ABSTRACT
Background
Adverse left ventricular (LV) remodeling predicts heart failure symptoms and overt LV dysfunction in patients with hypertrophic cardiomyopathy (HCM), but its influence on the occurrence of sudden cardiac death (SCD) is unknown. The aim of this study was to investigate the effect of adverse LV remodeling on SCD risk in patients with HCM.
Hypothesis
Adverse LV remodeling increases SCD in HCM patients.
Methods
This study included 41 patients with HCM who experienced SCD; each case was matched with 3 controls based on age, gender, and time of first contact. In this population of 164 patients, predictors of SCD were identified using univariable and multivariable logistic regression and expressed as odds ratio (OR) with 95% confidence interval (CI).
Results
Baseline characteristics, such as New York Heart Association (NYHA) class, systolic and diastolic left ventricular function, left ventricular wall thickness, left atrial size, atrial fibrillation, and established risk factors for SCD were similar in cases and controls. Independent predictors of SCD during follow‐up (median follow‐up, 7.7 ± 6.5 years) were: increase in NYHA class (OR: 8.7 [95% CI: 2.5‐30.5], P = 0.001), decrease of fractional shortening (per % decrease, OR: 1.09 [95% CI: 1.03‐1.14], P = 0.001), and decrease of diastolic function (OR: 3.5 [95% CI: 1.2‐10.2], P = 0.02).
Conclusions
This study shows that SCD risk in HCM increases when adverse remodeling occurs. Because cases and controls were similar at baseline, these findings emphasize the importance of vigilant follow‐up of HCM patients and could aid clinical decision making concerning implantable cardioverter‐defibrillator implantation, especially in patients with moderate risk for SCD.
Introduction
Sudden cardiac death (SCD) is the most devastating expression of hypertrophic cardiomyopathy (HCM). The annualized rate of SCD in HCM patients is presumed to be ±1% per year.1, 2 Implantable cardioverter‐defibrillators (ICDs) have proven to be an effective way of preventing SCD in HCM patients, both in primary as in secondary prevention.3, 4, 5 For secondary prevention, there is universal agreement that ICDs should be implanted. The SCD risk in primary prevention is assessed by risk stratification based on the work of Elliott et al in 20001, 6 and updated to its current form in the 2011 American College of Cardiology Foundation/American Hearth Association guidelines.7
HCM is not a static disease, and the guidelines recommend to repeat the risk stratification every 12 to 24 months. Recently, Olivotto et al8 identified 4 clinical stages of HCM: nonhypertrophic HCM, classic phenotype, adverse remodeling, and overt dysfunction. Adverse remodeling is characterized by the presence of unfavorable structural and functional changes, and patients with adverse remodeling are presumed to be at increased risk of heart failure and overt dysfunction.8, 9, 10, 11 Characteristics of adverse remodeling as described by Olivotto et al are the following: a decrease in systolic and diastolic left ventricular function (LVF),11 left atrial (LA) and left ventricular (LV) dilatation,9, 12 an increase in symptoms and functional limitations,9 the occurrence of atrial fibrillation (AF),13 reduction or loss of left ventricular outflow tract (LVOT) obstruction,10, 14 and thinning of LV walls.10 The SCD risk increases from 0.5% to 1% per year for patients with the classic phenotype to 10% per year for patients with overt dysfunction, but the SCD risk of patients with adverse remodeling is unknown.8 We performed, therefore, a case–control study to investigate the relation between adverse LV remodeling and the risk of SCD in HCM patients.
Methods
Study Design and Patient Population
This study included 41 patients with HCM who died because of SCD and 123 age‐ and gender‐matched control patients with HCM attending the adult outpatient clinic at the Thoraxcenter, Erasmus Medical Center, Rotterdam, the Netherlands between January 1, 1995 and December 31, 2011. Each patient had an established diagnosis of HCM, based on unexplained left ventricular hypertrophy of ≥15 mm.15 Patients with HCM linked to Noonan's syndrome, Fabry's disease, mitochondrial disease, or congenital heart defects were excluded. Patients younger than 16 years were excluded. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional review board.
Cases were defined as HCM patients with SCD and controls as HCM patients without SCD. Patients with a prior SCD event before the study period or first contact were excluded. Cases were identified at the time of SCD, and chart review was done retrospectively. SCD is defined as death occurring <1 hour from the onset of symptoms in patients who had previously experienced a relatively stable or uneventful clinical course. In this study, SCD also included successfully resuscitated cardiac arrest or appropriate implantable cardioverter‐defibrillator (ICD) intervention for ventricular fibrillation (VF) or fast (≥200 bpm) ventricular tachycardia (VT). Each case was matched with 3 controls based on the following parameters: age (±1 year) and gender, and year of first contact (±3 years).
Assessment of Adverse Remodeling
The following clinical characteristics and signs of adverse remodeling were examined at baseline and at last documented contact before SCD or end of follow‐up: New York Heart Association (NYHA) functional class, maximal left ventricular wall thickness (LVWT), end‐systolic diameter (ESD), end‐diastolic diameter (EDD), LA size, LVOT gradient during rest and/or exercise, systolic LVF, diastolic LVF, the occurrence of AF (either persistent or paroxysmal), medical therapy and a history of septal reduction therapy, and either alcohol septal ablation or myectomy.
Systolic LVF was evaluated by visual assessment of ejection fraction (EF) and scored as normal (EF >55%), mildly reduced (EF 45%–55%), moderate (EF 30%–45%), and poor LVF (EF <30%).16 Additionally, fractional shortening was calculated. Decrease of systolic LVF was defined as the decrease of >1 classification during follow‐up (eg, from normal to mildly reduced). Diastolic LVF was described as normal, abnormal relaxation (stage I), pseudonormalization (stage II), and restrictive filling (stage III) based on the latest guidelines.17, 18 Decrease of diastolic LVF was defined as the decrease of ≥1 stage (eg, from normal to abnormal relaxation).
Patient Follow‐Up
Mortality was provided from civil service population registers and information provided by general practitioners and at the center where follow‐up occurred. Clinical characteristics were retrieved from hospital patient records provided by the center where follow‐up occurred.
Echocardiographic evaluation was independently performed by cardiologists with extensive experience in reading echocardiograms who were blinded to clinical data. The administrative censoring date for follow‐up in the control group was November 1, 2012.
Statistical Analysis
SPSS version 20 (IBM, Armonk, NY) and Microsoft Excel 2010 (Microsoft Corp., Redmond, WA) were used for all statistical analyses. Categorical variables were summarized as percentages. Normally distributed continuous data were expressed as mean ± standard deviation, and non‐normally distributed data were expressed as median ± interquartile range. For comparing variables, means and medians χ2 test, Student t test, or Mann–Whitney U test were used for categorical and continuous data, respectively. To identify clinical predictors of SCD, univariable and multivariable logistic regression analysis were used. Clinical variables from last contact prior SCD or censoring date were selected for backward stepwise multivariable analysis if univariable P value was <0.1 and were expressed as odds ratio (OR) with 95% confidence interval (CI). The final number of variables was restricted according to the number of end points to avoid overfitting the multivariable model. A P value <0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 41 cases of SCD were identified. Seventeen patients died, 11 were successfully resuscitated after cardiac arrest, and 13 patients had appropriate ICD intervention for either VF or fast VT (>200 bpm). Three controls per case were identified based on age, gender, and year of first contact, and thus the total study population consisted of 41 cases and 123 controls (Table 1). The majority of cases and controls were male (112 [66%] patients) with an average age of 46 ± 15 years (range, 16–73 years) at baseline. No significant differences were described at baseline between cases and controls in NYHA class, diastolic LVF, EDD, ESCD, fractional shortening, LVOT gradient, maximal LVWT, and LA size. More cases had systolic impairment at baseline (9 [21%] patients and 9 [7%] patients, P = 0.03). Both groups had similar numbers of patients with a family history for SCD, unexplained syncope, and LVWT ≥30 mm, and distribution of major risk factors was comparable (Figure 1). The medication used to alleviate symptoms was not significantly different in both groups.
Table 1.
Baseline Characteristics Of 41 Scd Cases And 123 Controls (No Scd)
| SCD, n = 41 | No SCD, n = 123 | P | |
|---|---|---|---|
| Age, y | 45.6 ± 15 | 45.6 ± 15 | 1.0 |
| Male | 27 (66) | 81 (66) | 1.0 |
| NYHA III/IV | 18 (44) | 41 (33) | 0.2 |
| Maximum LVWT, mm | 22 ± 5 | 21 ± 5 | 0.4 |
| Left atrial size, mm | 48 ± 10 | 45 ± 8 | 0.07 |
| LVOT gradient, mm Hg | 54 ± 48 | 51 ± 44 | 0.9 |
| Left ventricular function | |||
| End diastolic diameter, mm | 45 ± 6 | 42 ± 6 | 0.07 |
| Fractional shortening, % | 45 ± 9 | 42 ± 9 | 0.2 |
| Reduced ejection fraction (<55%) | 9 (17) | 9 (7) | 0.02 |
| Diastolic dysfunction | 21 (51) | 70 (57) | 0.7 |
| Risk factors for SCD | |||
| SCD in family history | 9 (22) | 21 (17) | 0.5 |
| nsVT on Holter monitoring | 9 (22) | 19 (15) | 0.3 |
| Abnormal exercise BP response | 5 (12) | 7 (6) | 0.6 |
| Syncope | 12 (30) | 23 (19) | 0.08 |
| LVWT ≥30 mm | 8 (20) | 10 (8) | 0.2 |
| ≥2 risk factors | 11 (27) | 17 (14) | 0.06 |
| Medication | |||
| β‐Blocker | 14 (34) | 60 (49) | 0.1 |
| Calcium channel blocker | 11 (27) | 35 (28) | 0.8 |
| Amiodarone | 4 (10) | 4 (3) | 0.09 |
Abbreviations: BP, blood pressure; LVOT, left ventricular outflow tract; LVWT, left ventricular wall thickness; nsVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; SCD, sudden cardiac death.
Data are presented as number (percentage) unless stated otherwise.
Figure 1.
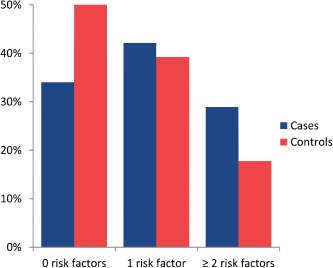
Distribution of risk factors showing the distribution of the number of established risk factors for sudden cardiac death in 41 cases and 123 controls.
Follow‐up
The median follow‐up was 7.7 ± 6.5 years, 6.6 ± 8.0 years for cases and 7.9 ± 5.8 years for controls (P = 0.01). Compared to the controls, the cases showed increase of NYHA class, decreased systolic and diastolic LVF, increased EDD, increased LA size, a decrease of fractional shortening, and a higher incidence of AF. Septal reduction therapy was performed in 19 (46%) cases and in 41 (33%) controls (P = 0.3) (Tables 2 and 3). LVOT gradient reduction in this group was from 93 ± 36 mm Hg at baseline to 28 ± 33 mm Hg at last follow‐up, and the procedure was successful in 45 patients (76%). During follow‐up, an ICD was implanted in 27 patients, 19 (46%) cases and 8 (7%) controls (P < 0.001).
Table 2.
Clinical Characteristics at Last Evaluation of 41 SCD Cases and 123 Controls (No SCD)
| SCD, n = 41 | No SCD, n = 123 | P | |
|---|---|---|---|
| Age, y | 51.9 ± 16 | 53.8 ± 15 | 0.4 |
| Duration of follow‐up, y | 6.6 ± 8.0 | 7.9 ± 5.8 | 0.01 |
| NYHA III/IV | 14 (34) | 7 (6) | < 0.001 |
| Maximum LVWT, mm | 20 ± 5 | 19 ± 4 | 0.09 |
| Left atrial size, mm | 52 ± 12 | 47 ± 8 | 0.01 |
| Atrial fibrillation | 15 (37) | 16 (13) | 0.001 |
| LVOT gradient, mm Hg | 29 ± 35 | 27 ± 30 | 0.8 |
| Left ventricular function | |||
| End‐diastolic diameter, mm | 50 ± 10 | 45 ± 7 | 0.002 |
| Fractional shortening, % | 32 ± 10 | 42 ± 10 | < 0.001 |
| Reduced ejection fraction (<55%) | 18 | 19 | < 0.001 |
| Diastolic dysfunction | 35 | 78 | < 0.02 |
| Septal reduction | |||
| Alcohol septal ablation | 10 (24) | 21 (17) | 0.3 |
| Myectomy | 9 (22) | 20 (16) | 0.4 |
Abbreviations: LVOT, left ventricular outflow tract; LVWT, left ventricular wall thickness; NYHA, New York Heart Association; SCD, sudden cardiac death.
Data are presented as number (percentage) unless stated otherwise.
Table 3.
Characteristics of Adverse Remodeling Between 41 SCD Cases and 123 Controls (No SCD)
| SCD, n = 41 | No SCD, n = 123 | P | |
|---|---|---|---|
| Increase of NYHA class | 13 (32) | 9 (7) | <0.001 |
| Decrease of LVWT, mm | 1 ± 5 | 2 ± 4 | 0.6 |
| Increase of left atrial size, mm | 4 ± 7 | 1 ± 5 | 0.04 |
| Increase of end‐diastolic diameter, mm | 6 ± 9 | 2 ± 5 | 0.04 |
| Decrease of fractional shortening, % | 10 ± 11 | 0 ± 10 | <0.001 |
| Decrease of systolic LV function | 17 (41) | 13 (11) | <0.001 |
| Decrease of diastolic LV function | 19 (46) | 29 (24) | 0.004 |
| Atrial fibrillation | 15 (37) | 16 (13) | 0.001 |
Abbreviations: LV, left ventricular; LVWT, left ventricular wall thickness; NYHA, New York Heart Association; SCD, sudden cardiac death.
Data are presented as number (percentage) unless stated otherwise.
Predictors of SCD
Table 4 summarizes the results of univariable and multivariable analysis. Characteristics of adverse remodeling, such as decrease in systolic and diastolic function, advancement of NYHA class, left atrial and ventricular dilation, and decrease of fractional shortening were all significant predictors in univariable analysis. In multivariable analysis, independent predictors for SCD were: fractional shortening (per % decrease, OR: 1.09 [95% CI: 1.03‐1.14], P = 0.001), decrease of diastolic function (OR: 3.5 [95% CI: 1.2‐10.1], P = 0.02), and increase of NYHA functional class (OR: 8.7 [95% CI: 2.5‐30.5], P = 0.001).
Table 4.
Analysis of Clinical Variables Associated With Sudden Cardiac Death in 164 Hypertrophic Cardiomyopathy Patients (41 Cases and 123 Controls)
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Predictor | OR | 95% CI | P | OR | 95% CI | P |
| Indicators of adverse LV remodeling | ||||||
| Increase of NYHA Class | 5.8 | 2.2‐14.9 | <0.001 | 8.7 | 2.5‐30.5 | 0.001 |
| Decrease of systolic left ventricular function | 6.0 | 2.6‐14.0 | <0.001 | |||
| Decrease of diastolic left ventricular function | 3.0 | 1.3‐6.4 | 0.005 | 3.5 | 1.2‐10.1 | 0.02 |
| Decrease of LVWT (per mm) | 1.0 | 0.9‐1.1 | 0.5 | |||
| Increase of end‐diastolic diameter (per mm) | 1.09 | 1.02‐1.15 | 0.008 | |||
| Decrease of fractional shortening (per %) | 1.09 | 1.04‐1.15 | <0.001 | 1.08 | 1.03‐1.14 | 0.003 |
| Increase of left atrial diameter (per mm) | 1.07 | 1.01‐1.14 | 0.02 | |||
| Atrial fibrillation | 3.9 | 1.7‐8.8 | 0.001 | 2.3 | 0.5‐10.7 | 0.3 |
| Additional predictors | ||||||
| Alcohol septal ablation | 1.6 | 0.7‐3.7 | 0.3 | |||
| Surgical myectomy | 1.4 | 0.6‐3.5 | 0.4 | |||
| ≥2 risk factors | 2.3 | 1.0‐5.4 | 0.06 | |||
Abbreviations: CI, confidence interval; LV, left ventricular; LVWT, left ventricular wall thickness; NYHA, New York Heart Association; OR, odds ratio.
The variables in the multivariable column are the variables selected for the final model.
Discussion
The findings of this study suggest that the presence of signs of adverse LV remodeling in HCM patients increases the risk for SCD. Additionally, deterioration of the NYHA functional class and decrease of systolic and diastolic LV function are predictors of SCD in HCM patients.
A decrease of systolic function (in this study identified by LV dilatation, a decrease in fractional shortening, and visual assessment of the EF) is more prevalent in the cases than in the controls. The increased risk of SCD in HCM patients with a low EF is already established, not only in general heart disease but also in HCM.9, 10, 11, 19 Our findings suggest that not only a low EF but the decrease of systolic function (EF <55%) on its own increases the risk of SCD. The same statement can be made for diastolic dysfunction. Not only is a severe diastolic dysfunction a strong independent predictor for SCD,20 but deterioration of diastolic LV function indicates an increased risk of SCD.
Current risk stratification for SCD is based on 5 major risk factors identified in the last 2 decades. This includes a detailed family history of SCD, a personal history of unexplained syncope, the assessment of maximal LVWT, Holter monitoring, and blood pressure response to exercise. HCM patients are at increased risk in cases of a family history of SCD in first‐degree relatives, an LVWT of ≥30 mm, and a personal history of syncope. In these patients, ICD implantation is considered reasonable (class IIa, level of evidence: C). Other indications are the presence of nonsustained ventricular tachycardia on Holter monitoring or abnormal blood pressure response during exercise testing, especially in the presence of other potential risk modifiers for SCD, such as the presence of LVOT obstruction ≥30 mm Hg, left ventricular apical aneurysms, or delayed enhancement on magnetic resonance imaging.7, 21 The presence of delayed enhancement correlates with electrocardiographic changes22 and has an trend toward significance for the risk of SCD.23 Although SCD does occur in patients with no risk factors, there is consensus not to implant an ICD in these patients.
Risk stratification in patients with only 1 risk factor remains a gray area, in which the presence of the aforementioned potential arbitrators may lead to implantation of an ICD. This is a relevant clinical challenge; in our population 72 (40%) patients had only 1 major risk factor (Figure 1). The presence of signs of adverse LV remodeling could aid clinical decision making in these patients. If signs of adverse LV remodeling are present, it could be considered an additional argument toward implanting an ICD. In patients with no classic risk factors, the presence of signs of adverse remodeling could trigger repeating the risk stratification.
Both guidelines1, 7 advise to repeat the risk stratification every 12 to 24 months. However, this is an arbitrary time interval, and with the results of our study it should be advised to repeat the risk stratification when the aforementioned signs of adverse LV remodeling are identified. Additionally, as our results imply that adverse LV remodeling is also a potential arbitrator, it should be evaluated at every repeated risk stratification.
Furthermore, it is important to note that at baseline, both cases and controls were similar, and no independent predictors for SCD could be identified. It was only during follow‐up that differences between both groups were identified. This underscores the importance of vigilant follow‐up of HCM patients, not only repeating the SCD risk stratification and evaluating systolic and diastolic LVF and symptoms to determine if adverse LV remodeling is present.
Limitations
This study has several limitations. This study was performed in a referral center for patients with HCM; therefore, selection bias may have influenced the study results. The findings in this study will not be helpful in the first assessment of the patient, as baseline characteristics should be known, and the patient should be followed over time. This made data collection limited to variables that were routinely collected, and novel developments during the follow‐up period were difficult to include. For this reason, advanced echocardiographic imaging such as strain rate imaging and cardiac magnetic resonance information was insufficient to use in this study. Rhythm documentation of the event was not available for all SCD cases. Considering appropriate ICD shocks as SCD end point could overestimate the SCD rate. A greater proportion of patients in the SCD group had septal ablation (24% vs 17%) or myectomy (22% vs 16%) than those without SCD. Although the incidence of septal reduction therapy was not statistically significant between the groups, the confounding effect of septal reduction therapy cannot be excluded, because the study may not be powered sufficiently to adjust for this variable in the multivariable model.
Conclusion
Adverse LV remodeling is not only a predictor for heart failure and overt dysfunction in HCM patients, but these patients are also at increased risk for SCD. This can only be identified during vigilant follow‐up of HCM patients, as initial screening might not show any signs of adverse remodeling. During follow‐up, not only should current risk stratification be repeated, but signs of adverse remodeling should be evaluated. It also implies that SCD occurs not only in the young and asymptomatic but especially when the disease progresses.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Maron BJ, McKenna WJ, Danielson GK, et al; Task Force on Clinical Expert Consensus Documents. American College of Cardiology; Committee for Practice Guidelines. European Society of Cardiology. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003;42:1687–713. [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. [DOI] [PubMed] [Google Scholar]
- 3. Maron BJ, Spirito P, Shen WK, et al. Implantable cardioverter‐defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA. 2007;298:405–412. [DOI] [PubMed] [Google Scholar]
- 4. Schinkel AF, Vriesendorp PA, Sijbrands EJ, et al. Outcome and complications after implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy: systematic review and meta‐analysis. Circ Heart Fail. 2012;5:552–559. [DOI] [PubMed] [Google Scholar]
- 5. Vriesendorp PA, Schinkel AF, Van Cleemput J, et al. Implantable cardioverter‐defibrillators in hypertrophic cardiomyopathy: patient outcomes, rate of appropriate and inappropriate interventions, and complications. Am Heart J. 2013;166:496–502. [DOI] [PubMed] [Google Scholar]
- 6. Elliott PM, Poloniecki J, Dickie S, et al. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36:2212–2218. [DOI] [PubMed] [Google Scholar]
- 7. Gersh BJ, Maron BJ, Bonow RO, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:2761–2796. [DOI] [PubMed] [Google Scholar]
- 8. Olivotto I, Cecchi F, Poggesi C, et al. Patterns of disease progression in hypertrophic cardiomyopathy: an individualized approach to clinical staging. Circ Heart Fail. 2012;5:535–546. [DOI] [PubMed] [Google Scholar]
- 9. Harris KM, Spirito P, Maron MS, et al. Prevalence, clinical profile, and significance of left ventricular remodeling in the end‐stage phase of hypertrophic cardiomyopathy. Circulation. 2006;114:216–225. [DOI] [PubMed] [Google Scholar]
- 10. Maron BJ, Spirito P. Implications of left ventricular remodeling in hypertrophic cardiomyopathy. Am J Cardiol. 1998;81:1339–1344. [DOI] [PubMed] [Google Scholar]
- 11. Olivotto I, Maron BJ, Appelbaum E, et al. Spectrum and clinical significance of systolic function and myocardial fibrosis assessed by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol. 2010;106:261–267. [DOI] [PubMed] [Google Scholar]
- 12. Nistri S, Olivotto I, Betocchi S, et al. Prognostic significance of left atrial size in patients with hypertrophic cardiomyopathy (from the Italian Registry for Hypertrophic Cardiomyopathy). Am J Cardiol. 2006;98:960–965. [DOI] [PubMed] [Google Scholar]
- 13. Olivotto I, Cecchi F, Casey SA, et al. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. 2001;104:2517–2524. [DOI] [PubMed] [Google Scholar]
- 14. Ciro E, Maron BJ, Bonow RO, et al. Relation between marked changes in left ventricular outflow tract gradient and disease progression in hypertrophic cardiomyopathy. Am J Cardiol. 1984;53:1103–1109. [DOI] [PubMed] [Google Scholar]
- 15. Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–276. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Bierig M, Devereux RB, et al; Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 17. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 18. Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. [DOI] [PubMed] [Google Scholar]
- 19. Melacini P, Basso C, Angelini A, et al. Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy. Eur Heart J. 2010;31:2111–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Biagini E, Spirito P, Rocchi G, et al. Prognostic implications of the Doppler restrictive filling pattern in hypertrophic cardiomyopathy. Am J Cardiol. 2009;104:1727–1731. [DOI] [PubMed] [Google Scholar]
- 21. Maron BJ. Contemporary insights and strategies for risk stratification and prevention of sudden death in hypertrophic cardiomyopathy. Circulation. 2010;121:445–456. [DOI] [PubMed] [Google Scholar]
- 22. Song BG, Yang HS, Hwang HK, et al. Correlation of electrocardiographic changes and myocardial fibrosis in patients with hypertrophic cardiomyopathy detected by cardiac magnetic resonance imaging. Clin Cardiol. 2013;36:31–35. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Green JJ, Berger JS, Kramer CM, et al. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012;5:370–377. [DOI] [PubMed] [Google Scholar]


