparison of the Framingham and Reynolds risk scores for global cardiovascular risk prediction in the multiethnic Women's Health Initiative. Circulation. 2012;125:1748–1756, S1–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joshi PH, Chaudhari S, Blaha MJ, et al. A point‐by‐point response to recent arguments against the use of statins in primary prevention: this statement is endorsed by the American Society for Preventive Cardiology. Clin Cardiol. 2012;35:404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tabas I, Williams KJ, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. [DOI] [PubMed] [Google Scholar]
- 15. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. [DOI] [PubMed] [Google Scholar]
- 16. Ford I, Murray H, Packard CJ, et al. Long‐term follow‐up of the West of Scotland Coronary Prevention Study. N Engl J Med. 2007;357:1477–1486. [DOI] [PubMed] [Google Scholar]
- 17. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. [DOI] [PubMed] [Google Scholar]
- 18. Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–1163. [DOI] [PubMed] [Google Scholar]
- 19. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐average cholesterol concentrations, in the Anglo‐Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT‐LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. [DOI] [PubMed] [Google Scholar]
- 20. Ridker PM, Danielson E, Fonseca FA, et al; for the JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 21. Brugts JJ, Yetgin T, Hoeks SE, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta‐analysis of randomised controlled trials. BMJ. 2009;338:b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ray KK, Seshasai SR, Erqou S, et al. Statins and all‐cause mortality in high‐risk primary prevention: a meta‐analysis of 11 randomized controlled trials involving 65 229 participants. Arch Intern Med. 2010;170:1024–1031. [DOI] [PubMed] [Google Scholar]
- 23. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ridker PM, Pradhan A, MacFadyen JG, et al. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alberton M, Wu P, Druyts E, et al. Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta‐analysis. QJM. 2012;105:145–157. [DOI] [PubMed] [Google Scholar]
- 26. Taylor AJ, Sullenberger LE, Lee HJ, et al. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double‐blind, placebo‐controlled study of extended‐release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110:3512–3517. [DOI] [PubMed] [Google Scholar]
- 27. Lee JM, Robson MD, Yu LM, et al. Effects of high‐dose modified‐release nicotinic acid on atherosclerosis and vascular function: a randomized, placebo‐controlled, magnetic resonance imaging study. J Am Coll Cardiol. 2009;54:1787–1794. [DOI] [PubMed] [Google Scholar]
- 28. Taylor AJ, Villines TC, Stanek EJ, et al. Extended‐release niacin or ezetimibe and carotid intima‐media thickness. N Engl J Med. 2009;361:2113–2122. [DOI] [PubMed] [Google Scholar]
- 29. Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary‐prevention trial with gemfibrozil in middle‐aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–1245. [DOI] [PubMed] [Google Scholar]
- 30. Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu FB, Bronner L, Willett WC, et al. Fish and omega‐3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–1821. [DOI] [PubMed] [Google Scholar]
- 32. Ascherio A, Rimm EB, Stampfer MJ, et al. Dietary intake of marine n‐3 fatty acids, fish intake, and the risk of coronary disease among men. N Engl J Med. 1995;332:977–982. [DOI] [PubMed] [Google Scholar]
- 33. Saito Y, Yokoyama M, Origasa H, et al. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub‐analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis. 2008;200:135–140. [DOI] [PubMed] [Google Scholar]
- 34. Kastelein JJ, Sager PT, de Groot E, et al. Comparison of ezetimibe plus simvastatin versus simvastatin monotherapy on atherosclerosis progression in familial hypercholesterolemia: design and rationale of the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial. Am Heart J. 2005;149:234–239. [DOI] [PubMed] [Google Scholar]
- 35. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo‐controlled trial. Lancet. 2011;377:2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qian YW, Schmidt RJ, Zhang Y, et al. Secreted PCSK9 downregulates low‐density lipoprotein receptor through receptor‐mediated endocytosis. J Lipid Res. 2007;48:1488–1498. [DOI] [PubMed] [Google Scholar]
- 37. Koren MJ, Scott R, Kim JB, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double‐blind, placebo‐controlled, phase 2 study. Lancet. 2012;380:1995–2006. [DOI] [PubMed] [Google Scholar]
- 38. Canoy D, Boekholdt SM, Wareham N, et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population‐based prospective study. Circulation. 2007;116:2933–2943. [DOI] [PubMed] [Google Scholar]
- 39. Balducci S, Zanuso S, Nicolucci A, et al. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES). Arch Intern Med. 2010;170:1794–1803. [DOI] [PubMed] [Google Scholar]
- 40. Estruch R, Martínez‐González MA, Corella D, et al. Effects of a Mediterranean‐style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145:1–11. [DOI] [PubMed] [Google Scholar]
- 41. Estruch R, Ros E, Salas‐Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 42. Sacks FM, Svetkey LP, Vollmer WM, et al; DASH‐Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 43. Appel LJ, Sacks FM, Carey VJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–2464. [DOI] [PubMed] [Google Scholar]
- 44. Bibbins‐Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mihaylova B, Emberson J, Blackwell L, et al.; Cholesterol Treatment Trialists' (CTT) Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta‐analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martin SS, Blumenthal RS, Miller M. LDL cholesterol: the lower the better [published correction appears in Med Clin North Am. 2012;96:xv–xvi]. Med Clin North Am. 2012;96:13–26. [DOI] [PubMed]
- 47. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 48. Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C‐reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population‐based cohort study. Lancet. 2011;378:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arad Y, Goodman KJ, Roth M, et al. Coronary calcification, coronary disease risk factors, C‐reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46:158–165. [DOI] [PubMed] [Google Scholar]
- 50. Kushner FG, Hand M, Smith SC, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241. [DOI] [PubMed] [Google Scholar]
- 51. Martin SS, Metkus TS, Horne A, et al. Waiting for the National Cholesterol Education Program Adult Treatment Panel IV Guidelines, and in the meantime, some challenges and recommendations. Am J Cardiol. 2012;110:307–313. [DOI] [PubMed] [Google Scholar]
- 52. Catapano AL, Reiner Z, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis. 2011;217(suppl 1):S1–S44. [DOI] [PubMed] [Google Scholar]
- 53. Davidson MH, Robinson JG. Safety of aggressive lipid management. J Am Coll Cardiol. 2007;49:1753–1762. [DOI] [PubMed] [Google Scholar]


