Abstract
Background
Cardiac resynchronization therapy (CRT) is an established therapy for patients with chronic heart failure (CHF) and a broad QRS complex. Gender‐related safety and efficacy data are necessary for informed patient decision‐making for female patients with CHF. The aim of the study was to assess the effects of gender on the outcome of CRT in highly symptomatic heart failure patients.
Hypothesis
Gender may have an effect on the outcome of heart failure patients undergoing cardiac resynchronisation therapy.
Methods
The study analyzed the 2‐year follow‐up of 393 New York Heart Association (NYHA) class III/IV patients with a class I CRT indication enrolled in the Management of Atrial Fibrillation Suppression in AF‐HF Comorbidity Therapy (MASCOT) study.
Results
In female patients (n = 82), compared with male patients (n = 311), CHF was more often due to dilated cardiomyopathy (74% vs 44%, respectively; P < 0.0001). Females also had a more impaired quality‐of‐life score and a smaller left ventricular end‐diastolic diameter (LVEDD). Women were less likely than men to have received a CRT defibrillator (35% vs 61%, respectively; P < 0.0001). After 2 years, the devices had delivered more biventricular pacing in women than in men (96% ± 13% vs 94% ± 13%, respectively; P < 0.0004). Women had a greater reduction in LVEDD than did men (−8.2 mm ± 11.1 mm vs −1.1 mm ± 22.1 mm, respectively; P < 0.02). Both genders improved similarly in NYHA functional class. Women reported greater improvement than men in quality‐of‐life score (−21.1 ± 26.5 vs −16.2 ± 22.1, respectively; P < 0.0001). After adjustment for cardiovascular history, women had lower all‐cause mortality (P = 0.0007), less cardiac death (P = 0.04), and fewer hospitalizations for worsening heart failure (P = 0.01).
Conclusions
Females exhibited a better response to CRT than did males. Because females have such impressive benefits from CRT, improved screening and advocacy for CRT implantation in women should be considered.
Introduction
Women and men respond differently to medical treatment of cardiovascular diseases.1, 2 The current management of patients with advanced chronic systolic heart failure (CHF) includes medical as well as nonpharmacological treatment to alleviate symptoms, prevent major morbidity, and lower mortality.3 Cardiac resynchronization therapy (CRT) is well established for patients with systolic CHF and bundle branch block on the resting electrocardiogram.4
Gender‐related differences have been observed for nonpharmacological treatments such as implantable cardioverter‐defibrillator therapy.5 Data from CRT studies such as Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) and Cardiac Resynchronization–Heart Failure (CARE‐HF) revealed no gender‐related differences in survival,6, 7 whereas the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy (MADIT‐CRT) demonstrated a greater benefit for women compared with men from CRT with defibrillator (CRT‐D) than from an implantable cardioverter‐defibrillator alone.8, 9 For proper patient decision‐making in female CHF patients, gender‐specific safety and efficacy data based on publications are mandatory.10 The purpose of the present analysis was to assess the effects of gender on the outcome of CRT.
Patient Population and Methods
Patients included in this analysis were enrolled in the Management of Atrial Fibrillation Suppression in AF‐HF Comorbidity Therapy (MASCOT) study and followed for 2 years.11 Patients were eligible for enrollment if they presented with New York Heart Association (NYHA) functional class III or IV despite optimal medical therapy, had a QRS duration ≥130 ms, a left ventricular ejection fraction (LVEF) ≤35%, and a left ventricular end‐diastolic diameter (LVEDD) ≥55 mm.4 Exclusion criteria included permanent atrial fibrillation (AF) and myocardial infarction, cardiac surgery, or a coronary revascularization procedure within the previous 3 months. Implanted devices were the CRT‐pacemakers (CRT‐P) Frontier (model 5510) or Frontier II (model 5596) or the CRT‐Ds Epic HF (model V‐350) or Atlas HF (model V‐341; all from St. Jude Medical, Sylmar, CA). The patients received a CRT‐P or CRT‐D device upon the clinical decision of each center.
Study Design
The MASCOT study was a multicenter, single‐blind, randomized, parallel study that examined the safety and efficacy of a specific atrial overdrive pacing algorithm in CRT recipients during the 2‐year follow‐up.11 For this purpose, the AF suppression algorithm was programmed either to “on” or “off.” Follow‐up visits were scheduled at hospital discharge and at 3, 6, 9, 12, and 24 months after device implantation. The study protocol was reviewed and approved by the institutional ethics committee of each participating medical center. All patients granted their informed consent to participate in the study.
Data Collection and Analysis
The present analysis included the patients who completed the 2‐year follow‐up or died during the follow‐up period. For each patient who died during the follow‐up period, the reason for study termination, including a patient death form with a death classification, was completed. At each follow‐up visit, the number of and reasons for hospital admissions were recorded.
All patients underwent echocardiography in the left lateral decubitus position before and 2 years after implantation. Echocardiographic changes after CRT were assessed by comparing left‐ventricular end‐systolic volume, left‐ventricular end‐diastolic volume (LVEDV), and LVEF from baseline measurements.
Changes in NYHA functional class and in the quality‐of‐life score were measured with the Minnesota Living with Heart Failure Questionnaire. These variables were used to describe the clinical outcome. Other variables for the clinical outcome were all‐cause mortality, cardiac death, sudden death, sudden cardiac death, hospitalization, and hospitalization for worsening CHF. The presence of AF was also recorded.
Statistical Analysis
Data collected from the 2 groups were compared at baseline and after final follow‐up. Normality of the data was verified using box‐and‐whisker plots, normal probability plots, and Kolmogorov‐Smirnov tests for normality. Baseline data for all patients with available data were included in the analysis presented in Table 1. Continuous variables from the normal distribution were compared using the 2‐sample t test for independent variables. The nonparametric Wilcoxon signed‐rank test was used for non‐normal variables. Categorical variables were compared using the Fisher exact test or the χ2 test, as applicable. A P value < 0.05 was considered significant.
Table 1.
Baseline Characteristics of Male and Female Subjects
| Male, n = 311 | Female, n = 82 | P Value | OR (95% CI) | |
|---|---|---|---|---|
| Demographic data | ||||
| Age, y | 67.80 ± 9.53 | 67.76 ± 9.47 | 0.90 | NA |
| Weight, kg | 78.48 ± 14.26 | 68.81 ± 13.21 | <0.0001 | NA |
| Height, m | 1.72 ± 0.07 | 1.61 ± 0.06 | 0.003 | |
| BMI, kg/m2 | 26.46 ± 4.08 | 26.67 ± 4.74 | 0.90 | |
| Body surface area, m2 | 1.91 ± 0.18 | 1.72 ± 0.16 | <0.0001 | |
| SBP, mm Hg | 119.74 ± 18.27 | 118.41 ± 19.34 | 0.69 | NA |
| DBP, mm Hg | 72.02 ± 10.78 | 69.39 ± 10.70 | 0.12 | NA |
| QoL score | 42.32 ± 20.91 | 54.18 ± 20.04 | <0.0001 | NA |
| NYHA functional class, n (%) | ||||
| III | 265 (85.21) | 73 (89.02) | 0.09 | NA |
| IV | 44 (14.15) | 7 (8.54) | ||
| CHF etiology, n (%) | <0.0001 | 0.27 (0.16‐0.47) | ||
| Ischemic etiology | 173 (55.63) | 21 (25.61) | ||
| Dilated cardiomyopathy | 138 (44.37) | 61 (74.39) | ||
| Medications, n (%) | ||||
| ACEI | 215 (69.13) | 65 (79.27) | 0.07 | 1.71 (0.95–3.07) |
| ARB | 57 (18.33) | 14 (17.07) | 0.79 | 0.92 (0.48–1.75) |
| β‐Blocker | 220 (70.74) | 63 (76.83) | 0.27 | 1.37 (0.78–2.42) |
| Diuretic | 294 (94.53) | 77 (93.90) | 0.82 | 0.89 (0.32–2.49) |
| Spironolactone | 63 (20.26) | 18 (21.95) | 0.74 | 0.9 (0.5–1.63) |
| Digitalis | 74 (23.79) | 33 (40.24) | 0.0029 | 2.16 (1.29–3.6) |
| Antiarrhythmic drugs | 108 (34.73) | 11 (13.41) | 0.0002 | 0.29 (0.15–0.57) |
| Comorbidities, n (%) | ||||
| DM | 89 (28.62) | 23 (28.05) | 0.92 | 1.03 (0.6–1.77) |
| HT | 143 (45.98) | 34 (41.46) | 0.46 | 1.2 (0.73–1.97) |
| Chronic renal insufficiency | 46 (14.79) | 7 (8.54) | 0.14 | 1.86 (0.81–4.29) |
| History of AF, n (%) | ||||
| Paroxysmal AF | 34 (10.93) | 10 (12.20) | 0.75 | 0.88 (0.42–1.87) |
| Persistent AF | 0 (0) | 0 (0) | NA | NA |
| 12‐lead ECG | ||||
| QRS duration, ms | 162.24 ± 25.98 | 168.25 ± 36.06 | 0.30 | NA |
| AF at baseline, yes, n (%) | 60 (19.29) | 10 (12.20) | 0.14 | 0.58 (0.28–1.19) |
| Echocardiographic parameters | ||||
| LVEDD, mm | 71.21 ± 9.85 | 66.89 ± 9.41 | 0.0009 | NA |
| LVEF, % | 25.22 ± 6.48 | 25.30 ± 7.11 | 0.68 | NA |
| Left atrial diameter, longitudinal view | 47.91 ± 8.59 | 44.93 ± 10.75 | 0.02 | NA |
| Device type, n (%) | ||||
| CRT‐P | 119 (38.26) | 53 (64.63) | <0.0001 | 0.34 (0.2–0.56) |
| CRT‐D | 192 (61.74) | 29 (35.37) |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; CHF, chronic heart failure; CI, confidence interval; CRT, cardiac resynchronization therapy; CRT‐D, CRT‐defibrillator; CRT‐P, CRT‐pacemaker; DBP, diastolic blood pressure; DM, diabetes mellitus; ECG, electrocardiogram; HT, hypertension; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; NA, not applicable; NYHA, New York Heart Association; OR, odds ratio; QoL, quality of life; SBP, systolic blood pressure.
Results
Between September 2003 and March 2006, 405 patients at 34 medical centers from 10 different countries were enrolled in the MASCOT trial (see Appendix). Twelve patients were excluded because they were not randomized in the MASCOT study and were excluded from the following analyses.
From the 393 patients analyzed, 21% of patients undergoing CRT implantation were female (Table 1). As compared with males, females had a lower body weight, a greater frequency of CHF due to dilated cardiomyopathy, a more impaired quality‐of‐life score, and a smaller LVEDD. Females more often received a CRT‐P rather than a CRT‐D.
Changes During Follow‐up
At the final follow‐up, women had more frequent biventricular pacing (Table 2). In the echocardiographic assessment, women had a greater reduction in LVEDD (Figure 1). Left‐ventricular ejection fraction improved in women, but men had a significantly greater improvement in LVEF. Both genders had a similar decrease of their NYHA functional class, with no statistically significant difference between women and men. Women reported an improvement in quality‐of‐life score that was nearly statistically significant.
Table 2.
Clinical Efficacy Outcome at 24 Months or Termination of the Study
| Male, n = 311 | Female, n = 82 | P Value | OR (95% CI) | |
|---|---|---|---|---|
| Atrial pacing (%) | 54.11 ± 40.36 | 58.26 ± 39.61 | 0.28 | NA |
| Ventricular pacing (%) | 94.53 ± 13.71 | 96.32 ± 13.68 | 0.0004 | NA |
| Effect on NYHA functional class | ||||
| Difference between baseline and last NYHA class | −1.04 ± 0.80 | −1.00 ± 0.70 | 0.79 | NA |
| Patients who improved ≥1 NYHA class, n (%) | 154 (78.57) | 41 (75.93) | 0.68 | 1.16 (0.57–2.37) |
| Effect on LVEF | ||||
| Difference between baseline and last LVEF (%) | 13.32 ± 13.02 | 7.23 ± 11.62 | 0.02 | NA |
| Patients who improved ≥5% in LVEF (%) | 15 (13.39) | 3 (10.34) | 0.14 | 0.52 (0.22–1.24) |
| Effect on LVEDD | ||||
| Difference between baseline and last LVEDD (%) | −1.14 ± 22.05 | −8.27 ± 11.14 | 0.02 | NA |
| Effect on QoL score | ||||
| Difference between baseline and final QoL (%) | −16.20 ± 22.19 | −21.19 ± 26.56 | <0.0001 | NA |
| Clinical outcome, n (%) | ||||
| All‐cause mortality | 62 (19.94) | 4 (4.88) | 0.0007 | 4.86 (1.71–13.8) |
| Cardiac death | 30 (9.65) | 2 (2.44) | 0.04 | 4.27 (1.0–18.3) |
| Sudden death | 14 (4.50) | 2 (2.44) | 0.54 | 1.89 (0.42–8.47) |
| Sudden cardiac death | 10 (3.22) | 1 (1.22) | 0.47 | 2.69 (0.34–21.3) |
| Hospitalization | 129 (41) | 31 (37) | 0.55 | 1.17 (0.71–1.92) |
| Hospitalization due to worsening HF | 73 (23.47) | 9 (10.98) | 0.01 | 2.49 (1.19–5.22) |
| AF at last visit | 27 (8.68) | 6 (7.32) | 0.82 | 1.2 (0.48–3.02) |
Abbreviations: AF, atrial fibrillation; CI, confidence interval; HF, heart failure; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; NA, not applicable; NYHA, New York Heart Association; OR, odds ratio; QoL, quality of life.
Figure 1.
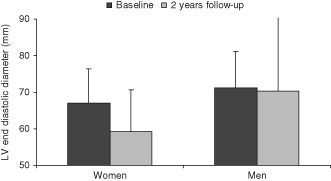
At baseline, women had a significantly smaller LV end‐diastolic diameter than did men (P = 0.0009); after 2‐year follow‐up, women had a significantly greater reduction of LV end‐diastolic diameter than did men (P < 0.02). Abbreviations: LV, left ventricular.
All‐Cause Mortality and Heart Failure Hospitalization at Final Follow‐up
After adjustment for cardiovascular history, women had lower all‐cause mortality, less cardiac death, and fewer hospitalizations due to worsening heart failure (Figure 2). No differences were detected for sudden death, all‐cause hospitalization, and the presence of AF at the last follow‐up visit.
Figure 2.
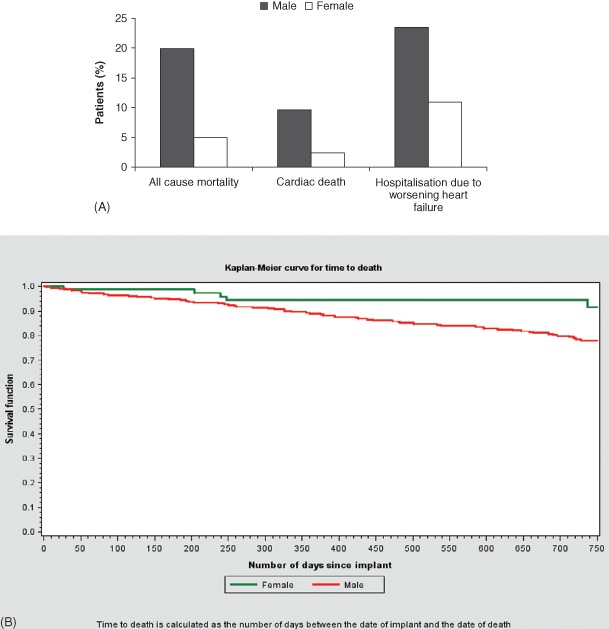
(A) After 2‐year follow‐up, women had lower all‐cause mortality (P = 0.008), lower cardiac mortality (P = 0.04), and fewer hospitalizations due to worsening heart failure (P = 0.045) than did men. (B) The Kaplan‐Meier curve for time to death shows a significantly better survival for women than for men (P = 0.006).
Discussion
Gender Distribution in Cardiac Resynchronization Therapy Implantation
In MASCOT, 21% of patients undergoing CRT implantation were female. The frequency of females in 6 CRT studies ranged from 15.2% to 38%, with a mean value of 23.8% (Table 3). Even in more recent trials, the percentage of women receiving CRT remained unchanged,12, 13 and similar gender distribution has been observed in Medicare patients.14 Our data contain similar rates as these reports with regard to gender distribution in CRT recipients.
Table 3.
Gender Distribution in Studies Assessing the Outcome of Cardiac Resynchronization Therapy Patients
| Author | Publication and Year | Total Population | Women, No. (%) | Men, No. (%) |
|---|---|---|---|---|
| Cleland et al7 | N Engl J Med 2005 | 409 | 105 (26) | 304 (74) |
| Bleeker et al18 | Pacing Clin Electrophysiol 2005 | 173 | 36 (20.8) | 137 (79.2) |
| Lilli et al23 | Pacing Clin Electrophysiol 2007 | 195 | 46 (23.5) | 149 (76.5) |
| Zardkoohi et al19 | Pacing Clin Electrophysiol 2007 | 117 | 26 (22.2) | 91 (77.8) |
| Alaeddini et al14 registry | Pacing Clin Electrophysiol 2008 | 2590 | 659 (25) | 1931 (75) |
| 26593 | 6928 (26) | 19646 (74) | ||
| 42196 | 11286 (27) | 30889 (73) | ||
| Moss et al8 | N Engl J Med 2009 | 1089 | 275 (25.3) | 814 (74.7) |
| Dickstein et al30 | Eur Heart J 2009 | 2438 | 659 (27) | 1779 (73) |
| Tang et al31 | N Engl J Med 2010 | 894 | 136 (15.2) | 758 (84.8) |
| Leyva et al20 | Pacing Clin Electrophysiol 2011 | 550 | 122 (22) | 428 (78) |
| Mooyaart et al12 | Am J Cardiol 2011 | 578 | 147 (25.4) | 431 (74.6) |
| Xu et al21 | J Cardiovasc Electrophysiol 2012 | 728 | 166 (22.8) | 562 (77.2) |
| Zabarovskaja et al13 | Europace 2012 | 619 | 118 (19) | 501 (81) |
| Celikyurt et al32 | Clin Cardiol 2013 | 105 | 40 (38) | 65 (62) |
| All (with exception of Alaeddini et al) | 7895 | 1876 (23.8) | 6019 (76.2) |
The prevalence of CHF is equally distributed between genders in epidemiological surveys.15, 16 Between 2002 and 2004, more US women than men were admitted to the hospital due to worsening CHF.14 Although there may be equal prevalence of CHF in men and women, women are more likely to have diastolic heart failure with preserved systolic function. It is possible that, in many cases, women may not be considered candidates for CRT based on having preserved systolic function. On the other hand, gender disparities and underutilization of therapies have been previously reported for other diagnostic and therapeutic procedures in women with cardiovascular diseases.17 It is not clear whether the observed differences are based on gender disparities or they result from underutilization similar to other procedures.
Not surprisingly, women had lower body weight and a smaller LVEDV. The most consistent gender‐related difference was the more frequent etiology of CHF due to dilated cardiomyopathy in women compared with men.12, 18, 19, 20, 21, 22 One exception is a report from Lilli and co‐workers.23 This difference may be the result of the greater incidence of coronary artery disease in men. As women had smaller heart diameters as measured by echocardiography (eg, the LVEDD), this may translate into gender‐specific effects on cardiac remodeling.24 The assumption, however, that lower body weight and decreased body height, and thus a smaller female body volume, accounts for the gender differences in response to CRT was not supported in the analysis of Cheng et al.25 In addition, patients with CHF of ischemic etiology had an established indication for an implantable defibrillator very early. These patients, who were mainly male, seemed to receive a CRT‐D more often than did patients whose CHF was due to dilated cardiomyopathy, who were mainly female.
Echocardiographic Changes After Cardiac Resynchronization Therapy Implantation
The echocardiographic assessments after 2‐year follow‐up revealed a greater reduction of LVEDD in women compared with men. A more pronounced improvement of LVEF, however, was seen in men. Both are valid echocardiographic findings of beneficial reverse remodeling and indicate gender‐related differences in cardiac remodeling. Two other investigations had similar findings but also demonstrated a greater improvement of the LVEF in women than in men.21, 23 An additional reason for these results may reflect the observation that ventricular remodeling occurs more often in patients with a smaller baseline LVEDD, which was more often present in women.26
Mortality and Morbidity After Cardiac Resynchronization Therapy Implantation
No significant gender‐related differences in overall survival were reported in the COMPANION and CARE‐HF trials, as well as in 3 additional investigations.6, 7, 18, 19, 23 A multinomial logistic regression analysis to identify risk factors for death after CRT implantation found a nonsignificant P value for male gender.27 In a retrospective cohort study, Xu et al reported fewer deaths in women compared with men over a median follow‐up of 3.7 years.21 After adjusting for multiple variables, the survival benefit from CRT was not statistically different between men and women.
Because ischemic etiology has been identified as a predictor of death after CRT implantation, we performed a multivariate analysis.17 Adjusting for this confounder, we observed an improved survival in women compared with men. Our results are similar to those from a study including 555 patients and with median follow‐up of 36.2 months. Female gender was independently associated with lower mortality after CRT, and the benefit continuously increased after device implantation.20 In the study from Mooyaart et al with 578 patients, women had a lower 2‐year all‐cause mortality rate compared with men (8% vs 15%, respectively).12 Our study supports the gender‐specific responses to CRT and points out that the additional benefit for women is evident as early as 2 years after implantation.
Possible explanations for these findings in our analysis can be the higher percentage of biventricular pacing in women. The importance of biventricular pacing with regard to outcomes has been previously reported.21 One reason for this difference may the trend toward more frequent AF in men compared with women, in combination with a better rate control in the case of AF. Women received digitalis more frequently than men, but the occurrence of AF is not significantly different and the prescription of β‐blockers was similar in both groups. Second, males had more often received antiarrhythmic drugs. Thirdly, as discussed previously, women may have a more robust response to CRT.28 Fourthly, the reduction in end‐diastolic volume in the MADIT‐CRT trial was a predictor for better prognosis after device implantation.29 In our study, these changes occurred more frequently in women than in men. Because the definitive underlying mechanisms remain unclear, there is a need to better understand the mechanisms responsible for these gender‐related differences.
Study Limitations
This is a post hoc analysis that was not part of the original endpoints of the MASCOT trial. The randomization in MASCOT evaluating the overdrive pacing algorithm should not have an impact on this post hoc analysis, because randomization was similar in men and women. The study was not powered to detect differences in mortality for the 2 groups. Finally, there were differences in the percentages of male and females receiving a CRT‐D. However, there is yet no evidence of improved patient survival with CRT‐D instead of CRT‐P.6
Conclusion
After adjusting for confounders, mainly for the etiology of CHF, female gender significantly contributed in our analysis to a better long‐term outcome of CRT. Because females have such impressive benefits from CRT, improved screening and advocacy for CRT implantation in women should be considered.
The following investigators and institutions participated in the Management of Atrial Fibrillation Suppression in AF‐HF Comorbidity Therapy Study (MASCOT): Fiorenzo Acquati, Ospedale Valduce, Como, Italy; Francesco Alessandrini, Università Cattolica del Sacro Cuore, Campobasso, Italy; Maria‐Grazia Bongiorni, Ospedale Cisanello, Pisa, Italy; Johannes Brachmann, Klinikum Coburg, Coburg, Germany; Valeria Calvi, Ospedale Ferrarotto, Catania, Italy; Ngai‐yin Chan, Princess Margaret Hospital, Hong Kong, China; Per Dahl Christensen, Sygehus Viborg, Viborg, Denmark; Pierre Fiorello, CMC Parly II, Le Chesnay, France; Daniel Flammang, Centre Hospitalier Général Girac, Saint Michel, France; Francesco Foti, Ospedale di Melegnano, Melegnano, Italy; Robert Frank, Hôpital Pitié‐Salpêtrière, Paris, France; Antonio Fusco, Clinica Pederzoli, Peschiera del Garda, Italy; Grahame Goode, Blackpool Victoria Hospital, Blackpool, United Kingdom; Daniel Gras, Nouvelles Cliniques Nantaises, Nantes, France; Michael Gruska, Hanusch Krankenhaus, Vienna, Austria; Gaël Jauvert, InParys, Saint‐Cloud, France; Salem Kachboura, CHU Abderrahmane Mami, Ariana, Tunisia; Gert Kaltofen, Klinikum Chemnitz gGmbH Krankenhaus, Chemnitz, Germany; Wolfgang Kiowski, Herzgefäss Zentrum Klinik im Park, Zürich, Zürich, Switzerland; Francesco Lisi, Azienda Ospedaliera Cannizzaro, Catania, Italy; Themistoklis Maounis, Onassis Cardiac Surgery Center, Athens, Greece; Eraldo Occhetta, Ospedale Maggiore della Carita, Novara, Italy; Luigi Padeletti, Ospedale Careggi, Florence, Italy; Olivier Piot, Centre Cardiologique du Nord, St. Denis, France; Jean‐Ernst Poulard, Centre Hospitalier Général, Abbeville, France; Jean‐Luc Rey, CHRU Hôpital Sud, Amiens, France; Nadir Saoudi, Centre Hospitalier Princesse Grace, Monaco; Andreas Schuchert, Universitätsklinikum Hamburg‐Eppendorf, Hamburg, Germany; Olivier Thomas, Clinique Ambroise Paré, Neuilly‐sur‐Seine, France; Bernardo Tuccillo, Ospedale Loreto Mare, Naples, Italy; Thomas Vesterlund, Aalborg Hospital, Aalborg, Denmark; Paul Vock, A.ö. KH der Stadt St. Pölten, St Pölten, Austria; Arnd Weide, Kardiologische Gemeinschaftspraxis, Hannover, Germany; Paolo Zecchi, Policlinico Gemelli, Rome, Italy.
A.S. was an advisory board member of the MASCOT study for St. Jude Medical. C.M. and T.M. have had no involvement that might raise the question of bias in the work reported. R.F. was an advisory board member of the MASCOT study for St. Jude Medical. R.E. and A.P. are full‐time employees of St. Jude Medical. L.P. is a consultant for Boston Scientific, Medtronic, St. Jude Medical, and Sorin Biomedica. The MASCOT study was sponsored by St. Jude Medical.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Shekelle PG, Rich MW, Morton SC, et al. Efficacy of angiotensin‐converting enzyme inhibitors and beta‐blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta‐analysis of major clinical trials. J Am Coll Cardiol. 2003;41:1529–1538. [DOI] [PubMed] [Google Scholar]
- 2. Rathore SS, Wang Y, Krumholz HM. Sex‐based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med. 2002;347:1403–1411. [DOI] [PubMed] [Google Scholar]
- 3. McMurray JV, Adamopoulos S, Stefan D, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Eur Heart J. 2012:33;1787–1847. [DOI] [PubMed] [Google Scholar]
- 4. Dickstein K, Vardas PE, Auricchio A, et al; 2010 Focused Update of ESC Guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC Guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Europace. 2010;12:1526–1536. [DOI] [PubMed] [Google Scholar]
- 5. Peterson PN, Daugherty SL, Wang Y, et al; National Cardiovascular Data Registry . Gender differences in procedure‐related adverse events in patients receiving implantable cardioverter‐defibrillator therapy. Circulation. 2009;119:1078–1084. [DOI] [PubMed] [Google Scholar]
- 6. Bristow MR, Saxon LA, Boehmer J, et al; Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) Investigators . Cardiac resynchronization therapy with or without implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 7. Cleland JG, Daubert JC, Erdmann E, et al; Cardiac Resynchronization–Heart Failure (CARE‐HF) Study Investigators . The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1349. [DOI] [PubMed] [Google Scholar]
- 8. Moss AJ, Hall WJ, Cannom DS, et al. Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med. 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]
- 9. Arshad A, Moss AJ, Foster E, et al. Cardiac resynchronization therapy is more effective in women than in men the MADIT‐CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy) trial. J Am Coll Cardiol. 2011;57:813–820. [DOI] [PubMed] [Google Scholar]
- 10. Dhruva SS, Redberg RF. Evaluating sex differences in medical device clinical trials: time for action. JAMA. 2012;307:1145–1146. [DOI] [PubMed] [Google Scholar]
- 11. Padeletti L, Muto C, Maounis T, et al; Management of Atrial Fibrillation Suppression in AF‐HF Comorbidity Therapy Study Group . Atrial fibrillation in recipients of cardiac resynchronization therapy device: 1‐year results of the randomized MASCOT trial. Am Heart J. 2008;156:520–526. [DOI] [PubMed] [Google Scholar]
- 12. Mooyaart EA, Marsan NA, van Bommel RJ, et al. Comparison of long‐term survival of men versus women with heart failure treated with cardiac resynchronization therapy. Am J Cardiol. 2011;108:63–68. [DOI] [PubMed] [Google Scholar]
- 13. Zabarovskaja S, Gadler F, Braunschweig F, et al. Women have better long‐term prognosis than men after cardiac resynchronization therapy. Europace. 2012;14:1148–1155. [DOI] [PubMed] [Google Scholar]
- 14. Alaeddini J, Wood MA, Amin MS, et al. Gender disparity in the use of cardiac resynchronization therapy in the United States. Pacing Clin Electrophysiol. 2008;31:468–472. [DOI] [PubMed] [Google Scholar]
- 15. Levy D, Kenchaiah S, Larson MG, et al. Long‐term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. [DOI] [PubMed] [Google Scholar]
- 16. Goldberg RJ, Spencer FA, Farmer C, et al. Incidence and hospital death rates associated with heart failure: a community‐wide perspective. Am J Med. 2005;118:728–734. [DOI] [PubMed] [Google Scholar]
- 17. Lansky AJ, Hochman JS, Ward PA, et al. Percutaneous coronary intervention and adjunctive pharmacotherapy in women: a statement for healthcare professionals from the American Heart Association. Circulation. 2005;111:940–953. [DOI] [PubMed] [Google Scholar]
- 18. Bleeker GB, Schalij MJ, Boersma E, et al. Does a gender difference in response to cardiac resynchronization therapy exist? Pacing Clin Electrophysiol. 2005;28:1271–1275. [DOI] [PubMed] [Google Scholar]
- 19. Zardkoohi O, Nandigam V, Murray L, et al. The impact of age and gender on cardiac resynchronization therapy outcome. Pacing Clin Electrophysiol. 2007;30:1344–1348. [DOI] [PubMed] [Google Scholar]
- 20. Leyva F, Foley PW, Chalil S, et al. Female gender is associated with a better outcome after cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2011;34:82–88. [DOI] [PubMed] [Google Scholar]
- 21. Xu YZ, Friedman PA, Webster T, et al. Cardiac resynchronization therapy: do women benefit more than men? J Cardiovasc Electrophysiol. 2012;23:172–178. [DOI] [PubMed] [Google Scholar]
- 22. Gasparini M, Lunati M, Santini M, et al; on behalf of the Insync/Insync ICD Italian Registry . Long‐term survival in patients treated with cardiac resynchronization therapy: a 3‐year follow‐up study from the InSync/InSync ICD Italian Registry. Pacing Clin Electrophysiol. 2006;29:S2–S10. [DOI] [PubMed] [Google Scholar]
- 23. Lilli A, Ricciardi G, Porciani MC, et al. Cardiac resynchronization therapy: gender related differences in left ventricular reverse remodeling. Pacing Clin Electrophysiol. 2007;30:1349–1355. [DOI] [PubMed] [Google Scholar]
- 24. Tamura T, Said S, Gerdes A. Gender‐related differences in myocyte remodeling in progression to heart failure. Hypertension. 1999;33:676–680. [DOI] [PubMed] [Google Scholar]
- 25. Cheng A, Gold MR, Waggoner AD, et al. Potential mechanisms underlying the effect of gender on response to cardiac resynchronization therapy: insights from the SMART‐AV multicenter trial. Heart Rhythm. 2012;9:736–741. [DOI] [PubMed] [Google Scholar]
- 26. Stellbrink C, Breithardt OA, Franke A, et al; PATCH‐CHF investigators . Impact of cardiac resynchronization therapy using hemodynamically optimized pacing on left ventricular remodeling in patients with congestive heart failure and ventricular conduction disturbances. J Am Coll Cardiol. 2001;38:1957–1965. [DOI] [PubMed] [Google Scholar]
- 27. Bai R, Di Biase L, Elayi C, et al. Mortality of heart failure patients after cardiac resynchronization therapy: identification of predictors. J Cardiovasc Electrophysiol. 2008;19:1259–1265. [DOI] [PubMed] [Google Scholar]
- 28. Kirubakaran S, Ladwiniec A, Arujuna A, et al. Male gender and chronic obstructive pulmonary disease predict a poor clinical response in patients undergoing cardiac resynchronisation therapy. Int J Clin Pract. 2011;65:281–288. [DOI] [PubMed] [Google Scholar]
- 29. Solomon SD, Foster E, Bourgoun M, et al. Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: Multicenter Automatic Defibrillator Implantation Trial: cardiac resynchronization therapy. Circulation. 2010;122:985–992. [DOI] [PubMed] [Google Scholar]
- 30. Dickstein K, Bogale N, Priori S, et al. The European cardiac resynchronization therapy survey. Eur Heart J. 2009;30:2450–2460. [DOI] [PubMed] [Google Scholar]
- 31. Tang AS, Wells GA, Talajic M, et al; Resynchronization‐Defibrillation for Ambulatory Heart Failure Trial Investigators. Cardiac‐resynchronization therapy for mild‐to‐moderate heart failure. N Engl J Med. 2010;363:2385–2395. [DOI] [PubMed] [Google Scholar]
- 32. Celikyurt U, Agacdiken A, Sahin T, et al. Number of leads with fragmented QRS predicts response to cardiac resynchronization therapy. Clin Cardiol. 2013;36:36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]


