Abstract
Background
The optimal duration of dual antiplatelet therapy (DAPT) after acute coronary syndrome (ACS) is not known. Factors influencing DAPT duration are not well described.
Hypothesis
We hypothesized that continued DAPT 12 months beyond ACS would be associated with patient factors such as stent type and that it may be associated with lower rates of ischemic events.
Methods
The TIMI 38 Coronary Stent Registry (CSR) followed patients who completed the TRITON‐TIMI 38 trial, received a stent, and were alive and event free. Continuation of DAPT was determined by the treating physician.
Results
The CSR enrolled 2110 patients (1679>12 months from index ACS) and followed for a median of 2.1 additional years. DAPT was continued in 554 (26%) and was more likely to be continued in patients with drug‐eluting stents (DES; 54%) and in North America. The rate of cardiovascular death, MI, or stroke was 2.35% per year, and 13 patients (0.6%) experienced Academic Research Consortium definite or probable ST. Recurrent ischemic events were similar between patients who continued thienopyridine therapy and those who stopped at registry entry (P = 0.74 for cardiovascular death/MI/stroke; P = 0.72 for definite or probable ST). After propensity score adjustment, there was no significant difference in cardiovascular death/MI/stroke (P = 0.55) or bleeding (P = 0.51) with prolonged DAPT.
Conclusions
Patients stabilized for a year after ACS and stenting have low rates of ST relative to overall cardiovascular events. The decision to continue DAPT maybe associated with stent type (DES vs bare‐metal stent) and region.
Introduction
Dual antiplatelet therapy (DAPT) with a P2Y12 receptor antagonist added to aspirin has been shown to reduce the risk of ischemic events in patients presenting with acute coronary syndromes (ACS) through 12 months.1, 2, 3 However, when expanded to chronic treatment in patients with stable atherosclerotic vascular disease or risk factors, DAPT has not significantly reduced ischemic events. Analyses in selected subgroups with established disease, particularly myocardial infarction (MI), do suggest a benefit of prolonged DAPT as chronic therapy.4, 5 In addition, observational studies have described the risk of late stent thrombosis (ST) in stable patients with coronary stents and have raised concerns about discontinuation of DAPT even after 12 months, particularly in patients who have drug‐eluting stents (DES).6, 7 The largest randomized trials evaluating prolonged DAPT in patients with coronary stents have not shown benefit.8, 9
The uncertainty in the optimal duration of DAPT after MI is reflected in professional guideline recommendations that do not define an exact duration of therapy after ACS.10 As a result, clinicians must determine the duration of DAPT for individual patients based on characterization of risk. We sought to describe the patterns and predictors of DAPT utilization after 1 year in patients with ACS treated with coronary stenting as well as the rate of recurrent events in this stable population.
Methods
Study Population
The TIMI 38 Coronary Stent Registry (CSR) was a prospective, investigator‐initiated, observational study designed as a follow‐on to the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis In Myocardial Infarction 38 (TRITON‐TIMI 38) clinical trial, which randomized 13 608 patients presenting across the spectrum of ACS to treatment with either prasugrel or clopidogrel. Full inclusion and exclusion criteria for TRITON‐TIMI 38 have been previously published.11, 12
To qualify for registry inclusion, patients had to have been treated with a coronary stent and could not have experienced MI or ST after their index event. Participation in the registry was elective on a site and subject level. Trial sites electing to participate in the registry were required to receive approval from their local ethics boards for participation in the CSR. Once the registry was approved, qualifying patients were approached for their consent for participation in the TIMI 38 CSR (Supplementary Figure 1).
Subjects from 155 of 702 TRITON‐TIMI 38 sites in 20 countries participated in the CSR. Patients enrolled in the registry were more likely to be white, have a history of hypercholesterolemia, were less frequently treated with coronary artery bypass grafting (CABG), and were more likely to receive a DES compared with patients who did not enroll (Supplementary Table 1). Compared with TRITON‐TIMI 38, there was greater representation in the CSR from North America and Western Europe and less representation from Eastern Europe (Supplementary Table 1).
Protocol
Subjects randomized in the TRITON‐TIMI 38 trial were followed for 6 to 15 months (median, 14.5 months) during the trial. On their completion, consenting patients immediately began follow‐up in the CSR. Treatment decisions, including continuation of DAPT, were at the discretion of the patient's treating physician and administered as open‐label therapy not provided by study staff. During the registry follow‐up period, prasugrel was not approved for clinical use, so if thienopyridine treatment was continued, clopidogrel or ticlopidine were used. Patients in the CSR were followed at 6‐month intervals through scheduled visits. Information was recorded regarding antiplatelet use as well as clinical events.
Registry follow‐up was continued for an additional 2 years after the completion of the TRITON‐TIMI 38 trial, which ended in July 2007, with a common analytic registry end date of June 30, 2009. The follow‐up period for patients participating in the CSR is detailed in Supplementary Figure 1.
Endpoints
Patients in the CSR were followed for major adverse cardiovascular and bleeding events. All potential endpoints were reviewed and adjudicated by trained adjudicators using definitions used for TRITON‐TIMI 38.11, 12 Endpoints of interest included cardiovascular death (CVD), total mortality, MI, ST (Academic Research Consortium [ARC] definite + probable definition),13 stroke, and TIMI bleeding (major, minor, or requiring medical attention) not related to CABG.
Statistical Analysis
Baseline characteristics were compared using the χ2 test for categorical and the Wilcoxon rank‐sum test for continuous variables. Outcome comparisons were performed on the basis of the time to the first event and expressed as Kaplan‐Meier estimates. Hazard ratios (HR) were calculated from Cox proportional‐hazard models.
To adjust for differences in the comparison groups, propensity scores were created using logistic regression modeling. Separate propensity scores were created for the continuation of thienopyridine therapy at registry entry as well as the use of DES or bare‐metal stent (BMS) at the index intervention (Supplementary Tables 2 and 3). The propensity score for the use of DES at index had a C‐statistic of 0.88 and the propensity thienopyridine continuation had a C‐statistic of 0.81. For both models, missing values were assumed to be missing at random.
Adjusted HRs were calculated from Cox proportional‐hazard models using propensity stratification. This included an interactive process to define and verify that the strata were balanced on score and covariates. The predicted exposure levels were then divided into propensity strata, and the Cox model was used to estimate the treatment effect within each propensity score stratum measured and averaged across the strata to estimate the effect on the outcome of continuing vs not continuing thienopyridine. A sensitivity analysis using propensity matching was also performed to assess the adequacy of the stratification process.
Tests for interaction between subgroup and continued treatment with thienopyridine or not and testing for interactions between subgroup and stent type for DES/BMS analysis were performed using Cox models.
Results
Subjects from 155 of 702 TRITON‐TIMI 38 sites in 20 countries participated in the CSR. Patients enrolled in the registry were more likely to be white, have a history of hypercholesterolemia, were less frequently treated with CABG, and were more likely to receive a DES compared with patients who did not enroll (Supplementary Table 1). Compared with TRITON‐TIMI 38, there was greater representation in the CSR from North America and Western Europe and less representation from Eastern Europe (Supplementary Table 1).
Baseline characteristics for the registry population are shown in Table 1. A total of 2110 patients were included in the registry. Eighty percent (1679) of these patients were ≥12 months beyond their index ACS, and patients were followed for a median of an additional 2.1 years after inclusion in the registry. Overall, 4416 patient‐years of follow‐up were collected in the CSR. Of the 2110 patients included in the registry, 1144 (54%) received ≥1 DES, whereas 966 (46%) received BMS only. A total of 554 (26%) patients were continued on thienopyridine therapy at trial completion (Table 1).
Table 1.
Baseline Characteristics and Continuation of Dual Antiplatelet Therapy
| Variable | Total, N = 2110a | Thienopyridine, n = 554 | No Thienopyridine, n = 1545 | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age ≥75 years | 283 (13) | 76 (14) | 204 (13) | 0.76 |
| Male sex | 1581 (75) | 407 (73) | 1168 (76) | 0.32 |
| White race | 2005 (95) | 522 (95) | 1473 (96) | 0.35 |
| Region | ||||
| North America | 700 (33) | 353 (64) | 347 (22) | <0.001 |
| South America | 101 (5) | 16 (3) | 83 (5) | |
| Western Europe | 598 (28) | 64 (12) | 526 (34) | |
| Eastern Europe | 386 (18) | 74 (13) | 311 (20) | |
| Rest of world | 325 (15) | 47 (9) | 278 (18) | |
| Risk factors | ||||
| DM | 453 (21) | 120 (22) | 330 (21) | 0.88 |
| Current smoker | 782 (37) | 183 (33) | 596 (39) | 0.021 |
| Hypertension | 1316 (62) | 363 (66) | 947 (61) | 0.078 |
| Hypercholesterolemia | 1295 (61) | 373 (67) | 915 (59) | <0.001 |
| Previous MI | 363 (17) | 111 (20) | 249 (16) | 0.036 |
| Previous CHF | 65 (3) | 16 (3) | 49 (3) | 0.74 |
| CrCl <60 mL/min | 215 (10) | 54 (10) | 160 (10) | 0.68 |
| TRITON‐TIMI 38 presentation and treatment | ||||
| Qualifying NSTE‐ACS | 1608 (76) | 488 (88) | 1110 (72) | <0.001 |
| CABG | 2 (0.09) | 0 (0) | 2 (0.13) | 0.40 |
| BMS only | 966 (46) | 128 (23) | 830 (54) | <0.001 |
| DES only | 1045 (50) | 397 (72) | 645 (42) | <0.001 |
| DES (any) | 1144 (54) | 426 (77) | 715 (46) | <0.001 |
| ACEI/ARB | 1501 (71) | 383 (69) | 1111 (72) | 0.22 |
| β‐Blocker | 1891 (90) | 496 (90) | 1385 (90) | 0.94 |
| Statin | 1934 (92) | 519 (94) | 1405 (91) | 0.045 |
| Randomization group (prasugrel) | 1070 (51) | 278 (50) | 785 (51) | 0.80 |
| Events and follow‐up in TRITON‐TIMI 38 | ||||
| TIMI major non‐CABG bleed | 28 (1.3) | 10 (1.8) | 18 (1.2) | 0.26 |
| TIMI major/minor non‐CABG bleed | 52 (2.5) | 15 (2.7) | 37 (2.4) | 0.69 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARC, Academic Research Consortium; BMS, bare‐metal stent; CABG, coronary artery bypass grafting; CHF, congestive heart failure; CrCl, creatinine clearance; DES, drug‐eluting stent; DM, diabetes mellitus; MI, myocardial infarction; NSTE‐ACS, non–ST‐segment elevation acute coronary syndrome; TIMI, Thrombolysis In Myocardial Infarction; TRITON‐TIMI 38, Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis In Myocardial Infarction 38.
Data are presented as n (%).
Thienopyridine status missing in 11 patients.
Correlates and Patterns of Thienopyridine Continuation
A total of 554 (26%) patients were continued on thienopyridine therapy at registry entry. This proportion remained relatively stable through the 24 months of follow‐up in the registry (Figure 1). The proportion was greater in patients who received any DES than in those who received BMS only, but it remained stable in both groups over time (Figure 1).
Figure 1.
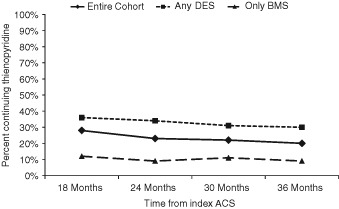
Percent of subjects who were continued on thienopyridine therapy over time for the overall cohort (diamonds), subjects who received only BMS (triangles), and subjects who received DES (squares). Abbreviations: ACS, acute coronary syndrome; BMS, bare‐metal stent; DES, drug‐eluting stent.
Patients in North America were more likely to be continued on thienopyridine therapy compared with all other regions (Table 1). This pattern remained stable during the 24 months of registry follow‐up (Figure 2). Patients who were not current smokers, those with hypercholesterolemia, and those with a history of prior MI or elective percutaneous coronary intervention (PCI) as well as those treated with DES were more frequently continued on thienopyridine therapy during registry follow‐up (Table 1).
Figure 2.
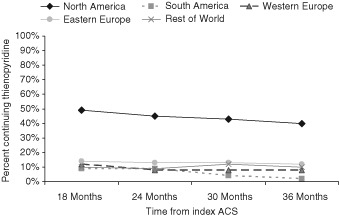
Percent of patients who were continued on thienopyridine therapy over time for North America (diamonds), South America (squares), Western Europe (triangles), Eastern Europe (circles), and rest of world (crosses). Abbreviations: ACS, acute coronary syndrome.
On multivariate analysis, continuation of thienopyridine therapy was significantly associated with North American region, treatment with DES, and history of elective PCI during trial participation (Supplementary Table 2A). Clinical and demographic factors including age, sex, hypertension, hypercholesterolemia, prior MI, or CABG were not associated with continued thienopyridine (Supplementary Table 2A). Similarly, angiographic factors such as the presence of multivessel disease and maximal stent length were not associated with continued thienopyridine therapy (Supplementary Table 2B).
Recurrent Events
Event rates for the total cohort over the period of registry follow‐up are shown in Figure 3. A total of 73 patients (3.0%) experienced a MI and 24 (1.1%) died of cardiovascular causes. Overall, the 2‐year rate of the composite of CVD, MI, or stroke was 4.4% (n = 103). Academic Research Consortium definite or probable ST occurred in 13 (0.60%) and TIMI major or minor bleeding in 17 (0.80%).
Figure 3.
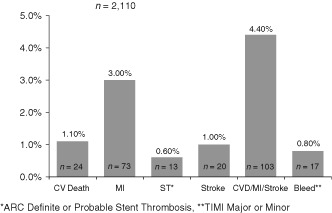
Cumulative incidence of endpoints at 2 years from registry entry. Abbreviations: ACS, acute coronary syndrome; ARC, Academic Research Consortium; CV, cardiovascular; CVD, cardiovascular death; KM, Kaplan‐Meier; MI, myocardial infarction; ST, stent thrombosis; TIMI, Thrombolysis In Myocardial Infarction.
Continued Thienopyridine Therapy and Outcomes
There was also no significant difference in rate of CVD, MI, or stroke in patients who continued thienopyridine therapy compared with those who did not (3.7% vs 4.7%; HR: 0.93, 95% confidence interval [CI]: 0.59‐1.45, P = 0.74; Table 2). Academic Research Consortium definite or probable ST was similar in patients who continued thienopyridine therapy compared with those who did not (0.8% vs 0.5%; HR: 1.24, 95% CI: 0.38‐4.01, P = 0.72).
Table 2.
Outcomes by Continued Thienopyridine Therapy
| Outcome | Thienopyridine, N = 554, N (2‐Year KM Rate) | No Thienopyridine, N = 1545, N (2‐Year KM Rate) | Adjusted HR (IQR)a | Adjusted P Valuea |
|---|---|---|---|---|
| Coronary death | 2 (0.2) | 14 (0.9) | 0.43 (0.09–2.07) | 0.30 |
| CVD | 3 (0.4) | 21 (1.3) | 0.32 (0.08–1.19) | 0.090 |
| All‐cause death | 8 (1.4) | 28 (1.7) | 0.73 (0.30–1.75) | 0.48 |
| MI | 21 (2.9) | 52 (3.1) | 1.08 (0.61–1.90) | 0.79 |
| Stroke | 4 (0.8) | 16 (1.1) | 0.61 (0.18–2.07) | 0.43 |
| ARC definite ST | 4 (0.8) | 7 (0.4) | 0.82 (0.21–3.15) | 0.77 |
| ARC definite/probable ST | 4 (0.8) | 9 (0.5) | 0.68 (0.19–2.43) | 0.55 |
| CVD/MI | 24 (3.3) | 67 (3.9) | 0.95 (0.56–1.60) | 0.84 |
| CVD/MI/stroke | 25 (3.7) | 78 (4.7) | 0.86 (0.52–1.42) | 0.55 |
| TIMI medical attention bleeding | 11 (2.1) | 16 (0.9) | 0.91 (0.38–2.18) | 0.83 |
| TIMI major bleeding | 1 (0.2) | 5 (0.4) | 0.39 (0.04–3.88) | 0.43 |
| TIMI major/minor bleeding | 7 (1.3) | 10 (0.6) | 1.46 (0.48–4.45) | 0.51 |
| Any TIMI bleed | 18 (3.5) | 24 (1.4) | 1.25 (0.62–2.53) | 0.54 |
Abbreviations: ARC, Academic Research Consortium; CVD, cardiovascular death; HR, hazard ratio; IQR, interquartile range; KM, Kaplan‐Meier; MI, myocardial infarction; ST, stent thrombosis; TIMI, Thrombolysis In Myocardial Infarction.
Data are presented as n (%).
Propensity stratified.
Unadjusted analyses showed TIMI major or minor bleeding was numerically higher in the thienopyridine group (1.3% vs 0.6%; HR: 1.99, 95% CI: 0.76‐5.22, P = 0.16;Table 2). When adjusted using propensity score stratification, there was no difference in the hazard of CVD or recurrent ischemic events for patients who continued thienopyridine therapy compared with those who discontinued it (Table 2). Similarly, there was no significant increase in the adjusted HR of TIMI major bleeding, TIMI minor bleeding, TIMI medical‐attention bleeding, or the composite of all 3 (Table 2).
Stent Type and Outcomes
The rate of CVD, MI, or stroke was similar in patients who received a DES at the index event compared with those who received BMS only (P = 0.36; Supplementary Table 3), and there were no significant differences in the rates of ARC definite or probable ST (P = 0.49) or bleeding (Supplementary Table 3). After propensity score adjustment, there were no significant differences in outcomes (ischemic or safety) by stent type (Supplementary Table 4).
When tested for an interaction between the continued use of thienopyridine therapy at registry entry and stent type (DES vs BMS), there was a significant interaction for CVD (P = 0.033; Supplementary Table 4) but no significant interaction for MI or ST (Supplementary Table 4).
For patients with DES, the adjusted hazard of CVD was lower (adjusted HR: 0.10, 95% CI: 0.01‐0.80, P = 0.031) in patients who continued thienopyridine therapy compared with those who did not; however, this difference was not seen in the BMS‐only group, where the hazard of CVD was similar for those who continued on thienopyridine therapy compared with those who did not (adjusted HR: 1.66, 95% CI: 0.32‐8.55, P = 0.54; Supplementary Table 4).
Discussion
The CSR prospectively recorded patterns of treatment and outcomes during long‐term follow‐up of a well‐characterized group of patients treated with coronary stenting for ACS. Greater than 80% of the cohort was treated with DAPT for >12 months from their index event, and all had survived the period before registry inclusion without experiencing an MI or ST.
Over the 2 years of registry follow‐up, we observed that patients were at much lower risk of CVD, MI, or stroke (2.0% at 1 year, 4.7% at 2 years) than during the acute phase observed during TRITON‐TIMI 38 (12.1% clopidogrel at 15 months) and during the initial phase after stabilization from ACS (TRITON‐TIMI 38 3‐day landmark 6.9%).12 Overall, ST was infrequent in the registry phase (13 ARC definite or probable events over 2 years) and was numerically similar to the rate of TIMI major bleeding. These findings indicate the clinical stability and low event rates of patients who have survived ≥1 year from their ACS and underscore the rarity of clinically detectable ST.
Approximately 26% of the CSR cohort was continued on thienopyridine therapy after 1 year. The rate of thienopyridine continuation was higher in patients with DES, those with recent PCI, and in patients from North America. Interestingly, continued therapy did not appear to be driven by demographic, clinical, or angiographic factors such as patient age, severity of coronary artery disease at index, maximal stent length, or history of recurrent MI. Patients who were continued on thienopyridine therapy tended to stay on therapy throughout follow‐up, with similar rates of use from 12 months forward through 36 months, suggesting that physicians tended to decide on a treatment strategy and remain consistent with that initial decision over time.
Observational studies have suggested that there is a higher rate of late ST in patients receiving DES compared with BMS.7, 14, 15 In the CSR, patients with DES were observed to experience higher rates of ST compared with patients with BMS only; however, after propensity score adjustment, this observation did not persist, suggesting, at least in part, that this finding was the result of patient characteristics rather than an intrinsic stent‐related risk. Use of DES, however, remained a significant predictor of continued thienopyridine use in the CSR, suggesting that treating clinicians identified this group as higher risk relative to those with BMS only. Similar findings have been shown in other registries of patients with atherosclerotic vascular disease.16
The optimal duration of DAPT after coronary stenting remains an open question. Randomized trials have not demonstrated benefit; however, they have been modestly powered for rare events such as late ST.8, 9 The DAPT Study17 (http://www.ClinicalTrials.gov NCT00977938), which has completed enrollment of approximately 20 000 patients with stents, and will randomize them to either continued DAPT or aspirin alone at 12 months, will provide important answers for patients who have undergone PCI. The CSR results underscore the low frequency of ST (0.6% at 2 years) in stable populations with coronary stents and the need for robustly sized trials.
The optimal treatment for patients with a history of ACS also remains an open question. This is particularly complex for the subset of ACS patients who have an indication for anticoagulation therapy, or triple antithromobotic therapy.18 The rate of CVD, MI, or stroke unrelated to coronary stenting in our population was approximately 5× greater than the rate of ARC definite or probable ST at 2 years (3% vs 0.6%). This suggests that prolonged DAPT, if beneficial, is more likely to show benefit for de novo events rather than stent‐related events and that those at risk for recurrent events may have the greatest opportunity to benefit. This hypothesis is supported by subgroup analyses from trials of DAPT in stable populations suggesting benefit in those with a history of MI.4 In addition, trials of novel antithrombotic agents in stable patients with atherosclerotic vascular disease have shown greatest benefit in patients with history of MI.19, 20
There are important limitations of this analysis. First, as an observational cohort study, differences between nonrandomized groups may be related to unmeasured differences that could not be controlled for with propensity scoring and adjustment. Second, the registry was modestly sized at 2110 and was likely underpowered to detect differences in infrequent events such as ST.
Conclusion
Overall, we found that patients who survive beyond 1 year from ACS have a low rate of death or recurrent ischemic events and a very low rate of ST. Prolonged DAPT was associated with use of a DES and North American residency and was not associated with risk factors or angiographic findings. Well‐powered randomized trials are needed to determine which patients may benefit from prolonged DAPT and the optimal duration of this therapy.
Sources of Funding
This study was supported by a grant from Daiichi Sankyo Co, Ltd, and Eli Lilly and Co.
Disclosures
The TIMI Study Group has received significant research grant support from Accumetrics, Amgen, AstraZeneca, Beckman Coulter, Bristol‐Myers Squibb, CV Therapeutics, Daiichi Sankyo Co Ltd, Eli Lilly and Co, GlaxoSmithKline, Integrated Therapeutics, Merck and Co, Nanosphere, Novartis Pharmaceuticals, Nuvelo, Ortho‐Clinical Diagnostics, Pfizer, Roche Diagnostics, Sanofi‐Aventis, Sanofi‐Synthelabo, Siemens Medical Solutions, and Singulex.
Dr. Nicolau has research grants from Sanofi, Novartis, GSK; consulting/honoraria from Bayer, AstraZeneca, Sanofi, Daiichi Sankyo, BMS
Debra Miller RN RCIS is an employee of and owns stock in Eli Lilly and Company
Dr Antman has received research grants from Eli Lilly, Daiichi Sankyo, and Sanofi‐Aventis
Dr Wiviott has received research funding from Eli Lilly, Daiichi Sankyo, Merck, Astra Zeneca, as well as consulting/CME Honoraria from AstraZeneca, ARENA, Bayer, Bristol Myers Squibb, Daiichi Sankyo, Merck, Novartis, Pfizer, Eli Lilly, Sanofi‐Aventis
The other authors report no conflicts.
Supporting information
Appendix S1. XXXXX
Disclosures and Sources of Funding can be found on past page 6 of this article.
References
- 1. Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation. N Engl J Med. 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 2. Chen ZM, Pan HC, Chen YP, et al. Early intravenous then oral metoprolol in 45 852 patients with acute myocardial infarction: randomised placebo‐controlled trial. Lancet. 2005;366:1622–1632. [DOI] [PubMed] [Google Scholar]
- 3. Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation. N Engl J Med. 2005;352:1179–1189. [DOI] [PubMed] [Google Scholar]
- 4. Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. [DOI] [PubMed] [Google Scholar]
- 5. Bhatt DL, Flather MD, Hacke W, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49:1982–1988. [DOI] [PubMed] [Google Scholar]
- 6. James SK, Stenestrand U, Lindback J, et al. Long‐term safety and efficacy of drug‐eluting versus bare‐metal stents in Sweden. N Engl J Med. 2009;360:1933–1945. [DOI] [PubMed] [Google Scholar]
- 7. Pfisterer M, Brunner‐La Rocca HP, Buser PT, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug‐eluting stents: an observational study of drug‐eluting versus bare‐metal stents. J Am Coll Cardiol. 2006;48:2584–2591. [DOI] [PubMed] [Google Scholar]
- 8. Valgimigli M, Campo G, Monti M, et al. Short‐ versus long‐term duration of dual‐antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125:2015–2026. [DOI] [PubMed] [Google Scholar]
- 9. Park SJ, Park DW, Kim YH, et al. Duration of dual antiplatelet therapy after implantation of drug‐eluting stents. N Engl J Med. 2010;362:1374–1382. [DOI] [PubMed] [Google Scholar]
- 10. Antman EM, Hand M, Armstrong PW, et al. 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST‐Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to review new evidence and update the ACC/AHA 2004 Guidelines for the Management of Patients With ST‐Elevation Myocardial Infarction, writing on behalf of the 2004 Writing Committee [published correction appears in Circulation. 2008;117:e162]. Circulation. 2008;117:296–329. [DOI] [PubMed] [Google Scholar]
- 11. Wiviott SD, Antman EM, Gibson CM, et al; TRITON‐TIMI 38 Investigators . Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis In Myocardial Infarction 38 (TRITON‐TIMI 38). Am Heart J. 2006;152:627–635. [DOI] [PubMed] [Google Scholar]
- 12. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 13. Mauri L, Hsieh WH, Massaro JM, et al. Stent thrombosis in randomized clinical trials of drug‐eluting stents. N Engl J Med. 2007;356:1020–1029. [DOI] [PubMed] [Google Scholar]
- 14. Bavry AA, Kumbhani DJ, Helton TJ, et al. Late thrombosis of drug‐eluting stents: a meta‐analysis of randomized clinical trials. Am J Med. 2006;119:1056–1061. [DOI] [PubMed] [Google Scholar]
- 15. Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus‐eluting and paclitaxel‐eluting stents in routine clinical practice: data from a large two‐institutional cohort study. Lancet. 2007;369:667–678. [DOI] [PubMed] [Google Scholar]
- 16. Rodriguez F, Cannon CP, Steg PG, et al; for REACH Registry Investigators . Predictors of long‐term adherence to evidence‐based cardiovascular disease medications in outpatients with stable atherothrombotic disease: findings from the REACH registry. Clin Cardiol. 2013; doi: 10.1002/clc22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mauri L, Kereiakes DJ, Normand SL, et al. Rationale and design of the Dual Antiplatelet Therapy Study: a prospective, multicenter, randomized, double‐blind trial to assess the effectiveness and safety of 12 versus 30 months of dual antiplatelet therapy in subjects undergoing percutaneous coronary intervention with either drug‐eluting stent or bare‐metal stent placement for the treatment of coronary artery lesions. Am Heart J. 2010;160:1035–1041, 1041.e1. [DOI] [PubMed] [Google Scholar]
- 18. Reed GW, Cannon CP. Triple oral antithrombotic therapy in atrial fibrillation and coronary artery stenting. Clin Cardiol. 2013;36:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morrow DA, Braunwald E, Bonaca MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404–1413. [DOI] [PubMed] [Google Scholar]
- 20. Scirica BM, Bonaca MP, Braunwald E, et al. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2°P‐TIMI 50 trial. Lancet. 2012;380:1317–1324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. XXXXX


