Abstract
Background:
Contrast‐induced nephropathy (CIN) has been generally considered to be transient and associated with unfavorable clinical outcomes.
Hypothesis:
The aim of this study was to investigate whether Mehran risk score could predict CIN with persistent renal dysfunction and long‐term clinical outcomes in acute myocardial infarction (AMI) patients undergoing percutaneous coronary intervention (PCI).
Methods:
We analyzed the clinical data of 1041 AMI patients. The primary end point was defined as major adverse cardiovascular and cerebrovascular event (MACCE) including death, reinfarction, target vessel revascularization, heart failure requiring hospital admission, and stroke. Patients were categorized into 4 groups according to risk scores: low (≤ 5, n = 596), moderate (6–10, n = 265), high (11–15, n = 111), and very high (≥16, n = 69).
Results:
Among the 148 patients (14.2%) who developed CIN, persistent renal dysfunction was observed in 68 patients. Presence in high‐ or very high‐risk groups was the most important independent risk factor of CIN with persistent renal dysfunction (odds ratio: 3.35, 95 confidence interval [CI]: 1.89–5.92, P < 0.001). Furthermore, patients in higher‐risk groups experienced significantly more MACCE and mortality 2 years after PCI. Using multivariate analysis, significant increase in the hazard ratio (HR) for MACCE was noted in moderate‐ (HR: 1.40, 95% CI: 0.97–2.03, P = 0.075), high‐ (HR 1.96, 95% CI: 1.22–3.15, P = 0.006), and very high‐risk (HR 2.40, 95% CI: 1.36–4.21, p = 0.002) groups, compared with the low‐risk group. The very high‐risk group had approximately 6‐fold increase in mortality over the low‐risk group (HR: 6.22, 95% CI: 2.77–13.95, P < 0.001).
Conclusions:
Mehran risk score predicted CIN with persistent renal dysfunction and long‐term clinical outcomes in patients with AMI.
Drs. Jin Wi and Young‐Guk Ko contributed equally to the preparation of the article.
This study was supported partly by grants from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (No. A085012, A102064, and A110879); the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (No.A08 5136); Yonsei University (6‐2009‐0008); Korea Institute of Medicine; and the Cardiovascular Research Center, Seoul, Korea.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Contrast‐induced nephropathy (CIN) is a generally transient and reversible form of acute renal failure that develops after angiographic procedures using contrast medium.1 However, CIN has been reported to be associated with poor clinical outcomes, including increased short‐ and long‐term mortality and morbidity.1, 2 Important predisposing factors for CIN include older age, preexisting renal failure, hemodynamic instability, congestive heart failure, diabetes mellitus, anemia, and large‐volume contrast media.3, 4, 5 Mehran CIN risk score, including 8 clinical and procedural variables, was initially developed for the simple evaluation of an individual patient's risk to develop CIN.5 The risk for CIN is significantly higher among patients with acute myocardial infarction (AMI) undergoing percutaneous coronary intervention (PCI) than among the general population undergoing elective PCI, and CIN is an important predictor of clinical outcomes in the AMI population.6 A recent study reported that Mehran risk score could predict short‐ and long‐term outcomes in patients with ST‐segment elevation myardial infarction (STEMI).7 Recently, we reported that over 45% of AMI patients who developed CIN after PCI had persistent renal dysfunction without recovery, and this patient group had more adverse long‐term outcomes than the patients without CIN or the patients experiencing CIN with transient renal dysfunction.8 Thus, the purpose of our study was to investigate whether the Mehran risk score is valid in the prediction of CIN with persistent renal dysfunction and adverse long‐term clinical outcomes in STEMI and non–ST‐segment elevation myocardial infarction (NSTEMI) patients.
Methods
Study Population
The registry of the Infarction Prognosis Study, a prospective, single‐center, cohort study is maintained at the Severance Cardiovascular Hospital according to an institutional review board‐approved protocol. It includes demographic, clinical, and procedural data for patients with AMI. From the registry, we identified all patients ≥20 years old who underwent PCI from May 2005 to July 2009. Patients exposed to contrast medium within 7 days before PCI or patients with end‐stage renal disease requiring chronic dialysis treatment were excluded from the analysis. Written informed consent was obtained from each patient before enrollment and investigations were in accordance with the Declaration of Helsinki
Definitions
CIN was defined as >25% or >0.5 mg/dL increase in serum creatinine level within 48 hours after administration of contrast medium when no other major kidney insult was identified. Recovery of renal function in CIN patients was defined as return to serum creatinine level ≤25% or ≤0.5 mg/dL above the baseline level at 1 month, as detected by single measurement. Transient renal dysfunction referred to partial or complete recovery of renal function to the baseline level within 1 month, whereas persistent renal dysfunction referred to impaired renal function more than 1 month after PCI.
Mehran CIN risk score included 8 clinical and procedural variables: hypotension, intra‐aortic balloon pump (IABP), congestive heart failure, chronic kidney disease, diabetes, age >75 years, anemia, and volume of contrast.5 Patients were categorized into 4 risk groups based on Mehran risk score: low‐ (≤5), moderate‐ (6–10), high‐ (11–15), and very high‐ (≥16) risk group. The primary end point was defined as major adverse cardiovascular and cerebrovascular event (MACCE), including all‐cause death, nonfatal AMI, target‐vessel revascularization, heart failure requiring hospital admission, and cerebrovascular events.
Study Protocol
Patients were treated according to the international treatment guidelines.9 Serum creatinine concentration was routinely measured before PCI and 24 hours, 48 hours, and 1 month after PCI. Glomerular filtration rate was estimated using the Cockcroft‐Gault formula. Patients received intravenous hydration with 0.9% normal saline for 12 hours before and after PCI. All patients underwent echocardiographic evaluation within 24 hours of hospital admission. Relevant baseline and follow‐up laboratory and clinical data were recorded during the hospital stay. After hospital discharge, patients were followed up in the clinic at 1 month and every 3 months thereafter.
Percutaneous Coronary Intervention
PCI was performed by the femoral or radial approach using standard methods. Patients received 250 mg aspirin, 300 mg clopidogrel (600 mg in case of STEMI), and a bolus of 5000 U heparin immediately after admission, followed by an additional bolus of heparin for maintaining activated clotting time of >250 s. Nonionic, iso‐osmolality, contrast medium, iodixanol (Visipaque; GE Healthcare, Princeton, NJ), was used almost exclusively (>99% of procedures). After PCI, dual antiplatelet therapy with 100 mg/d aspirin and 75 mg/d clopidogrel was generally maintained for at least 12 months. Angioplasty technique, the type of stents, supportive pharmacological therapies, and contrast medium dose were left to the discretion of the cardiologist.
Statistical Analysis
Continuous data were reported as mean ± standard deviation and compared by the analysis of variance test. Categorical data were expressed as absolute value and percentage and compared by the χ 2 or Fisher exact test. A multivariate logistic regression was used to determine independent risk factors of CIN and CIN with persistent renal dysfunction. The cumulative incidence of clinical events was estimated using the Kaplan‐Meier method, and the significance of curves was tested using the log‐rank test. Univariate and multivariate Cox regression analyses using forward stepwise selection were performed to identify independent predictors of MACCE and mortality. Variables included in Mehran CIN risk score were excluded in multivariate analysis to avoid redundancy. For patients who experienced multiple events, the first episode was taken as the standard. Statistical significance was defined as P < 0.05. In addition, 95% confidence interval (CI) was calculated for treatment differences in all efficacy variables. Statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL).
Results
Baseline Characteristics
Of 1200 patients from the registry cohort, 159 patients were excluded for the following reasons: PCI was not performed (n = 59), serum creatinine level was not properly checked after PCI (n = 76), or chronic dialysis was performed (n = 24). Therefore, 1041 patients (751 male [72%]; mean age, 62.7 ± 12.2 years) remained for the analysis. Among them, STEMI was diagnosed in 515 (49%) and NSTEMI in 526 (51%) patients. There was no difference in Mehran risk score between patients with STEMI and those with NSTEMI.
Patients were categorized into 4 groups by Mehran risk score: low, moderate, high, and very high. Table 1 shows the baseline characteristics of each CIN risk group. With increasing risk of CIN, patients were older and more likely to be female. Comorbidities such as diabetes, hypertension, anemia, and chronic renal insufficiency were also more frequently observed in higher‐risk groups. Patients in higher‐risk groups exhibited more frequently previous history of revascularization and multivessel coronary artery disease. Heart failure, hypotension, use of an IABP insertion, and lower left ventricular ejection fraction (LVEF) were more frequently present in higher‐risk groups. In addition, patients in higher‐risk groups showed higher baseline and peak serum creatinine levels and received more contrast medium volume during PCI.
Table 1.
Comparison of Baseline Clinical Characteristics Among Mehran CIN‐Risk Groups
| Variables | Overall (n = 1041) | Low (n = 596) | Moderate (n = 265) | High (n = 111) | Very High (n = 69) | P Value |
|---|---|---|---|---|---|---|
| Mehran CIN‐risk score | 6.0 ± 5.4 | 2.4 ± 1.5 | 7.5 ± 1.6 | 12.7 ± 1.6 | 20.0 ± 3.7 | <0.001 |
| Male | 748 (72%) | 457 (77%) | 184 (69%) | 67 (60%) | 40 (58%) | <0.001 |
| Age, y | 62.7 ± 12.2 | 59.2 ± 11.3 | 65.0 ± 11.5 | 69.1 ± 11.6 | 72.6 ± 11.4 | <0.001 |
| Age >75 years | 171 (16%) | 30 (5%) | 66 (25%) | 39 (35%) | 36 (52%) | <0.001 |
| BMI, kg/m2 | 24.1 ± 3.5 | 24.5 ± 3.6 | 23.8 ± 3.2 | 23.3 ± 3.3 | 22.7 ± 2.7 | <0.001 |
| BMI <24 kg/m2 | 459 (49%) | 236 (43%) | 125 (51%) | 60 (64%) | 38 (73%) | <0.001 |
| Smokers | 394 (38%) | 258 (43%) | 89 (34%) | 29 (26%) | 18 (26%) | <0.001 |
| Diabetes mellitus | 269 (26%) | 61 (10%) | 118 (45%) | 46 (41%) | 44 (64%) | <0.001 |
| Hypertension | 509 (49%) | 253 (42%) | 148 (56%) | 66 (60%) | 42 (61%) | <0.001 |
| Dyslipidemia | 397 (38%) | 248 (42%) | 90 (34%) | 35 (32%) | 24 (35%) | 0.058 |
| eGFR <60 mL/min | 377 (36%) | 123 (21%) | 119 (45%) | 77 (69%) | 58 (84%) | <0.001 |
| Previous stroke | 61 (6%) | 28 (5%) | 18 (7%) | 8 (7%) | 7 (10%) | 0.172 |
| Previous MI | 59 (6%) | 32 (5%) | 9 (3%) | 9 (8%) | 9 (13%) | 0.016 |
| Previous PCI | 137 (13%) | 69 (12%) | 31 (12%) | 23 (20%) | 14 (20%) | 0.015 |
| Previous CABG | 22 (2%) | 6 (1%) | 8 (3%) | 5 (4%) | 3 (4%) | 0.012 |
| Clinical presentation | ||||||
| STEMI | 515 (49%) | 294 (49%) | 123 (46%) | 58 (52%) | 40 (58%) | 0.343 |
| NSTEMI | 526 (51%) | 302 (51%) | 142 (54%) | 53 (48%) | 29 (42%) | 0.343 |
| Cardiogenic shock | 166 (16%) | 0 (0%) | 53 (20%) | 53 (48%) | 60 (87%) | <0.001 |
| IABP | 59 (6%) | 0 (0%) | 0 (0%) | 22 (20%) | 37 (54%) | <0.001 |
| LV dysfunction (LVEF <40%) | 214 (23%) | 87 (16%) | 51 (21%) | 42 (42%) | 34 (57%) | <0.001 |
| Mean LVEF, % | 48.4 ± 12.7 | 51.0 ± 11.8 | 48.0 ± 11.8 | 42.5 ± 13.4 | 36.0 ± 13.1 | <0.001 |
| Multivessel disease | 632 (61%) | 338 (57%) | 163 (62%) | 73 (66%) | 58 (84%) | <0.001 |
| Contrast volume, mL | 219 ± 72 | 210 ± 68 | 228 ± 75 | 229 ± 77 | 251 ± 79 | <0.001 |
| Contrast volume >300 mL | 180 (17%) | 82 (14%) | 49 (19%) | 27 (24%) | 22 (32%) | <0.001 |
| Hemoglobin, g/dL | 13.9 ± 2.1 | 14.6 ± 1.6 | 13.5 ± 2.1 | 12.6 ± 2.2 | 11.8 ± 2.6 | <0.001 |
| Anemiaa | 208 (20%) | 27 (5%) | 82 (31%) | 53 (48%) | 46 (67%) | <0.001 |
| Stent type | ||||||
| DES | 999 (96%) | 571 (96%) | 255 (96%) | 106 (95%) | 67 (97%) | 0.959 |
| BMS | 42 (4%) | 25 (4%) | 10 (4%) | 5 (5%) | 2 (3%) | 0.959 |
| Medication | ||||||
| Aspirin | 1,011 (97%) | 580 (97%) | 255 (96%) | 107 (96%) | 69 (100%) | 0.373 |
| Clopidogrel | 934 (90%) | 541 (91%) | 232 (88%) | 97 (87%) | 64 (93%) | 0.332 |
| Beta blocker | 829 (80%) | 500 (84%) | 201 (76%) | 82 (74%) | 46 (67%) | <0.001 |
| ACEi/ARB | 874 (84%) | 517 (87%) | 212 (80%) | 87 (78%) | 58 (84%) | 0.028 |
| Statin | 910 (87%) | 523 (88%) | 231 (87%) | 93 (84%) | 63 (91%) | 0.502 |
Abbreviations: ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BMS, bare metal stents; CABG, coronary artery bypass graft; CIN, contrast‐induced nephropathy; DES, drug‐eluting stents; eGFR, glomerular filtration rate estimated by Cockcroft‐Gault formula; IABP, intra‐aortic balloon pump; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non–ST‐elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐elevation myocardial infarction.
Defined as hemoglobin <13 g/dL for males and <12 g/dl for females.
Overall, CIN was observed in 148 (14.2%) patients: 53 (9%) in the low‐risk group, 31 (12%) in the moderate‐risk group, 39 (35%) in the high‐risk group, and 25 (36%) in the very high‐risk group (P < 0.001), respectively (Table 2). At multivariable analysis, the odds ratio (OR) for CIN was 4.89 (95% CI: 2.55–9.40, P < 0.001) in very high‐risk group and 4.60 (95% CI: 2.71–7.81, P < 0.001) in high‐risk group, as compared with the low‐risk group. There was no difference between the moderate‐risk and low‐risk group. Female gender (OR: 1.95, 95% CI: 1.30‐2.91, P = 0.001) was another independent predictor of CIN.
Table 2.
Changes in Renal Function and Incidence of CIN in Mehran CIN Risk Groups
| Variables | Overall (n = 1,041) | Low (n = 596) | Moderate (n = 265) | High (n = 111) | Very High (n = 69) | P Value |
|---|---|---|---|---|---|---|
| Pre‐PCI SCr, mg/dL | 1.06 ± 0.41 | 0.97 ± 0.21 | 1.04 ± 0.32 | 1.39 ± 0.77 | 1.46 ± 0.65 | <0.001 |
| Post‐PCI SCr, mg/dL | 1.16 ± 0.62 | 1.01 ± 0.28 | 1.11 ± 0.47 | 1.67 ± 1.27 | 1.79 ± 0.88 | <0.001 |
| ΔSCr (Post‐ Pre‐PCI), mg/dL | 0.10 ± 0.36 | 0.05 ± 0.19 | 0.08 ± 0.33 | 0.29 ± 0.70 | 0.32 ± 0.57 | <0.001 |
| Pre‐PCI eGFR, mL/min | 70.1 ± 26.7 | 79.1 ± 24.5 | 65.6 ± 22.8 | 50.1 ± 24.3 | 42.0 ± 22.2 | <0.001 |
| Post‐PCI eGFR, mL/min | 67.3 ± 27.6 | 76.8 ± 25.1 | 63.1 ± 23.3 | 46.0 ± 24.4 | 35.7 ± 20.6 | <0.001 |
| Pre‐PCI eGFR <30 mL/mina | 53 (5.1%) | 5 (0.8%) | 8 (3.0%) | 20 (18.0%) | 20 (29.0%) | <0.001 |
| Post‐PCI eGFR <30 mL/mina | 79 (7.6%) | 10 (1.7%) | 15 (5.7%) | 28 (25.2%) | 26 (37.7%) | <0.001 |
| SCr at 1 month, mg/dL | 1.23 ± 0.72 | 1.10 ± 0.53 | 1.19 ± 0.56 | 1.50 ± 0.64 | 1.78 ± 1.33 | <0.001 |
| CIN | 148 (14.2%) | 53 (8.9%) | 31 (11.7%) | 39 (35.1%) | 25 (36.2%) | <0.001 |
| Transient renal dysfunction | 80 (54.1%) | 32 (60.4%) | 12 (38.7%) | 19 (48.7%) | 17 (68.0%) | 0.102 |
| Persistent renal dysfunction | 68 (45.9%) | 21 (39.6%) | 19 (61.3%) | 20 (51.3%) | 8 (32.0%) | 0.102 |
Abbreviations: CIN, contrast‐induced nephropathy; eGFR, glomerular filtration rate estimated by Cockcroft‐Gault formula; PCI, percutaneous coronary intervention; SCr, serum creatinine.
Denotes stage IV or V renal failure.
Among the 148 patients with CIN, persistent renal dysfunction was observed in 68 (46%) patients. In univariate analysis, categorization in high‐ or very high CIN‐risk groups, hypertension, elevated pre‐PCI serum creatinine level (>1.5 mg/dL), anemia, IABP, decreased LVEF (<40%), age >75 years, female gender, and contrast volume >300 mL were associated with persistent CIN. In multivariate analysis, categorization in high‐ or very high CIN‐risk groups and hypertension were identified as significant independent predictors of persistent CIN (Table 3).
Table 3.
Independent Predictors of CIN With Persistent Renal Dysfunction: Multivariate Logistic Regression Analysis
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| CIN risk group | ||||||
| Low and moderate risk | 1.00 | Reference | N/A | 1.00 | Reference | N/A |
| High and very high risk | 3.78 | 2.26–6.32 | <0.001 | 3.35 | 1.89‐5.92 | <0.001 |
| Hypertension | 2.47 | 1.46–4.20 | 0.001 | 2.21 | 1.23‐3.99 | 0.008 |
| SCr >1.5 mg/dL | 3.44 | 1.85‐6.39 | <0.001 | |||
| Anemia | 2.51 | 1.49–4.22 | 0.001 | |||
| IABP | 2.41 | 1.09–5.31 | 0.029 | |||
| LVEF <40% | 2.31 | 1.35–3.95 | 0.002 | |||
| Age >75 years | 2.09 | 1.20–3.66 | 0.009 | |||
| Female gender | 2.00 | 1.21–3.30 | 0.007 | |||
| Contrast volume >300 mL | 1.80 | 1.03–3.17 | 0.041 | |||
Abbreviations: Anemia is defined as hemoglobin < 13 g/dL for males and < 12 g/dl for females. CI, confidence interval; CIN, contrast‐induced nephropathy, IABP, intra‐aortic balloon pump; LVEF, left ventricular ejection fraction; N/A, not available; OR, odds ratio; SCr, serum creatinine level.
Clinical Outcomes
Patients were followed up for a mean duration of 22.8 ± 15.9 months. Clinical outcomes at 1 month and 2 years are summarized in Table 4. Two‐year MACCEs were observed in 166 patients (15.9%): all‐cause death in 84, reinfarction in 25, target vessel revascularization in 31, heart failure in 15, and stroke in 11 patients. Patients in higher‐risk groups showed both significantly higher rates of MACCE and mortality than those in lower‐risk group. There were significant differences in the cumulative rates of MACCE and mortality among all CIN‐risk groups (Figure 1). In addition, aggravation of renal function from stage I, II, or III to stage IV or V renal failure after PCI occurred in 30 patients, including 5 patients with renal failure requiring dialysis.
Table 4.
Clinical Outcomes Among Contrast‐Induced Nephropathy Risk Groups
| Clinical Events | Overall (n = 1041) | Low (n = 596) | Moderate (n = 265) | High (n = 111) | Very High (n = 69) | P Value |
|---|---|---|---|---|---|---|
| MACCE at 1‐month follow‐up | 53 (5.1%) | 13 (2.2%) | 9 (3.4%) | 12 (12.6%) | 19 (27.5%) | <0.001 |
| Death, all‐cause | 43 (4.1%) | 5 (0.8%) | 8 (3.0%) | 12 (10.8%) | 18 (26.1%) | <0.001 |
| Reinfarction | 6 (0.6%) | 4 (0.7%) | 1 (0.4%) | 0 (0.0%) | 1 (1.4%) | 0.574 |
| TVR | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.000 |
| Heart failurea | 1 (0.1%) | 1 (0.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.000 |
| Cerebrovascular events | 3 (0.3%) | 3 (0.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.749 |
| MACCE at 2‐year follow‐up | 166 (15.9%) | 66 (11.1%) | 42 (15.8%) | 29 (26.1%) | 29 (42.0%) | <0.001 |
| Death, all‐cause | 84 (8.1%) | 14 (2.3%) | 23 (8.7%) | 22 (19.8%) | 25 (36.2%) | <0.001 |
| Reinfarction | 25 (2.4%) | 15 (2.5%) | 6 (2.3%) | 1 (0.9%) | 3 (4.3%) | 0.518 |
| TVR | 31 (3.0%) | 20 (3.4%) | 8 (3.0%) | 2 (1.8%) | 1 (1.4%) | 0.867 |
| Heart failurea | 15 (1.4%) | 10 (1.7%) | 2 (0.8%) | 3 (2.7%) | 0 (0.0%) | 0.355 |
| Cerebrovascular events | 11 (1.1%) | 7 (1.2%) | 3 (1.1%) | 1 (0.9%) | 0 (0.0%) | 1.000 |
Abbreviations: MACCE, major adverse cardiovascular and cerebrovascular events; TVR, target vessel revascularization.
Heart failure requiring hospitalization.
Figure 1.
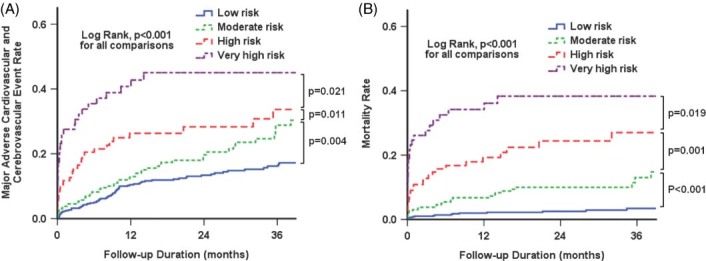
Cumulative event rates stratified by Mehran contrast‐induced nephropathy risk groups. (A) Major adverse cardiovascular and cerebrovascular events. (B) Mortality.
Multivariate Cox regression analysis revealed that CIN risk stratification by Mehran score was a significant independent predictor of MACCE and mortality (Table 5). Significant increase in HR both for MACCE and mortality was noted from moderate to high‐ and very high CIN‐risk groups, compared with the low‐risk group. Previous stroke, CIN, or body mass index <24 kg/m2 were other independent predictors of MACCE and mortality.
Table 5.
Multivariate Cox Regression Analysis
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Major adverse cardiovascular and cerebrovascular events | ||||||
| CIN risk group | <0.001 | 0.003 | ||||
| Low risk of CIN | 1.00 | Reference | N/A | 1.00 | Reference | N/A |
| Moderate risk of CIN | 1.65 | 1.17‐2.34 | 0.005 | 1.40 | 0.97‐2.03 | 0.075 |
| High risk of CIN | 2.85 | 1.89‐4.31 | <0.001 | 1.96 | 1.22‐3.15 | 0.006 |
| Very high risk of CIN | 5.38 | 3.52‐8.23 | <0.001 | 2.40 | 1.36‐4.21 | 0.002 |
| Previous stroke | 1.77 | 1.16‐2.70 | 0.008 | 1.68 | 1.01‐2.80 | 0.045 |
| CIN | 2.27 | 1.64‐3.15 | <0.001 | 1.66 | 1.12‐2.45 | 0.011 |
| BMI <24 kg/m2 | 1.89 | 1.41‐2.54 | <0.001 | 1.44 | 1.04‐1.99 | 0.026 |
| SCr >1.5 mg/dL | 3.52 | 2.47‐5.02 | <0.001 | |||
| IABP | 2.93 | 1.84‐4.67 | <0.001 | |||
| Anemia | 2.30 | 1.70‐3.10 | <0.001 | |||
| Shock | 2.18 | 1.56‐3.03 | <0.001 | |||
| Age >75 years | 1.94 | 1.40‐2.69 | <0.001 | |||
| Female gender | 1.81 | 1.40‐2.34 | <0.001 | |||
| LVEF <40% | 1.71 | 1.23‐2.38 | 0.001 | |||
| Multivessel disease | 1.49 | 1.11‐2.00 | 0.009 | |||
| Hypertension | 1.38 | 1.07‐1.79 | 0.013 | |||
| Mortality | ||||||
| CIN risk group | <0.001 | <0.001 | ||||
| Low risk of CIN | 1.00 | Reference | N/A | 1.00 | Reference | N/A |
| Moderate risk of CIN | 4.08 | 2.16‐7.71 | <0.001 | 2.85 | 1.43‐5.69 | 0.003 |
| High risk of CIN | 10.16 | 5.33‐19.39 | <0.001 | 5.26 | 2.52‐10.97 | <0.001 |
| Very high risk of CIN | 20.31 | 10.67‐38.64 | <0.001 | 6.22 | 2.77‐13.95 | <0.001 |
| CIN | 5.67 | 3.53‐9.12 | <0.001 | 2.89 | 1.69‐4.95 | <0.001 |
| Previous stroke | 2.51 | 1.53‐4.13 | <0.001 | 2.63 | 1.36‐5.09 | 0.004 |
| BMI <24 kg/m2 | 3.56 | 2.16‐5.87 | <0.001 | 1.78 | 1.01‐3.13 | 0.046 |
| SCr >1.5 mg/dL | 8.22 | 5.36‐12.61 | <0.001 | |||
| IABP | 5.76 | 3.43‐9.69 | <0.001 | |||
| Shock | 4.40 | 2.89‐6.70 | <0.001 | |||
| Anemia | 4.01 | 2.65‐6.07 | <0.001 | |||
| LVEF <40% | 3.83 | 2.38‐6.16 | <0.001 | |||
| Age >75 years | 3.07 | 1.99‐4.72 | <0.001 | |||
| Female gender | 1.67 | 1.18‐2.37 | 0.004 | |||
| Hypertension | 1.59 | 1.12‐2.26 | 0.010 | |||
Abbreviations: Anemia is defined as hemoglobin < 13 g/dL for males and < 12 g/dl for females. BMI, body mass index; CI, confidence interval; CIN, contrast‐induced nephropathy; HR, hazard ratio; IABP, intra‐aortic balloon pump; LVEF, left ventricular ejection fraction; N/A, not available; SCr, serum creatinine.
Discussion
The major findings of this study are as follows: (1) Mehran risk score was able to predict the risk of CIN in patients both with STEMI and NSTEMI; (2) high Mehran risk score (≥11) was an independent predictor of CIN with persistent renal dysfunction; and (3) Mehran risk score was an important independent prognostic predictor of long‐term MACCE and mortality in AMI patients treated with PCI. Patients in higher‐risk groups had higher rates of MACCE and mortality than those in lower‐risk groups.
Mehran risk score was initially developed to predict CIN after nonurgent PCI.5 In the original study, AMI patients were excluded from the analysis. However, various studies have reported that the risk of CIN is significantly higher among AMI patients undergoing PCI than among the general population undergoing elective PCI.6, 9, 10, 11, 12 Systemic perfusion impairment or hemodynamic instability caused by left ventricular dysfunction, large volume of contrast medium, and insufficient time of prophylactic hydration for renal protection are potential factors contributing to higher CIN risk in AMI patients.10, 11, 13, 14 Although CIN was reported to be generally associated with increased morbidity and mortality in patients undergoing PCI, only a few studies have focused on the clinical implications of CIN among AMI patients. Marenzi et al first reported that CIN was associated with a higher in‐hospital complication rate and mortality in AMI patients undergoing PCI.6 Similar findings were also observed later by Wickenbrock et al.12 However, similar to our study results, they found no difference in the incidence of CIN between STEMI and NSTEMI patients. Recently, Sgura et al reported that Mehran CIN risk score was able to predict not only CIN, but also short‐ and long‐term MACCE in patients with STEMI.7 We also reported that CIN has an adverse impact on long‐term outcomes in AMI patients.8 Because the Mehran score was developed to estimate the risk of CIN, it is plausible that the Mehran score can also predict adverse long‐term outcomes. Therefore, a new aspect of the present study was to investigate whether the application of Mehran score could be expanded to our AMI study cohort including both STEMI and NSTEMI for the prediction of CIN and the long‐term outcomes. As demonstrated by the results of the present study, Mehran risk score is a valuable predictor of future adverse clinical outcomes in all patients with AMI undergoing PCI.
CIN has been generally considered benign and transient pathology. However, the Dartmouth Dynamic Registry demonstrated that renal function in 54.6% of patients with CIN did not return to baseline after 2 weeks.15 We also reported that over 45% of AMI patients experiencing CIN had persistent renal dysfunction.8 Moreover, we demonstrated that persistent CIN was associated with significantly higher rates of adverse clinical outcomes than transient CIN or absence of CIN in AMI patients. Patients who developed transient CIN, however, had a higher event rate compared to those without CIN. Thus, the reversibility of renal function after development of CIN has significant implication for clinical outcomes among AMI patients. The question arises whether Mehran score can also predict persistent CIN. Interestingly, a higher Mehran risk score (≥11) was identified as the most important predictor of persistent CIN. Thus, patients at high risk for CIN are more likely to develop persistent CIN and to suffer more adverse clinical events than those at lower risk. This finding is another new aspect of our study.
The present study has several limitations. First, our study is a retrospective study with inherent limitations. Second, the incidence of CIN might be underestimated because patients in whom creatinine was not adequately checked or creatinine increased after 48 hours were not included in the analysis. In particular, AMI patients who died within 48 hours after PCI were excluded from the analysis. Third, the precise cause of renal dysfunction after PCI was difficult to determine. Whether other factors such as ischemia or atheroembolism contributed to the renal dysfunction remains largely unknown. Finally, our study does not provide any etiological link between renal function impairment after PCI and the increased risk of MACCE and death. We believe that multiple risk factors related to CIN indirectly contributed to the adverse clinical events rather than renal dysfunction directly caused increased MACCE and mortality. However, this needs to be explored in further studies.
Conclusion
Mehran risk score is an important independent predictor of CIN with persistent renal dysfunction and long‐term MACCE in AMI patients undergoing PCI.
References
- 1. McCullough PA, Adam A, Becker CR, et al. Epidemiology and prognostic implications of contrast‐induced nephropathy. Am J Cardiol. 2006;98:5K–13K. [DOI] [PubMed] [Google Scholar]
- 2. Best PJ, Lennon R, Ting HH, et al. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2002;39: 1113–1119. [DOI] [PubMed] [Google Scholar]
- 3. Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast material‐induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989;320:143–149. [DOI] [PubMed] [Google Scholar]
- 4. Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. [DOI] [PubMed] [Google Scholar]
- 5. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast‐induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 6. Marenzi G, Lauri G, Assanelli E, et al. Contrast‐induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–1785. [DOI] [PubMed] [Google Scholar]
- 7. Sgura FA, Bertelli L, Monopoli D, et al. Mehran contrast‐induced nephropathy risk score predicts short‐ and long‐term clinical outcomes in patients with ST‐elevation myocardial infarction. Circ Cardiovasc Interv. 2010;3:491–498. [DOI] [PubMed] [Google Scholar]
- 8. Wi J, Ko YG, Kim JS, et al. Impact of contrast‐induced acute kidney injury with transient or persistent renal dysfunction on long‐term outcomes of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Heart. 2011;97: 1753–1757. [DOI] [PubMed] [Google Scholar]
- 9. Van de Werf F, Ardissino D, Betriu A, et al. Management of acute myocardial infarction in patients presenting with ST‐segment elevation. The Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2003;24:28–66. [DOI] [PubMed] [Google Scholar]
- 10. Marenzi G, Assanelli E, Marana I, et al. N‐acetylcysteine and contrast‐induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–2782. [DOI] [PubMed] [Google Scholar]
- 11. Senoo T, Motohiro M, Kamihata H, et al. Contrast‐induced nephropathy in patients undergoing emergency percutaneous coronary intervention for acute coronary syndrome. Am J Cardiol. 2010;105:624–628. [DOI] [PubMed] [Google Scholar]
- 12. Wickenbrock I, Perings C, Maagh P, et al. Contrast medium induced nephropathy in patients undergoing percutaneous coronary intervention for acute coronary syndrome: Differences in STEMI and NSTEMI. Clin Res Cardiol. 2009;98: 765–772. [DOI] [PubMed] [Google Scholar]
- 13. Marenzi G, Assanelli E, Campodonico J, et al. Contrast volume during primary percutaneous coronary intervention and subsequent contrast‐induced nephropathy and mortality. Ann Intern Med. 2009;150:170–177. [DOI] [PubMed] [Google Scholar]
- 14. Zhao JL, Yang YJ, Zhang YH, et al. Effect of statins on contrast‐induced nephropathy in patients with acute myocardial infarction treated with primary angioplasty. Int J Cardiol. 2008;126: 435–436. [DOI] [PubMed] [Google Scholar]
- 15. Brown JR, Malenka DJ, DeVries JT, et al. Transient and persistent renal dysfunction are predictors of survival after percutaneous coronary intervention: insights from the Dartmouth Dynamic Registry. Catheter Cardiovasc Interv. 2008; 72:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]


